Abstract
Objective
The purpose of this pilot study was to determine the scientific and process feasibility in an effort to direct future larger trials.
Methods:
Scientific Feasibility: Twelve subjects were randomly allocated to an intervention and a control group. The intervention protocol consisted of intraoral vibration therapy on the muscles of mastication bilaterally for a period of 1 minute per muscle. Process Feasibility: Several feasibility outcomes were examined including recruitment and retention rates and consent.
Results:
Scientific Feasibility: Large effect sizes were generated for both mouth opening and VAS in favour of the intervention group. Process Feasibility: a recruitment ratio of 2.3 respondents to 1 participant was determined, along with a retention to loss ratio of 13:1 and a consent to loss ratio of 12:0.
Conclusion:
Scientific Feasibility: The scientific results should be interpreted with caution due to the small sample sizes employed. The study seems to support the scientific feasibility of a future larger single treatment trial. Process Feasibility: Recruitment and retention rates and ratios seem to support future studies. Utilizing the feasibility results of the current study to direct a future larger multiple treatment trial consistent with other comparable TMD studies however is limited.
Keywords: temporomandibular joint, vibration therapy, mouth opening, chiropractic
Abstract
Objectif :
L’objectif de ce projet pilote était de déterminer la faisabilité scientifique et la faisabilité du processus afin de planifier des essais cliniques de plus grande envergure.
Méthodes :
Faisabilité scientifique : Douze sujets étaient répartis de manière aléatoire dans un groupe intervention et un groupe témoin. Le protocole d’intervention comprenait la thérapie par vibration intraorale appliquée bilatéralement durant une minute par muscle sur les muscles de mastications. Faisabilité du processus : Plusieurs mesures de faisabilité ont été estimées, incluant les taux de recrutement, de rétention et de consentement.
Résultats :
Faisabilité scientifique : Des effets de grandes tailles ont été observés dans le groupe d’intervention pour l’ouverture de la bouche et l’échelle analogue de douleur (VAS). Faisabilité de processus : Le ratio de recrutement était de 2.3 répondants pour 1 participant, le ratio de rétention et de perte de 13:1 et de consentement et de perte de 12:0.
Conclusion :
Faisabilité scientifique : Les résultats doivent être interprétés avec prudence considérant le petit échantillon de sujets. L’étude semble appuyer la faisabilité de mener un essai clinique de plus grande envergure avec une intervention unidimensionnelle. Faisabilité du processus : Les taux et ratios de recrutement et de rétention observés semblent également appuyer les études futures. Cependant, l’emploie des résultats du présent projet de faisabilité dans le but d’élaborer un futur essai clinique avec une intervention multidimensionnelle similaire à d’autres études comparables sur les désordres temporomandibulaires est limité.
Keywords: articulation temporo-mandibulaire, thérapie par vibration, ouverture de la bouche, chiropratique
Background
Temporomandibular disorders (TMD) are a class of disorders affecting the muscles of mastication and/or the temporomandibular joint (TMJ).1,2 The prevalence of TMD is reported as 10–30%3,4 in the general population but can be as high as 42.9% in university students5. Symptoms can present as muscle and joint tenderness, popping or clicking, decreased jaw movement, altered mechanics or occasionally ear symptoms.3
There are various ways to classify TMD. The National Institute of Dental and Craniofacial Research (NIDCR), a branch of the National Institutes of Health (NIH), classifies TMD into three main categories: myofascial pain, internal derangement and degenerative joint disease. Internal derangement of the joint includes a dislocated jaw, displaced disc or injured condyle, while degenerative joint disease incorporates arthritides of the TMJ.6 Myofascial pain presents as discomfort or pain in the muscles controlling jaw movements as well as the neck and shoulder muscles.7 Stohler (2000), attributes up to 50% of all TMDs to masticatory myalgias or masticatory muscle disorders.7 However, a study of the incidence and prevalence of myofascial pain in the jaw and face of dental students in Sweden reported a 4% incidence and a 19% prevalence over the period of 1 year.8 In a systematic review, Manfredini et al. found that when using the research diagnostic criteria/temporomandibular disorders (RCD/TMD) Axis 1 diagnostic criteria, myofascial pain constituted 6–12.9% of the diagnoses while disc displacement with reduction comprised 8.9–15.8%.9 Inflammatory-degenerative disorders were found to be uncommon in the TMD population.9
TMD is reported more frequently and graded as more severe among the female population.3 The ratio of female to male prevalence is generally found to be 2:1 while the ratio of those seeking care is 5:1.3
Manual treatment of TMD has been shown to be clinically effective10,11 and includes soft tissue therapy (myofascial release techniques, myofascial trigger point pressure release), mobilization techniques, manipulation and therapeutic exercises that focus on the soft tissues in the masticatory region10,11. Soft tissue therapy can be applied both extraorally or intraorally.10,11
Aside from traditional manual therapy, many dentists recommend the use of an occlusal appliance like a splint. Occlusal splints are manufactured in various materials, sizes, shapes, and have been shown to be beneficial, although the therapeutic mechanism remains unclear.12 Low-level laser therapy, and electrical modalities, have shown some promise in the treatment of TMD, but once again the overall evidence is lacking.13,14,15 Acupuncture has been demonstrated as having limited evidence of benefit compared to sham acupuncture in alleviating pain and masseter muscle tenderness in TMD.16 Pharmacological intervention with the use of NSAIDs is also commonplace in the treatment of TMD.17 However, chronic NSAID reliance has been linked to gastrointestinal side effects ranging from dyspeptic symptoms to life-threatening bleeding, especially in the elderly.18
In order to effectively treat the soft tissues surrounding the temporomandibular joint, the anatomy must be properly understood. The TMJ is a synovial condyloid joint between the mandibular condyle and the articular eminence of the temporal bone.19,20 It contains a dense fibrocartilagenous disc to increase the ease of movement and decrease the concentration of joint stresses.19,20 Primary muscles that move the mandible include the temporalis, masseter, and the medial and lateral pterygoids.19–21 The superior portion of the lateral pterygoid is inaccessible to palpation whereas palpation of the inferior lateral pterygoid remains a contentious issue.22–24 Recent MRI evidence indicates the possibility of direct palpation whereas older inquiries involving cadaveric dissection and x-ray analysis suggest otherwise.22–25
Vibration therapy has demonstrated the potential to aid in the management of acute soft tissue injuries, disuse or immobilization-related muscular deconditioning and to enhance muscular performance.26–31 A study by Rittweger et al in 2002, showed improvements in chronic lower back pain with whole-body vibration although the mechanism of the pain reduction was not clearly understood.32 To our knowledge, despite the positive effects of vibration on other areas of the body, the localized application of vibration to the muscles of mastication specifically has not been investigated.
According to Thabane et al. (2010), there are several reasons for conducting a pilot study.33 These reasons can be grouped into broad categories including: process, resources, management and scientific. The process assesses the feasibility of the steps taken in the pilot that will take place as part of the main study and includes aspects such as recruitment rates and retention. The resource aspect deals with assessing time and budgetary requirements that may hinder the main study. Management refers to the potential human and data optimization issues such as personnel and data management in multicentre trials. The scientific aspect deals with the assessment of treatment safety, determination of dose levels and response and the estimation of treatment effect and its variance.33
The purpose of this pilot study was to determine the scientific and process feasibility in an effort to direct future, larger trials. The feasibility of the scientific aspect included: the proof of concept of localized, intraoral, vibration therapy on the muscle of mastication for the treatment of reduced mouth opening with respect to range of motion and pain; the assessment of treatment safety; and the efficiency of the study methodology including process time and the limitation of bias during the study and; the effect size and variability to determine future sample size calculations. The feasibility of the process aspect included: recruitment to participant, retention to loss and consent to loss of consent rates and ratios during the trial.
Methods
Scientific Feasibility
Trial Design
The study design was that of a randomized, clinical trial pilot study consisting of two participant groups: a therapeutic intervention group and a control group. Both groups consisted of individuals who experienced decreased mouth opening due to pain/dysfunction of the temporomandibular joint and its associated muscles and had a myofascial component associated with the decreased opening. The intervention group was treated with a onetime single intervention of localized, intraoral, vibration therapy to three muscles bilaterally: the medial and lateral pterygoid, and the masseter. The control group was a non-treatment control.
The principal investigator delivered the localized vibration therapy and was not involved in the measurement of mouth opening or VAS. The same co-investigator performed the mouth opening measurement throughout the study using the Therabite® Range of Motion Scale and gathered the VAS. This co-investigator was not blinded to the group the participant was assigned.
Participants
The study was undertaken at a chiropractic institution in Toronto, Ontario.
-
Inclusion Criteria:
Volunteers were included if they were within the ages of 20–60, had an inability to open their mouth the standard three finger width34, had a 10 mm or greater amount pain on the VAS with mouth opening and the elicitation of a 1 or greater on an 11 point scale (0 (no pain) to 10 (maximum pain)) of pain during the light to moderate palpation of 3 of 6 of the muscles of mastication to be treated including the medial and lateral pterygoid bilaterally and the masseter bilaterally.
-
Exclusion Criteria:
Volunteers were excluded if they: (1) had current/previous injury, fracture or surgery to their jaw; (2) were receiving concurrent treatments from another practitioner (eg. medical doctor, chiropractor, dentist or physiotherapist) for TMJ issues within the last three months; (3) had anatomical deformities of the jaw or were missing their front teeth; (4) were diagnosed as having a tumour, infection, or inflammatory disease affecting the jaw; (5) had a previous history of TMJ disc pathology, capsule pathology or joint locking; (6) had a history of TMD issues related to the cervical spine; (7) had any known previous adverse reactions to vibration (eg. aggravation of sinus problems) (8) were involved in workers compensation or motor vehicle accident claims; (9) were using medications or other supplements that may have affected muscular health; (10) suffered from any neurological disorder which may have affected jaw function or the muscles of mastication such as Bell’s palsy or trigeminal neuralgia; (11) neck, upper back and low back pain at the time of presentation that would preclude the participant from lying on their back for the period of the intervention; (12) were currently taking bisphosphonates (can predispose the patient to osteonecrosis of the jaw); (13) any open wounds in or around the mouth; (14) any canker sores, cold sores or other pathologies in or around the mouth.
Interventions
There were two groups in the study – an intervention group and a control group.
Intervention group
Time 0: participant is supine, mouth opening to the point of pain or maximum opening (whichever comes first), measurement by the co-investigator with the Therabite® ROM Scale, participant completes VAS (VAS-base1).
-
Intervention (described below)
Time 1: participant is supine, mouth opening to the point of ROM measurement at Time 0 by the co-investigator using Therabite® ROM Scale, participant completes VAS(VASbase2). Mouth opening is then continued to the point of pain or maximum opening (if greater than point of last measurement) and is measured by the co-investigator using the Therabite® ROM Scale, participant completes another VAS(VASfinal).
Following the study, participants in the intervention group were asked to fill out a post-study questionnaire.
Control group
Time 0: participant is supine, mouth opening to the point of pain or maximum opening (whichever comes first), measurement by the co-investigator using the Therabite® ROM Scale, participant completes VAS(VASbase1).
6-minute rest time (equal to intervention time) – supine, participant is asked to refrain from full opening and limited talking.
Time 1: participant is supine, mouth opening to the point of ROM measurement at Time 0 by the co-investigator using Therabite® ROM Scale, participant completes VAS(VASbase2). Mouth opening is then continued to the point of pain or maximum opening (if greater than point of last measurement) and is measured by the co-investigator using the Therabite® ROM Scale, participant completes another VAS(VASfinal).
Treatment Intervention
A standardized head position was maintained having the participant supine with their head supported by a thin pillow with neck lordosis support. This lordosis support consisted of a rolled up towel (rolled to a level of comfort for the participant). A standardized body position was maintained by having a second pillow under the participant’s knees. These positions were maintained throughout the study.35
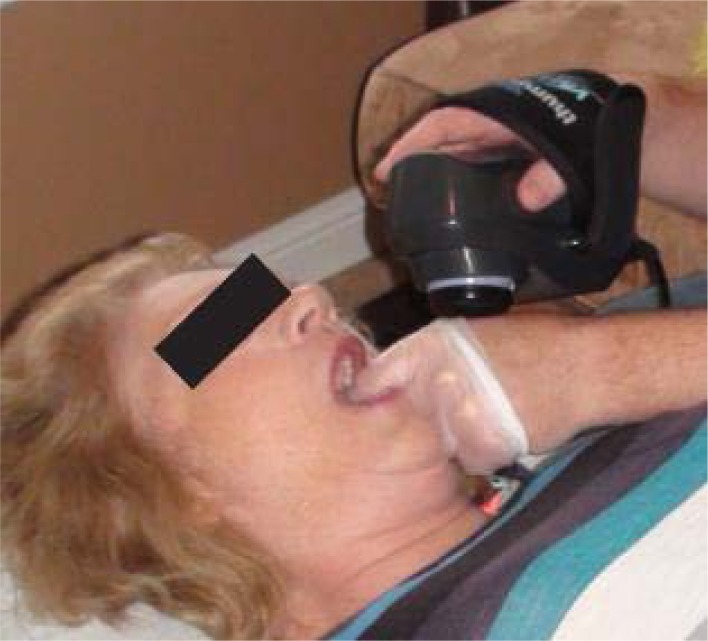
The intervention involved palpating the medial and lateral pterygoid, and the masseter intraorally with a non-latex gloved index finger with the participant in a supine position. The single ball vibration unit is applied to the 1st webspace of that hand and the index finger applies tolerable pressure to the muscle for a period of 1 minute. All three muscles were treated ipsilaterally and then the procedure was repeated on the contralateral side (see Figure 1 below).
Figure 1:
Localized Intraoral Vibration Therapy
Note: The application of the vibration was on the investigator’s hand – applying the vibration through a latex or vinyl glove may cause irritation to the skin beneath the vibration unit.
The control group in this study did not initially receive the intervention, however following completion of the data collection they were offered the intervention as per their choice. After the study, participants were returned to their regular and customary care.
ROM Measurement Procedure
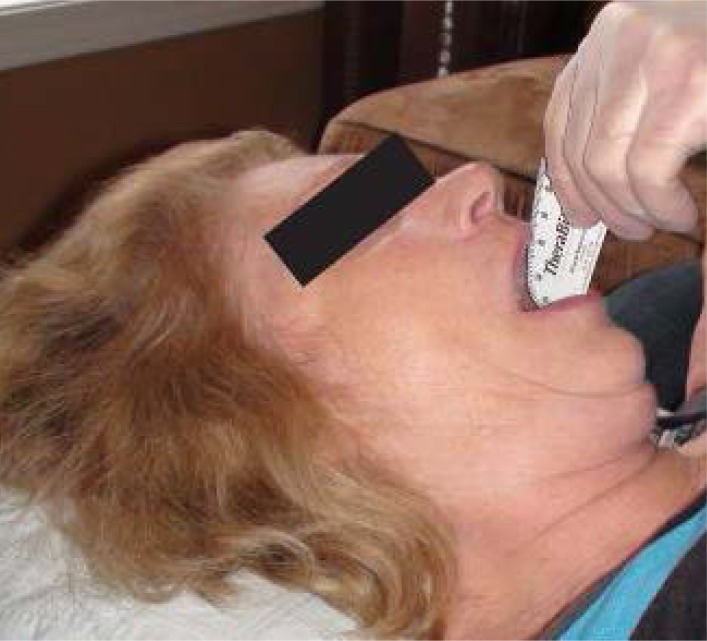
The measurement of maximum interincisal opening involves placing one end of the Therabite® Scale against the incisal edge of one central mandibular incisor with the other end against the incisal edge of the opposing maxillary central incisor in a manner consistent with the protocol of La Touche et al (2011).35 (see Figure 2 below)
Figure 2:
Therabite ® Range of Motion Scale and its application
Scientific Outcome Measures
The three scientific outcome measurements investigated were:
The amount of mouth opening in millimeters (measured using the Therabite® Range of Motion Scale). The Therabite® Range of Motion Scale was used to measure maximum interincisal opening. The Therabite® Scale has been used in several studies to measure maximum interincisal opening and may be considered the industry standard for clinical use.35–39 In a recent study done by La Touche et al (2011), the investigators found that the intra-rater reliability of using the Therabite® Scale was excellent with ICC values from 0.92 to 0.94.35 Interestingly, they also found that there was a statistically significant difference in maximum interincisal opening when measured in different head posture positions when seated.35 This suggests care must be taken to ensure a similar head posture position when taking pre and post treatment measures of maximum interincisal opening.
The amount of pain associated with mouth opening (using a VAS in millimeters). VAS and mouth opening were measured and recorded by the co-investigators. The visual analogue scale (VAS) was used to assess pain ratings. It has been used in previous TMD studies as reliability and validity has previously been established.40–42
A post-study questionnaire was issued for all participants involved in the intervention group (See Appendix A for the questionnaire).
Sample Size
A minimum of 12 healthy volunteers was required for participation in the study. No formal sample size calculation was used. The sample size was determined as a “rule of thumb” measure of 12 participants due to the unknown effect sizes of a scientifically untested treatment intervention.33 In looking at a similar pilot study done by Kalamir et al. in 2010, no sample size estimate was reported.43 In their study, 30 participants were randomized into 3 groups – 10 per group.43 The age demographic was 20 to 60 years and was chosen in an attempt to reduce the number of comorbidities that may influence a lack of mouth opening or its measurement (eg. lack of teeth).
Randomization
Participants were randomized using a computer generated random numbers table into respective intervention and control groups. The random numbers table was generated by a member of the Research Ethics Board (REB), who had no other direct involvement in the study. A co-investigator enrolled the participants and assigned them to the group based on the sealed envelopes received from the aforementioned member of REB.
Blinding
The principal investigator was not involved in the measurement of mouth opening and VAS during the experimental procedure. The principal investigator’s main focus was to deliver the treatment protocol and therefore had no impact on the measuring or recording of the data. The chosen co-investigator alone executed the measurement tasks (ROM and VAS) and recorded the data. The co-investigator executing the measurement tasks, however, was not blinded to the groups which may have increased bias during the measurement tasks. The participants were not blinded to the intervention and no sham intervention was attempted.
Statistical Analysis
Three scientific outcomes were examined, including: 1) change in mouth opening range of motion (ROMfinal – ROMbase1); 2) a) change in pain for each group when measured for a second time at the baseline ROM measurement (VASbase1 – VASbase2) and b) change in pain for each group when measured at the new final ROM (VASbase1 – VASfinal); 3) post study questionnaire for those in the intervention group.
For the first two outcomes, all measurements recorded (ROM(final – base), VAS(base1 – base2), VAS(base1 – final) were analyzed to determine mean scores, standard deviation and effect size according to Cohen.44 Cohen’s d equation is the following:
where (where x̅ is the mean score, s is the standard deviation, n is the number of participants in that group). The third outcome was reported as descriptive statistics based on the questionnaire.
Ethical Considerations and Funding
The Research Ethics Board at the Canadian Memorial College granted approval for this study on February 7, 2012 with the approval number of 1201A02.
All participants were required to complete consent form prior to participation ensuring that he/she is well informed of all study details, including possible risk, benefits and procedures.
No funding was received for this study.
Process Feasibility
Process Outcomes
Recruitment and retention rates, and consent were collected and monitored during the study and are outlined using descriptive statistics including flow charts and tables. Ratios including recruitment to participant, retention to loss, and consent to loss of consent were also calculated.
Results
Patient Flow
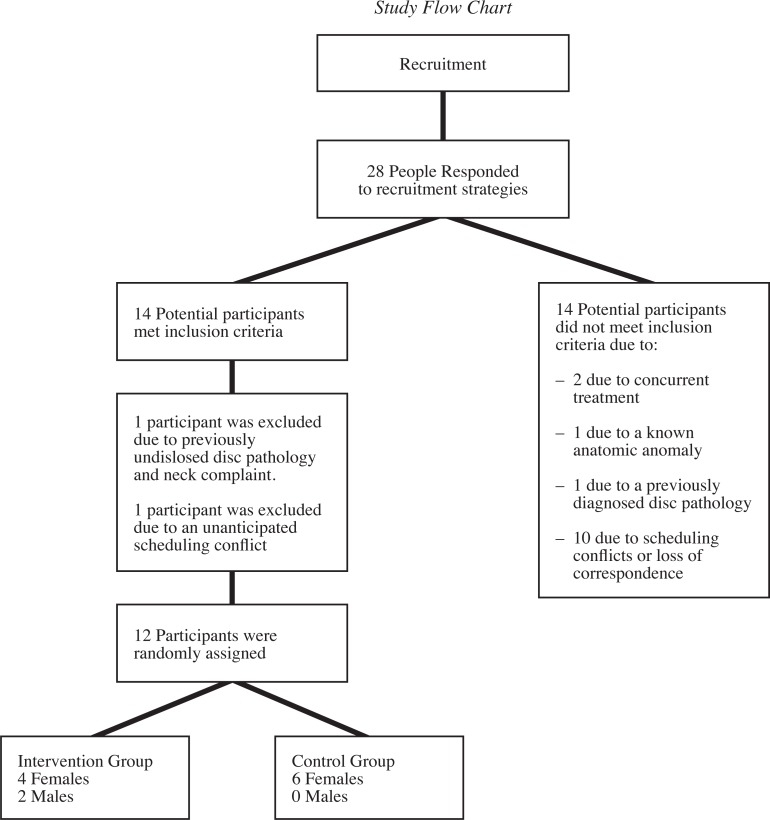
A total of 28 people responded to the recruitment strategies. Fourteen potential participants met all of the inclusion criteria and indicated they were available during the study’s testing period. Of the 14 participants that were excluded, two were excluded due to concurrent treatment, one due to a known anatomic anomaly, one due to previously diagnosed disc pathology, and ten participants were unavailable due to scheduling conflicts or loss of correspondence. A review of the inclusion criteria with each of the remaining 14 participants identified one participant with previously undisclosed disc pathology and cervical spine related complaint. This individual was then excluded from the study. One other participant was unable to attend testing due to an unanticipated scheduling conflict. In total, 12 participants were randomized using a computer generated random numbers table into respective intervention and control group. (See Figure 3 for the Study Flow Chart).
Figure 3.
Study Flow Chart
Recruitment
Recruitment occurred over a period of 3 weeks. Participants were recruited through email, posters and class presentations directed at students, faculty and staff.
Baseline Data
The participants’ baseline characteristics are presented in Table 1.
Table 1:
Baseline characteristics of the study participants
| Characteristic | Control Group (n = 6) | Intervention Group (n = 6) |
|---|---|---|
| Age (y)a | 25 (2.8) | 24 (1.6) |
| Sex, male:female | 0:6 | 2:4 |
| VASbase1b | 19.5 (2.7) | 28.2 (22.3) |
| ROMb | 37.7 (4.6) | 38.5 (8.9) |
Means (standard deviations in parentheses).
Means in millimeters.
At baseline, there were no significant differences in participant age or ROM. The intervention group included two males whereas no males were randomized to the control group. There also appears to be a difference in the VASbase1 between the two groups.
Scientific Outcomes
Outcome 1 and 2.
The change scores for the outcome measures of mouth opening ROM and pain are tabulated in Table 2 and 3.
Table 2:
Change scores by participant.
| Participant | ROM (Final – Base) | VASbase (Base1–Base2) | VASfinal (Base1-Final) | |
|---|---|---|---|---|
| ControlM | 1 | 0 | 3 | 3 |
| 2 | 0 | −2 | 3 | |
| 3 | −2 | 1 | −1 | |
| 4 | 0 | 2 | 3 | |
| 5 | −1 | 3 | 2 | |
| 6 | 4 | 15 | 5 | |
| InterventionM | 7 | 5 | 2 | −3 |
| 8 | 2 | 12 | −2 | |
| 9 | 5 | 9 | 3 | |
| 10 | 5 | 56 | 50 | |
| 11 | 8 | 15 | 9 | |
| 12 | 14 | 23 | 9 |
score in millimeters
Table 3:
Baseline and change scores for the two groups
| Baseline ROM | ROM (Final – Baseline) | Baseline VAS | VASbase (VASbase1 –VASbase2) | VASfinal (VASbase1 – VASfinal) | |
|---|---|---|---|---|---|
| ControlM (n=6) | 37.7 (4.6) | 0.17 (2.04) | 19.5 (2.7) | 3.7 (5.85) | 2.5 (1.97) |
| InterventionM (n=6) | 38.5 (8.9) | 6.5 (4.14) | 28.2 (22.3) | 19.5 (19.17) | 11.0 (19.79) |
| Effect Sizea | 2.12 | 1.19 | 0.66 |
Mean in millimeters (standard deviations in parentheses)
Effect size calculated using Cohen’s d Equation44
Cohen’s d Equation is: where
Note: The results of the changes in ROM and pain need to be viewed and interpreted with caution due to the small sample size associated with each of the groups as well as the differences in baseline characteristics (male/ female ratio and VAS). Because of the small sample sizes the effect sizes may be considered unstable and may be due to chance.
In Table 3, the mean change in the mouth opening ROM for the control group was 0.17 mm while the intervention group was 6.5 mm although the standard deviations were somewhat large for both. Based on Cohen’s effect size calculation, there was a large effect size of 2.12 which suggests the treatment intervention yielded positive effects in increasing mouth opening compared to the control group.
The same was true for the first measure of pain with the control group having a mean of 3.7 mm of change and the intervention group being 19.5 mm. Again, the two means had large standard deviations. This resulted in a smaller effect size than the ROM but was still considered a large effect size of 1.19. In examining the raw change scores (Table 2), participant 10 had a large decrease in pain at the baseline ROM which dramatically changed both the mean (12.2 mm when removed compared to 19.5) and the standard deviation (7.72 versus 19.17). This suggests a positive effect in decreasing the amount of pain in the intervention group when opening to the initial mouth opening ROM.
The pain rating at the final opening ROM was 2.5 mm in the control group while the intervention group had a change of 11.0 mm. The intervention group had a very large standard deviation compared to the control group (1.97 compared to 19.79). Again, examining the raw data, participant 10 in the intervention group had a large difference in pain scores at final opening ROM causing a dramatic change in mean (3.2 mm when removed versus 11.0) and standard deviation (5.76 versus 19.79). This resulted in a moderate effect size of 0.66 according to Cohen’s d Equation. This suggested that there was a small difference between the groups with regard to pain at the end range mouth opening.
The dramatic change in means and standard deviations with one outlier removed from the calculation again reinforces the limitation of having small sample size numbers.
Outcome 3.
The analysis of the post-study questionnaire is presented in Table 4.
Table 4:
Post-study questionnaire results, Intervention Group (n=6)
| Percentage of Favorable Responses | |
|---|---|
| 1. Rating of the experience: | 67% |
| 2. Rating of each subjects likelihood of pursing the study treatment in the future: | 83% |
| 3. Rating of the comfort of the procedure: | 67% |
The results of the post-study questionnaire for the intervention group (Table 4) yield positive results with respect to the participants’ subjective evaluation of the experience, likelihood to pursue a similar treatment in the future, and the comfort of the procedure. Sixty-seven percent of the participants rated their experience and the comfort of the procedure as favorable. Eighty-three percent of participants responded favorably as to their likelihood to pursue this treatment modality in the future.
Harms
There were no adverse events reported by any participants during or after the course of this study.
Process Feasibility Outcomes
The process outcomes are outlined in Table 5.
Table 5:
Process feasibility outcomes: Recruitment, retention and consent
| Recruitment | Retention | Consent | |||
|---|---|---|---|---|---|
| Initial Response (IR) | 28 | Met Criteria |
13 | Initial Consent | 12 |
| Did not meet criteria | 15 | Lost before participation | 1 | Consent withdrawn during trial | 0 |
| Met Criteria (MC) | 13 | Retention: Loss Ratio | 13:1 | Consent: Loss Ratio | 12:0 |
| Recruitment Ratio (IR:MC) | 2.3:1 | ||||
Discussion
The purpose of this pilot study was to determine the scientific and process feasibility in an effort to direct future, larger trials.
Scientific Feasibility
The results of the current study suggests that a single treatment of localized, intraoral vibration therapy directed at the muscles of mastication does have a beneficial effect with regard to an immediate increase in mouth opening and decrease in pain. Again, caution should be noted with regard to the interpretation of this data due to the small sample sizes in each arm of the study. This was consistent with our original hypothesis that vibration would have a positive effect on these two outcomes. With regard to the pain level at the finishing end range, it was somewhat consistent with our original hypothesis in that there was a moderate effect size demonstrated. We would have expected a small effect size if any at all because both groups were instructed to open to the point of pain or to maximum opening at which point that range and pain were measured. If the participants were being consistent in following the investigators instructions, the pain level should have remained consistent as well.
One caveat to this could possibly occur and may have been demonstrated by Participant number 10 in the intervention group (see Table 2). It could be speculated that during baseline ROM, the VAS for participant 10 was larger than the average. Following treatment, the second VAS at the baseline ROM had decreased significantly. Participant 10’s ROM increased by 5 mm and may have put them to maximal opening. If they did not reach the point of pain then the significantly lower VAS at final opening would be much different than that of baseline thus explaining the large difference. In future studies, if VAS is measured in the same manner, the participant should be asked and documented if they reached maximal opening or the point of pain in order to account for this possible scenario.
A study done by Kalamir et al, (2012), investigated whether intraoral myofascial therapy (IMT), education and self-care were better than no treatment.11 Five outcome measures were used, including interincisal opening range, measured in millimeters using vernier callipers. Participants were randomized into 1 of 3 groups: IMT consisting of 2 treatment interventions per week for 5 weeks, IMT plus education and self-care exercises (IMTESC), and wait-list control.11 The study found statistically significant differences in resting, opening and clenching pain, as well as opening scores and global reporting of change in both treatment groups as compared to the control group at six months and one year. The results suggest IMTESC and IMT are superior to no-treatment over the one-year study follow-up with IMTESC also being superior to IMT.11 A follow-up study done by the same group in 2013 compared IMT and ESC directly over a 6 week period and found that although the change in pain scores were significant in favour of the IMT group, the results were not clinically significant ie. greater than a 2 point difference in a numerical pain rating scale.45 In a study done by Ibanez-Garcia et al (2009), they measured pre and post treatment pain levels using VAS.46 The externally applied treatments of neuromuscular technique and strain-counterstrain of the masseter muscle in those subjects with myofascial trigger points occurred one time per week for three weeks. The VAS measured local pain of the masseter using a mechanical pressure algometer set at 2.5kg/cm2. The study found that there was a statistically significant change (p<0.001) in VAS in the neuromuscular group versus the strain-counterstrain and control groups. The change in VAS in the neuromuscular group was 13 mm suggesting a moderate effect size.46 In the current study, pain was measured using the VAS. It has been suggested that the smallest detectable difference for “actual pain” using the VAS is 28 mm.47 Our study showed a 19.5 mm change although our standard deviation was 19.17 suggesting that we may not have met clinical significance with regard to change in pain scores. Our study also had a low level of pain (at least 10 mm of pain or greater on a 100 mm scale) as a minimum for inclusion. This leaves little room for improvement on the pain scale and considering that 28 mm is considered the smallest detectable difference47, this was a limitation. As a result, future studies should have a minimum of 30 mm or greater on the VAS for inclusion.
The follow-up study by Kalamir et al. (2000) did not find statistically or clinically significant changes in mouth opening either. This was defined as a 5 mm change in mouth opening.45,47 Kropmans et al. (1999) suggested that a 5 mm change in mouth opening was clinically significant and generalizable for healthy subjects and to those patients with restricted mouth opening.47 However, in 2000, the same group studied those with painful restrictions in their TM and found that a statistically and clinically significant change of 9 mm was necessary unless repeated measures was performed in which case 6 mm improvement would be necessary.48 In our study, we found a difference of mouth opening of 6.5 mm which was greater than the 5 mm change45,46 but less than the 9 mm change suggested by Kropmans48. When examining the change scores by participant (Table 2), only one participant in the intervention group did not achieve a 5 mm change while none of the control group reached 5 mm of change. The study by Ibanez-Garcia et al (2009) also measured changes in mouth opening and although they reached pre and post treatment statistical significance in both the neuromuscular technique and strain-counterstrain groups, neither group reached the 5mm threshold (4mm change in both groups).46
None of the above studies looked at acute changes in pain or mouth opening similar to the current study. All of the studies ranged in follow-up from 1 month to 1 year and involved 3 or more treatments.11,45,46
The post-study questionnaire given to the intervention group suggests the treatment technique is safe, comfortable and favored by patients although, again, the study had a small sample size in the intervention group. There were no adverse reactions reported by any of the participants during the course of this study.
The methodology employed during the study was adequate and efficient according to our post-study questionnaire but does require some changes. The principal investigator performing the intervention found the clinically meaningful treatment time to be roughly 15–30 seconds per muscle. This would shorten the intervention time furthering the efficiency of the study methodology. The use of mechanical pressure algometers similar to Ibanez-Garcia et al.46 may also help to reduce the bias associated with subjective pain scores. However, the use of a pressure algometer may be limited to external musculature only, and to potential budgetary restrictions associated with future studies.
The present study was also limited due to its inability to verify the presence of unknown factors such as a bony anomaly, an arthritide or degenerative changes in the TMJ. Future studies may investigate the effect of vibration on patients with known joint and bone issues in those patients having had previous advanced imaging.
The main limitation of this study, as with most pilot studies33,43, was the small sample size and the lack of statistical power. The interpretation of any results should thus be viewed with caution. Thabane et al. (2010) suggests that pilot studies are a “good opportunity to assess feasibility of large full-scale studies” and are a way to “enhance the likelihood of success of the main study and potentially help to avoid doomed main studies”.33 From this standpoint, even with our small sample size and potentially unstable effect sizes, the large effect sizes generated during the current study, would support a future, larger, single treatment trial utilizing localized intraoral vibration therapy as a treatment intervention, as well as help in the generation of future sample size estimates that would provide adequate statistical power.
Another limitation is the difference in the groups at baseline with regard to gender (no males in the control group). This is due to ineffective randomization which occurs when the sample size is too small. Another seeming difference of the baseline scores is the VAS score – 19.5 mm for the control group versus 28.2 mm for the intervention group. Although the difference seems large, it does not meet what Kropmans et al. suggests is the smallest detectable difference of 28 mm.47 Therefore a difference of 8.7 mm may not be a clinically significant difference between the two groups.
One potentially critical limitation of the study methodology is its lack of blinding for the co-investigator in charge of measuring ROM and VAS. In an effort to correct this oversight and minimize its potential bias during future, similar studies, the co-investigator should be blinded to the participant group. This could be done by having the co-investigator leave the room and the vibration machine being turned on during the rest period for the control group so that the co-investigator wouldn’t be able to guess the group based on the sound of the machine.
Another key limitation of the current study is its lack of follow up measures and thus its generalizability for patients seeking longer term results. Although the change in ROM for the intervention group appears to reach clinically significant levels47, follow up measures would be needed to see how long these levels were sustained. The current methodology only applied a single treatment and only measured acute changes in ROM and pain. In order to accommodate those patients seeking longer term results and to be consistent with other comparable studies11,45, the methodology should be expanded to include several treatments over a period of weeks with longer follow-up times. The current study only examined the feasibility of a single treatment model and, as such, its results cannot be reliably extrapolated to a larger, multiple treatment model and any attempts to do so should be done with caution.
Process Feasibility
From a recruitment standpoint, the current study yielded a 2.3:1 ratio for respondents to those meeting the criteria (Table 5). The current study also had a retention to loss rate of 13:1 and a perfect consent to loss of consent rate during the trial of 12:0. The recruitment process occurred over a 3 week period. The participant group had a gender ratio of 6 females to 1 male (Table 1) which is similar to patients seeking care for TMD related problems in other studies.3 In a pilot study by Kalamir et al.43, they advertised and recruited over a 6 month period yielding 66 respondents and ultimately 30 study participants. This would suggest that our recruitment strategies were adequate for a pilot study.
In the larger trial by Kalamir et al.11 following their pilot study43, they received 221 enquiries and were able to assess 134 potential participants with 93 ultimately being enrolled. This suggests a recruitment ratio of 1.7:1. The recruitment period for this trial was 1 year. Based on our recruitment ratio of 2.3:1 (over a 3 week period), in order to accommodate a future trial with 93 participants, it would take approximately 214 responses. This would take approximately 6 months to complete based on our recruitment strategy but it is suggested that 6 months to 1 year would be needed based on the existing literature.
A limitation of this calculation is that our recruitment, retention and consent rates are based on a single treatment intervention model compared to a multiple intervention and extended follow up study.11 However, in the study done by Kalamir et al.11, they only had one drop out during their 1 year follow up suggesting that multiple treatments and extended follow up times may not be a limiting factor for recruitment, retention and consent rates.
Conclusion
The scientific feasibility of this pilot study should be viewed and interpreted with caution due to the small sample sizes employed during the study and the unbalanced arms (male/female ratio and VAS). However, the large effect size for changes in ROM and VAS (at the baseline ROM) in favour of the intervention group is encouraging that there is a positive treatment effect associated with the use of localized, intraoral vibration on the muscles of mastication. Also, there were no adverse events reported suggesting the procedure is safe and the majority of the participants in the intervention group found the intervention comfortable and would pursue the use of the treatment in the future. However, there were many limitations with regard to the methodology utilized and these would need to be considered during future, larger trials. The study also seems to support the feasibility of a future, larger, single treatment trial from a process feasibility standpoint. Utilizing the feasibility results of the current study to direct a future, larger, multiple treatment trial consistent with other comparable TMD studies however is limited.
Appendix A. Post Study Questionnaire
Post Study Questionnaire
Participant Name: __________________________________
Date: _____________________
| Please circle the number that you feel most reflects your view. | ||
| 1. Would you rate this experience as: | ||
| Unsatisfactory |

|
Very Satisfactory |
| 2. How would you rate your likelihood of pursuing the study treatment in the future? | ||
| Very Unlikely |

|
Very Likely |
| 3. Did you receive the study treatment? | □Yes | □No |
| If yes, how comfortable would you rate this treatment? | ||
| Very Uncomfortable |

|
Very Comfortable |
Footnotes
Potential conflicts of interest/commercialization of research
Dr. Brad Muir, the principal investigator of this research project, is a member of the VMTX Vibromax Therapeutics team that utilizes vibration therapy as the main component of its treatment technique. As a result, a potential conflict of interest arises as positive results could influence future sales of the VMTX equipment, manuals and courses.
Institution where work was performed: The experimental data were collected at the Canadian Memorial Chiropractic College, Toronto, Canada.
Declaration: The lead author and principal investigator Dr. Brad Muir, is a member of the VMTX Vibromax Therapeutics team.
References
- 1.McNeill C. Epidemiology. In: McNeill C, editor. Temporomandibular Disorders: Guidelines for Classification, Assessment, and Management. 2nd ed. Chicago, Ill: Quintessence Publishing Co; 1993. pp. 19–22. [Google Scholar]
- 2.Di Fabio RP. Physical therapy for patients with TMD: a descriptive study of treatment, disability, and health status. J Orofac Pain. 1998;12:124–135. [PubMed] [Google Scholar]
- 3.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 4.Gremillion H, Mahon P. The prevalence and etiology of temporomandibular disorders and orofacial pain. Texas Dental J. 2000;117:30–39. [PubMed] [Google Scholar]
- 5.Shiau YY, Kwan HW, Chang C. Prevalence of temporomandibular disorder syndrome (TMD) in university students: a third year report of the epidemiological study in Taiwan. Chin Dent J. 1989;8:106–116. [PubMed] [Google Scholar]
- 6.National Institutes of Health; National Institute of Dental and Craniofacial Research. Bethesda, MD: National Institute of Dental and Craniofacial Research; 2006. TMJ Disorders NIH Publication No. 06-3487. [Google Scholar]
- 7.Stohler C. Masticatory myalgias. In: Fonseca RJ, et al., editors. Oral and Maxillofacial Surgery Temporomandibular Disorders. Philadelphia: WB Saunders; 2000. pp. 38–45. [Google Scholar]
- 8.Marklund S, Wanman A. Incidence and prevalence of myofascial pain in the jaw-face region. A one-year prospective study on dental students. Acta Odontologica Scandinavica. 2008;66:113–121. doi: 10.1080/00016350802010372. [DOI] [PubMed] [Google Scholar]
- 9.Manfredini D, Guarda-Nardini L, Winocur E, Piccotti F, Ahlberg J, Lobbezoo F. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:453–462. doi: 10.1016/j.tripleo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer SM, Brismee JM, Sizer PS, Courtney CA. Temporomandibular disorders. Part 2: conservative management. J Man Manipul Ther. 2014;22(1):13–23. doi: 10.1179/2042618613Y.0000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalamir A, Bonello R, Graham PL, Vitiello AL, Pollard H. Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized, clinical trial. J Manip Physiol Ther. 2012;35:26–37. doi: 10.1016/j.jmpt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Jokstad A, Krogstad A, Krogstad B. Clinical comparison between two different splint designs for temporomandibular disorder therapy. Acta Odontologica Scandinavia. 2005;63:218–226. doi: 10.1080/00016350510019982. [DOI] [PubMed] [Google Scholar]
- 13.Nunez S, Garcez A, Suzuki S, Ribeiro M. Management of mouth opening in patients with temporomandibular disorders through low-level laser therapy and transcutaneous electrical neural stimulation. Photomed Laser Surg. 2006;24:45–49. doi: 10.1089/pho.2006.24.45. [DOI] [PubMed] [Google Scholar]
- 14.Cetiner S, Kahraman SA, Yucetas S. Evaluation of low-level laser therapy in the treatment of temporomandibular disorders. Photomed Laser Surg. 2006;24(5):637–641. doi: 10.1089/pho.2006.24.637. [DOI] [PubMed] [Google Scholar]
- 15.Kulekcioglu S, Sivrioglu K, Ozcan O, Parlak M. Effectiveness of low-level laser therapy in temporomandibular disorder. Scandinavian Rheumatology Res. 2003;32:114–8. doi: 10.1080/03009740310000139. [DOI] [PubMed] [Google Scholar]
- 16.Turp JC. Limited evidence that acupuncture is effective for treating temporomandibular disorders. Evid Based Dent. 2011;12(3):89. doi: 10.1038/sj.ebd.6400816. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler A. Myofascial pain disorders; theory to therapy. Drugs. 2004;64(1):45–62. doi: 10.2165/00003495-200464010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Buttgereit F, Burmester G, Simon L. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2- specific inhibitors. Am J Med. 2001;110(3A):13S–19S. doi: 10.1016/s0002-9343(00)00728-2. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer SM, Brismee JM, Sizer PS, Courtney CA. Temporomandibular disorders. Part 1: anatomy and examination/diagnosis. J Man Manip Ther. 2014;22(1):2–12. doi: 10.1179/2042618613Y.0000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693–705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 21.Matsunga K, Usui A, Yamaguchi K, Akita K. An anatomical study of the muscles that attach to the articular disc of the temporomandibular joint. Clinical Anatomy. 2009;22(8):932–40. doi: 10.1002/ca.20865. [DOI] [PubMed] [Google Scholar]
- 22.Stratmann U, Mokrys K, Meyer U, Kleinheinz J, Joos U, Dirksen D, Bollmann F. Clinical anatomy and palpability of the inferior lateral pterygoid muscle. J Prosthetic Dent. 2000;83(5):548–54. doi: 10.1016/s0022-3913(00)70013-8. [DOI] [PubMed] [Google Scholar]
- 23.Stelzenmuller W, Weber D, Ozkan V, Freesmeyer W, Umstadt H. Is the lateral pterygoid muscle palpable? Internl Poster J Dental Oral Med. 2006;8(1):301. [Google Scholar]
- 24.Johnstone D, Templeton M. The feasibility of palpating the lateral pterygoid muscle. J Prosthetic Dent. 1980;44(3):318–23. doi: 10.1016/0022-3913(80)90020-7. [DOI] [PubMed] [Google Scholar]
- 25.Barriere P, Lutz JC, Zamanian A, Wilk A, Rhiem S, Veillon F, Kahn JL. MRI evidence of lateral pterygoid muscle palpation. Internl J Oral Maxillofascial Surg. 2009;38:1094–95. doi: 10.1016/j.ijom.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35(1):23–40. doi: 10.2165/00007256-200535010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Delecluse C, Roelants M, Verschueren S. Strength increase after whole body vibration compared with resistance training. Med Science Sports Exer. 2003;35:1033–1004. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- 28.Bosco C, Colli R, Introni E, Cardinale M, Iacovelli M, tihanyi J, von Duvillard S, Viru A. Adaptive responses of human skeletal muscle to vibration exposure. Clinical Physiology. 1999;19(2):4–13. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 29.Bosco C, Cardinale M, Tsarpela O, Lacatelli E. New trends in training science: the use of vibrations for enhancing performance. Eur J Applied Physiology. 1984;53:275–284. [Google Scholar]
- 30.Cardinale M, Wakeling J. Whole body vibration exercise: are vibrations good for you? Br J Sports Med. 2005;39:585–589. doi: 10.1136/bjsm.2005.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre I, Kazemi M. Treatment of posttraumatic arthrofibrosis of the radioulnar joint with vibration therapy (VMTX Vibromax Therapeutics™): A case report and narrative review of the literature. J Can Chiropr Assoc. 2008;52(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 32.Rittweger J, Just K, Kautzsch K, Reeg P, Felsenberg D. Treatment of chronic lower back pain with lumbar extension and whole-body vibration exercise: a randomized controlled trial. Spine. 2002;27:1829–34. doi: 10.1097/00007632-200209010-00003. [DOI] [PubMed] [Google Scholar]
- 33.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodology. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zawawi KH, Al-Badawi EA, Lobo SL, Melis M, Mehta NR. An index for the measurement of normal maximum mouth opening. J Can Dent Assoc. 2003;69(11):737–41. [PubMed] [Google Scholar]
- 35.La Touche R, Paris-Alemany A, von Piekartz H, Mannheimer JS, Fernandez-Carnero J, Rocabado M. The influence of cranio-cervical posture on maximal mouth opening and pressure pain thresholds in patients with myofascial temporomandibular pain disorders. Clinical J Pain. 2011;27:48–55. doi: 10.1097/AJP.0b013e3181edc157. [DOI] [PubMed] [Google Scholar]
- 36.Zawawi KH, Al-Badawi EA, Lobo S, Melis M, Mehta NR. An index for the measurement of normal maximum mouth opening. J Can Dent Assoc. 2003;69(11):737–741. [PubMed] [Google Scholar]
- 37.Kent ML, Brennan MT, Noll JL, Fox PC, Burri SH, Hunter JC, Lockhart PB. Radiation-induced trismus in head and neck cancer patients. Support Care Cancer. 2008;16:305–309. doi: 10.1007/s00520-007-0345-5. [DOI] [PubMed] [Google Scholar]
- 38.Mercuri LG, Edibam NR, Giobbie-Hurder A. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofacial Surg. 2007;65:1140–1148. doi: 10.1016/j.joms.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 39.van der Molen L, van Rossum MA, Ackerstaff AH, Smeele LE, Rasch C, Hilgers F. Pretreatment organ function in patients with advanced head and neck cancer: clinical outcome measures and patients’ views. BMC Ear, Nose and Throat Disorders. 2009;9:10. doi: 10.1186/1472-6815-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clinical Nursing. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 41.Yuill E, Howitt S. Temporomandibular joint: conservative care of TMJ dysfunction in a competitive swimmer. J Can Chirop Assoc. 2009;53(3):165–172. [PMC free article] [PubMed] [Google Scholar]
- 42.Jerjes W, Upile T, Abbas S, Kafas P, Vourvachis M, Rob J, McCarthy E, Angouridakis N, Hopper C. Muscle disorders and dentition-related aspects in temporomandibular disorders: controversies in the most commonly used treatment modalities. Internl Arch Med. 2008;1:23. doi: 10.1186/1755-7682-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalamir A, Graham PL, Vitiello AL, Bonello R, Pollard H. Intra-oral myofascial therapy for chronic, myogenous temporomandibular disorder: a randomized, controlled pilot study. J Man Manip Ther. 2010;18(3):139–146. doi: 10.1179/106698110X12640740712374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J. In: Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Cohen J, editor. Hampshire UK: Routledge; 2013. [Google Scholar]
- 45.Kalamir A, Graham PL, Vitiello AL, Bonello R, Pollard H. Intra-oral myofascial therapy versus education and self-care in the treatment of chronic, myogenous temporomandibular disorder: a randomized, clinical trial. Chiropractic and Manual Therapies. 2013;21:17. doi: 10.1186/2045-709X-21-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibanez-Garcia J, Alburquerque-Sendin F, Rodriguez-Blanco C, Girao D, Atienza-Meseguer A, Planella-Abella S, Fernandez-de-las Penas C. Changes in masseter muscle trigger points following strain-counterstrain or neuro-muscular technique. J Bodywork Movement Therapies. 2009;13:2–10. doi: 10.1016/j.jbmt.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Kropmans ThJB, Dijkstra PU, Stegenga B, Stewart R, de Bont LGM. Smallest detectable difference in outcome variables related to painful restriction of the temporomandibular joint. J Dent Res. 1999;78(3):784–789. doi: 10.1177/00220345990780031101. [DOI] [PubMed] [Google Scholar]
- 48.Kropmans ThJB, Dijkstra PU, Stegenga B, Stewart R, de Bont LGM. Smallest detectable difference of maximal mouth opening in patients with painfully restricted temporomandibular joint function. Eur J Oral Sci. 2000;108:9–13. doi: 10.1034/j.1600-0722.2000.00747.x. [DOI] [PubMed] [Google Scholar]