Abstract
In the United States, marijuana is one of the drugs most abused by adolescents, with females representing a growing number of users. In previous studies, treatment of adolescent female rats with morphine significantly altered brain reward systems in future offspring. As both cannabinoid and opioid systems develop during adolescence, it was hypothesized that early exposure to cannabinoids would induce similar transgenerational effects. In the current study, female rats were treated with the cannabinoid receptor (CB1/CB2) agonist WIN 55,212-2 or its vehicle for three consecutive days during adolescent development (30 days of age), and were subsequently mated in adulthood (60 days of age). The adolescent and adult male offspring of these WIN 55,212-2 (WIN-F1)- or vehicle (VEH-F1)-treated females were tested for their response to morphine using the conditioned place preference (CPP) paradigm. Both adolescent and adult WIN-F1offspring exhibited greater sensitivity to morphine CPP than their VEH-F1 counterparts. Collectively, the findings provide additional evidence of transgenerational effects of adolescent drug use.
Keywords: Opioid, WIN 55, 212-2, locomotor sensitization, marijuana, CB-1 receptor, morphine, development
Introduction
Marijuana is the most widely abused illicit drug in the United States. Its attractiveness to users is attributed to its psychoactive effects, mediated primarily by delta-9-tetrahydrocannabinol, a CB1 and CB2 receptor agonist (Pertwee, 1997). Of the 2.4 million people that tried marijuana for the first time in 2010, 58.5% were below 18 years of age (SAMHSA, 2011). In addition, while males still use marijuana at higher rates than females, marijuana is currently used by 6.4% of the female adolescent population (as compared with 8.3% of males). Certainly, marijuana use presents significant health risks for both males and females (including addiction and mental illness), yet the nature of these risks may be sex-dependent (SAMHSA, 2011).
In addition to concerns related to adolescent marijuana use, data also suggest that use of this drug during pregnancy can significantly affect offspring development. For example, in utero cannabinoid exposure has been associated with impairment of executive function (Fried, 2002), increased rates of depression and anxiety, and decreased reading comprehension and motivation in offspring (Goldschmidt et al., 2004). Other studies have also suggested that in utero exposure to cannabinoids may be predictive of future offspring use (Day et al., 2006). Rodent models of in utero cannabinoid exposure have been informative in this regard. Indeed, such studies have revealed memory impairment (Mereu et al., 2003) and increased morphine sensitization in a conditioned place preference (CPP) procedure (Rubio et al., 1998) in adolescent and adult offspring of rats treated with cannabinoids during pregnancy. Thus, human and rodent literature indicates that alterations in the development of cognitive, emotional, and reinforcement circuitry may be a consequence of cannabinoid exposure during the prenatal period.
While cannabinoid use during pregnancy may alter offspring development, it is unknown whether cannabinoid use occurring prior to pregnancy can impact future offspring. Previous studies have shown that the future offspring of female rats treated with morphine during adolescence exhibit augmented morphine sensitization (Byrnes, 2005). That is, increased sensitivity to morphine is observed even though the offspring themselves were never directly exposed to the drug. As sensitization to drugs of abuse has been used to model abuse vulnerability (Robinson and Berridge, 1993), these findings suggest that maternal drug history can have significant implications for subsequent generations. Whether these transgenerational effects of opiates are unique to this drug class, or represent a property shared by other drugs of abuse is unknown. However, as both cannabinoid and opioid systems develop during adolescence (Ellgren et al., 2008; Rodriguez de Fonseca et al., 1993), it was hypothesized that early exposure to cannabinoids would induce similar transgenerational effects.
The purpose of the present study was to determine whether exposure to cannabinoids during adolescent development induces transgenerational effects in future offspring. As significant interactions between cannabinoids and opioids have been observed in brain reward processes (Lopez-Moreno et al., 2008, 2010), the current study examined morphine CPP in the male offspring of females rats that were previously treated with the CB1/CB2 receptor agonist WIN 55,212-2 (WIN) during adolescence. To determine the developmental time course of any transgenerational effects, testing was conducted in separate groups of adolescent (40 days of age) and adult (60 days) offspring. The results suggest that male offspring of adolescent WIN-exposed females may be more sensitive to the rewarding aspects of opioids.
Methods
Experimental animals
Twenty-three-day-old female Sprague–Dawley rats were purchased from Charles River Laboratories (Kingston, NY). All animals were housed in standard plastic laboratory cages (40 cm × 20 cm × 18 cm) on a 12:12 h light:dark cycle (lights on at 07:00 h) in temperature-controlled (21–24°C) rooms. Food and water were provided ad libitum. Animals were acclimated to the housing conditions for at least seven days prior to experimentation. At 30 days of age, 16 adolescent females began twice daily subcutaneous (s.c.) injections with the CB1/CB2 receptor agonist, (R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone (WIN 55,212-2; Sigma-Aldrich, St Louis, MO) at increasing doses (1.0 mg/kg on day 1; 2.0mg/kg on day 2; 4mg/kg on day 3). Animals were weighed daily to monitor any effects of drug treatment on body weight. A similar brief, increasing dose regimen was shown previously to significantly increase subsequent heroin sensitization (Pontieri et al., 2001). A set of control females (n=16) were administered the vehicle solution (0.9% NaCl with 0.1% Tween 80, 1 mL/kg). At 60 days of age (i.e. 28 days after the last WIN 55,212-2 injection), all females were housed and mated with colony males. At parturition, all litters were weighed and culled to five males and five females. All male offspring (VEH-F1 and WIN-F1) were weaned and group-housed on postnatal Day 21, and remained undisturbed until the time of testing. All animal use was approved by the Institutional Animals Care and Use Committee of Tufts University, and carried out in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals.
Conditioned place preference
At either 40 (adolescent) or 60 (adult) days of age, male offspring were tested for CPP responses following conditioning with morphine sulfate (1 or 5 mg/kg, s.c.) or saline (0.9% NaCl, 1 mL/kg) administration. Only one animal per litter was used in each treatment group to avoid potential litter effects. All conditioning and testing was performed using an automated three chamber apparatus (side A, middle chamber, side B), equipped with infrared photobeams to track animal position and movement (Hamilton-Kinder, Poway, CA). Each side of the chamber was fitted with a removable plastic insert containing either horizontally or vertically (black) striped walls, and a smooth or textured floor. Each group tested was counterbalanced with regard to the insert used in side A and side B. On Day 1 (preconditioning), each animal was placed into the middle chamber of the apparatus and provided with open access to all three chambers. Time (s) spent in each chamber was measured for 15 min. The purpose of the Day 1 procedure was to determine preconditioning place preferences and to examine locomotor activity (photobeam breaks) in a novel environment. On the morning of Day 2, all animals were weighed, administered saline (1 mL/kg, s.c.), and immediately placed into “side A” for 30 min (unpaired chamber). Approximately five hours later, animals received the assigned conditioning treatments (morphine or saline, above) and were immediately placed into “side B” for 30 min (paired chamber). Conditioning took place in this manner for a total of three days (Days 2–4). On Day 5 (post-conditioning CPP), all animals were placed in the middle chamber of the apparatus (with open access to all three chambers), and time spent in each chamber was measured for 15 min. CPP was calculated as the difference in time spent in the drug-paired chamber as compared with the unpaired chamber during the postconditioning test (i.e. paired time - unpaired time).
Context-independent locomotor sensitization in response to morphine
In adult males only, 10 days after the last exposure to morphine, a subset of adult animals that had been conditioned to either saline or morphine (5 mg/kg) were examined for their locomotor response to a low dose of morphine (2 mg/kg). As testing was conducted in standard activity chambers, rather than in the CPP apparatus, any enhanced locomotor activity following an acute dose of morphine in those animals exposed to morphine during conditioning trial would be considered context-independent sensitization. On the day of testing, animals were weighed, administered saline (1 mL/kg, s.c.), and placed into a clear Plexiglas open field (45 cm × 25 cm × 20 cm). Locomotor activity was monitored for a total of 60 min using an automated 32-beam infrared photobeam frame which surrounded the open field (SmartFrame® Activity Cage Rack System; Hamilton-Kinder, Poway, CA). Each animal was then removed, administered morphine (2 mg/kg, s.c.), and returned to the activity cage for an additional 120 min of monitoring. Activity data were analyzed as beam breaks across time for pre- and post-morphine treatments.
Statistical analyses
Daily body weight in adolescent females during and after drug treatment was analyzed using a repeated measures analysis of variance (RM-ANOVA), with age (in days) as the within subject factor, and drug treatment (VEH or WIN) as the between subject factor. Pup body weights and litter sizes were analyzed using Student’s t-tests. Both pre- and postconditioning place preferences as well as locomotor activity were analyzed using a two-way ANOVA with drug dose (0, 1, and 5 mg/kg morphine) and maternal drug history (VEH or WIN) as factors. Finally, changes in locomotor activity measured 10 days after CPP testing following either saline or morphine (2 mg/kg) treatment were analyzed using a two-way ANOVA, with pretreatment condition (saline or 5 mg/kg morphine) and maternal drug history (VEH or WIN) as factors. For all data, post-hoc analyses were conducted using Tukey’s test. Statistical significance was defined at p < 0.05.
Results
Direct effects of adolescent WIN 55,212-2 exposure
As shown in Table 1, there were no significant effects of WIN administration on body weight of adolescent females as measured during the three day exposure period (Days 30–32) or at the time of mating in adulthood (Day 60). Both VEH and WIN groups had identical fertility rates, with 13/16 WIN and 13/16 VEH females becoming pregnant after 14 days of cohabitation with colony males. Finally, there were no significant effects of drug treatment on pup body weight, litter size, or gender composition on postnatal Day 1 (all p values > 0.1; data not shown).
Table 1.
Mean (±SEM) body weights (g) of females administered either vehicle (VEH) or WIN 55,212-2 (WIN) twice daily during early adolescence.
| Treatment | Day 30 | Day 31 | Day 32 | Day 60 |
|---|---|---|---|---|
| VEH (N=16) | 107.7 (±4.3) | 113.6 (±4.4) | 120.1 (±4.5) | 247.4 (±7.4) |
| WIN (N=16) | 104.0 (±3.7) | 109.8 (±3.7) | 115.1 (±3.8) | 244.6 (±5.9) |
Preconditioning
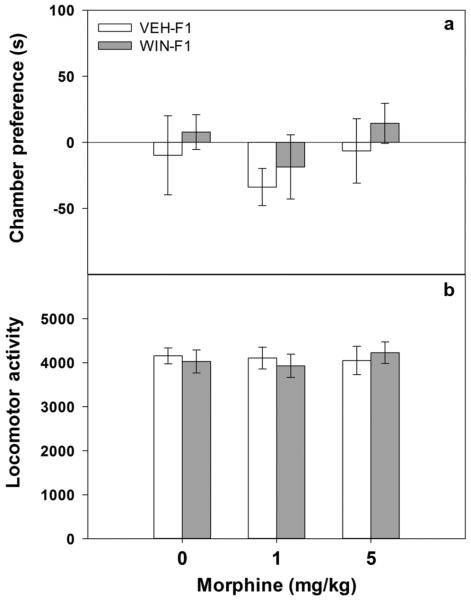
As shown in Figure 1 (panel (a)), prior to conditioning (Day 1) no significant differences in chamber preferences as a function of maternal drug history were observed (all p values > 0.3). In addition, no significant differences in locomotor activity during preconditioning were observed between any of the treatment groups (Figure 1, panel (b); all p values > 0.8).
Figure 1.
Preconditioning (Day 1) in adolescent male offspring. Adolescent male offspring (VEH-F1 and WIN-F1) were tested inside the three-compartment conditioned place preference (CPP) chamber for 15 min to determine Day 1 preconditioning place preference (panel (a)) and locomotor activity (panel (b)). Panel (a) data are differences in the time spent in seconds (mean± SEM) in the chamber to be paired with drug from the unpaired chamber. Panel (b) locomotor activity (mean number of beam breaks ± SEM) during CPP pre-testing. For both panels sample sizes are as follows: 7–9 animals per dose VEH-F1 and 10–12 animals per dose WIN-F1 animals.
Postconditioning
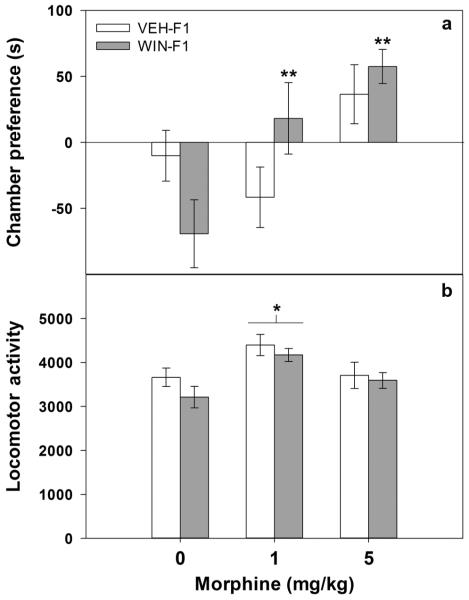
As shown in Figure 2 (panel (a)), there was a significant main effect of morphine dose on CPP (F[2,57] = 7.33, p < 0.01), as well as a significant interaction between morphine dose and maternal drug history (F[2,57] = 3.6, p = 0.03) . Post hoc analyses revealed a significant increase in CPP following either 1 or 5 mg/kg of morphine when compared with saline, an effect that was only observed in WIN-F1 males (both p values < 0.01). In additional, there was a trend toward significant differences between VEH-F1 and WIN-F1 animals administered either saline (p=0.06) or 1 mg/kg morphine (p=0.07). With regard to locomotor activity during postconditioning testing, there was a significant main effect morphine dose; F[2,57] = 7.72, p = 0.001 (Figure 2, panel (b)). Post hoc analyses indicated that this effect was due to a significant increase in activity in males conditioned with the 1 mg/kg dose of morphine as compared with groups conditioned with either saline or 5 mg/kg morphine (p < 0.05). There was no significant effect of maternal treatment, as both VEH-F1 and WIN-F1 animals demonstrated a similar increase in activity at this dose. Thus, while adolescent VEH-F1 animals appeared to be less sensitive to the effects of morphine on CPP, they did exhibit enhanced locomotor activity in response to conditioning with the 1 mg/kg dose of the drug.
Figure 2.
Postconditioning (Day 5) in adolescent male offspring. Following three days of place conditioning with either saline or morphine (1 or 5 mg/kg), VEH-F1 and WIN-F1 adolescent males were tested for conditioned place preference (CPP) and locomotor activity for 15 min. Panel (a) data are differences in the time spent in seconds (mean± SEM) in the chamber paired with drug from the unpaired chamber. **p< 0.01 vs. WIN-F1 saline-treated group. Panel (b) locomotor activity (mean number of beam breaks ± SEM) during CPP testing. *p< 0.05 as compared with 5 mg/kg morphine groups, collapsed across maternal drug history. For both panels sample sizes are as follows: 7–9 animals per dose VEH-F1 and 10–12 animals per dose WIN-F1 animals.
Morphine CPP in adult male offspring
Preconditioning
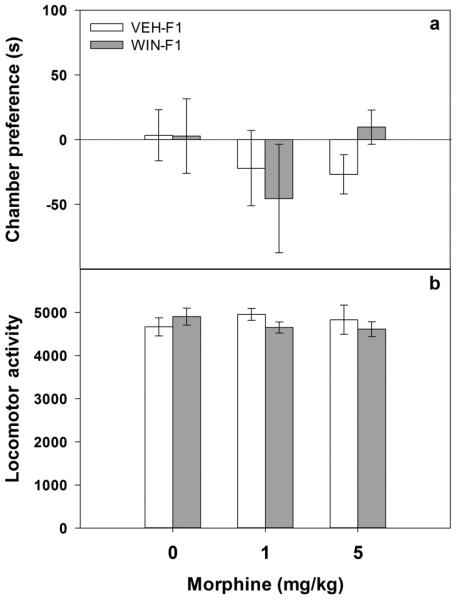
As shown in Figure 3 (panel (a)), prior to conditioning (Day 1) no significant differences in chamber preferences as a function of maternal drug history were observed (all p values > 0.2). In addition, no significant differences in locomotor activity during preconditioning were observed between any of the treatment groups (Figure 3, panel (b); all p values > 0.5).
Figure 3.
Preconditioning (Day 1) in adults male offspring. Adult male offspring (VEH-F1 and WIN-F1) were tested inside the three-compartment conditioned place preference (CPP) chamber for 15 min to determine Day 1 preconditioning place preference (panel (a)) and locomotor activity (panel (b)). Panel (a) data are differences in the time spent in seconds (mean± SEM) in the chamber to be paired with drug from the unpaired chamber. Panel (b) locomotor activity (mean number of beam breaks ± SEM) during CPP pre-testing. For both panels sample sizes are as follows: 7–11 animals per dose VEH-F1 and 7–11 animals per dose WIN-F1 animals.
Postconditioning
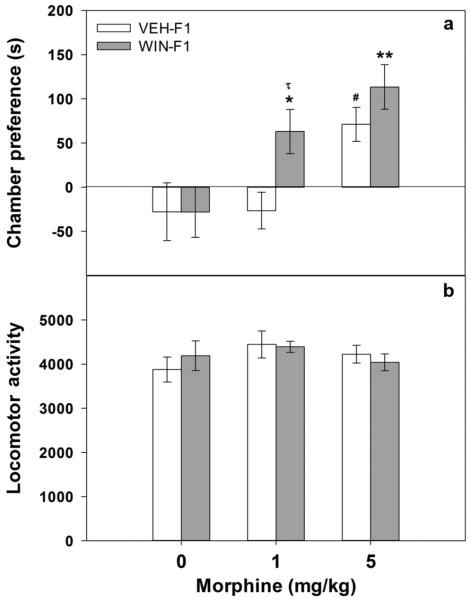
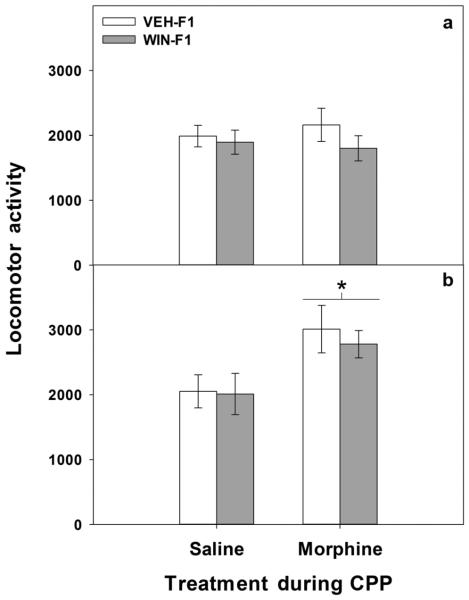
As shown in Figure 4 (panel (a)), there was a significant main effect of morphine dose on CPP (F[2,51] = 12.3, p < 0.001) as well as a significant main effect of maternal drug history (F[1,51] = 4.25, p < 0.05). Post hoc analyses revealed a significant increase in CPP following either 1 or 5 mg/kg of morphine in WIN-F1 males (p values < 0.05 and 0.01, respectively). In contrast, VEH-F1 males only demonstrated significant CPP when conditioned with the 5 mg/kg dose of morphine (p < 0.05 as compared with both 0 and 1 mg/kg). In addition, significant differences between VEH-F1 and WIN-F1 animals were observed at the 1 mg/kg dose (p=0.03). With regard to locomotor activity, no significant effects of prior conditioning on locomotor activity during the postconditioning were observed in adult males (all p values > 0.3; see Figure 4, panel (b)).
Figure 4.
Postconditioning (Day 5) in adult male offspring. Following three days of place conditioning with either saline or morphine (1 or 5 mg/kg), VEH-F1 and WIN-F1 adult males were tested for conditioned place preference (CPP) and locomotor activity for 15 min. Panel (a) data are differences in the time spent in seconds (mean± SEM) in the chamber paired with drug from the unpaired chamber. *p< 0.05 and **p <0.01 vs. WIN-F1 saline-treated group; #p <0.05 vs. VEH-F1 saline-treated group; τ <0.05 as compared with VEH-F1 1 mg/kg-treated group. Panel (b) locomotor activity (mean number of beam breaks ± SEM) during CPP testing. For both panels sample sizes are as follows: 7–11 animals per dose VEH-F1 and 7–11 animals per dose WIN-F1 animals.
Context-independent locomotor sensitization in response to morphine in adult male offspring
A subgroup of adult animals conditioned with either saline or morphine (5 mg/kg) was examined for locomotor responses to a low dose of morphine (2 mg/kg) 10 days after the last conditioning session. As shown in Figure 5 (panel (a)), locomotor activity in response to a saline injection was similar in all F1 males regardless of maternal drug history or conditioning treatment (i.e. saline or 5 mg/kg morphine; all p values > 0.6). However, as shown in Figure 5 (panel (b)), after animals were administered an acute dose of morphine (2 mg/kg), F1 males conditioned with morphine (5 mg/kg) demonstrated significantly increased locomotor activity when compared with those conditioned with saline (main effect of conditioning treatment; F[1,25] = 8.36, p< 0.01). This effect was similar in VEH-F1 and WIN-F1 males.
Figure 5.
Context-independent locomotor sensitization in response to morphine in adult male offspring. Ten days after conditioning with either saline or morphine (5 mg/kg), adult males were treated acutely with saline and monitored for locomotor activity (60 min). Animals were then treated with morphine (2 mg/kg), and monitored for locomotor activity for an additional 120 min. Data are locomotor activity (mean number of beam breaks ± SEM) following acute saline treatment (panel (a)) and acute morphine treatment (panel (b)) for groups of 6–7 animals. *p<0.01 compared with saline-conditioned groups, collapsed across maternal drug history.
Discussion
The current findings demonstrate significant transgenerational effects of adolescent female WIN 55,212-2 treatment on male off-spring behavior. WIN-F1 males exhibited increased CPP in response to morphine when tested during either adolescence or adulthood. These effects occurred in the absence of any direct exposure to this compound in utero, and provide evidence for transgenerational effects of adolescent maternal drug use, even in the absence of continued use during pregnancy. Moreover, the nature of these effects suggests that maternal drug history may increase the reinforcing effects of opiate exposure and potentially enhance substance abuse vulnerability in future progeny.
In the current set of findings, WIN-F1 males displayed more robust CPP when compared with age-matched VEH-F1 males. These effects were largely mediated by differences at the lower morphine dose (1.0 mg/kg), suggesting a shift in the sensitivity of the endogenous opioid system in these offspring. However, while effects of maternal drug history on CPP were observed in both adolescent and adult males, the nature of these effects appeared qualitatively different. Specifically, in adolescent males, the significant effects of morphine on CPP were due in part to decreased time spent in the paired chamber by saline-treated WIN-F1 animals. As these subjects did not demonstrate any significant preference for either chamber during preconditioning (see Figure 1, panel (a)), it is unclear what led to the appearance of decreased preference during postconditioning testing. One possibility may be that the “paired” chamber was the last chamber in which the animals received an injection and thus may have been more strongly associated with that experience. In addition, the saline injection in the paired chamber was always administered during the afternoon, a period associated with rising levels of corticosterone (Gibson and Krieger, 1981). Thus, differences in associative memory and/or corticosterone secretion in WIN-F1 males could have influenced their subsequent response in the paired compartment. Future studies would be needed to determine what processes underlie the observed effects of maternal drug history on the development of adolescent CPP. Nonetheless, the current findings demonstrate that transgenerational effects of cannabinoid exposure are discernible in adolescent male offspring.
Previous studies in adolescent male rats found that morphine CPP was observed only when the drug was paired with the non-preferred chamber (Campbell et al., 2000). Thus, the failure to establish morphine CPP in adolescent VEH-F1 males may have been due to the use of a non-biased design. In adult animals, both VEH-F1 and WIN-F1 males developed morphine-induced CPP. However, WIN-F1 males were more sensitive to the rewarding effects of the 1 mg/kg dose of morphine when compared with VEH-F1 males. These adult data suggest that WIN-F1 males are more sensitive to the rewarding effects of morphine. Together these findings demonstrate that while the nature of the morphine CPP response observed in adolescent and adult WIN-F1 males is different, at both ages CPP is modulated by maternal drug history.
Age-dependent effects were also observed with regard to locomotor activity. Specifically, adolescent males conditioned with the lower dose of morphine displayed increased locomotor activity during postconditioning (i.e. in the absence of drug). This effect was observed in both VEH-F1 and WIN-F1 adolescents, but not in adult animals. Consistent with this finding, adolescent male rats acutely treated with low doses of morphine (0.3 or 1.0 mg/kg) have been shown to have larger locomotor responses when compared with similarly treated adults (White et al., 2008). It is likely that the increased activity observed in adolescent males during postconditioning was due to the contextual conditioning associated with activating effects of this dose. Moreover, this effect does not appear to be influenced by maternal drug history.
In adults, both VEH-F1 and WIN-F1 groups displayed similar levels of context-independent locomotor sensitization in response to morphine when measured 10 days after the final conditioning session. The absence of significant differences in locomotor activity between VEH-F1 and WIN-F1 adults in response to a novel environment as tested during preconditioning as well as following a saline injection during the locomotor sensitization paradigm suggests that maternal drug history does not significantly alter novelty and/or stress-induced locomotor activity. Moreover, the failure to observe transgenerational effects on context-independent morphine-induced locomotor sensitization suggests that the effects of maternal drug history on morphine CPP in male offspring are not likely to be due to nonspecific effects on locomotor activity and/or sensitization processes. It would be interesting to determine whether differences in morphine-induced locomotor sensitization between VEH-F1 and WIN-F1 males would emerge using a context-dependent paradigm. Such findings would help determine whether alterations in contextual learning underlie the effects on CPP observed in WIN-F1 males.
The current study revealed increased morphine CPP without any significant effect on context-independent locomotor sensitization. Interestingly, previous studies have dissociated the effects of cannabinoids on the rewarding and behaviorally-sensitizing effects of morphine. Specifically, CB1 antagonists block the acquisition of morphine CPP, but do not alter morphine-induced locomotor sensitization (Singh et al., 2004). CB1 receptor knockout mice, however, demonstrate disturbances in both morphine CPP as well as morphine sensitization (Martin et al., 2000). These effects may be associated with the significant up-regulation of striatal D2 receptors, or altered endogenous opioid peptide levels observed in CB1 knockouts (Houchi et al., 2005; Steiner et al., 1999), and suggest that compensatory mechanisms play a role in the disrupted locomotor sensitization. Thus, though the mechanism(s) is not completely clear, the present findings may be indicative of alterations in the interaction between endogenous cannabinoid and opioid systems in WIN-F1 males.
Several studies have examined the effects of perinatal cannabinoid exposure on phenotypic modifications in offspring (Campolongo et al., 2011). While many of these effects depend upon the timing, dose, and duration of exposure, a number of studies have revealed significant effects on the endogenous opioid system (Rubio et al., 1998; Singh et al., 2004; Vela et al., 1995, 1998). In addition, more recent findings suggest long-term modification of endogenous opioid systems following cannabinoid exposure during adolescent development (Ellgren et al., 2007). These effects are perhaps not surprising given the significant interaction between endocannabinoids and endogenous opioids, particularly with regard to neural plasticity and development (Ramos et al., 2005; Spano et al., 2010)
The current model utilized increasing doses of the CB1/CB2 receptor agonist WIN 55,212-2 over 3 days during adolescent development. This increasing dose regimen was adapted from previous studies demonstrating long-term effects of a similar brief, increasing dose regimen on the endogenous opioid system (Pontieri et al., 2001), and on the regulation of reward pathways when administered to adolescent males (Pistis et al., 2004). The timing of the exposure during early adolescence coincides with a period of increased vulnerability to both the neural and behavioral effects of cannabinoids (Klugmann et al., 2011; Schneider, 2008, 2009; Schneider et al., 2008). In addition, in females, hypothalamic levels of the endogenous cannabinoid, N-arachidonoylethanolamide (AEA) peak prior to puberty (Wenger et al., 2002) and CB1 receptor activation regulates gonadotropin releasing hormone (GnRH) secretion (Gammon et al., 2005). These findings suggest that endocannabinoids play a role in the reorganization of the neuroendocrine axis in females during the early adolescent period. Thus, it is possible that increased CB1 receptor stimulation during this vulnerable period induces long-term alterations in the neuroendocrine axis which could manifest as subtle changes in either the pre- and/or postnatal environment. Moreover, given the recent evidence for the localization and function of brain CB2 receptors in reward pathways (Xi et al., 2011), it is possible these receptors also play a role in the effects observed herein. As such, brief cannabinoid exposure in the female could shift the developmental trajectory of future offspring, possibly via the disruption of a number of systems which may include cannabinoid-opioid interactions. Additionally, effects could also be related to direct epigenetic modifications in the unfertilized egg, which would be present during the adolescent exposure. Future studies are necessary to determine whether the observed effects represent direct or indirect epigenetic processes. Overall, these data indicate that activation of CB1 and/or CB2 receptors during adolescent development can alter the rewarding effects of opiates in both adolescent and adult male offspring. As such, these findings provide evidence that substance use occurring prior to pregnancy can impact the development of future male offspring. Future studies will determine whether similar effects can be observed in female offspring.
Acknowledgments
The authors would like to thank Michelle Stewart for her technical assistance.
Funding
This work was supported by the National Institutes of Health (grant numbers NIH 5T35RR029724 (MES) and NIH R01DA25674 (EMB)).
Footnotes
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: Effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, et al. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology (Berl) 2011;214:5–15. doi: 10.1007/s00213-010-1892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–1322. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA. Adolescents prenatally exposed to marijuana: Examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol. 2002;42:97S–102S. doi: 10.1002/j.1552-4604.2002.tb06009.x. [DOI] [PubMed] [Google Scholar]
- Gammon CM, Freeman GM, Jr, Xie W, et al. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology. 2005;146:4491–4499. doi: 10.1210/en.2004-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MJ, Krieger DT. Circadian corticosterone rhythm and stress response in rats with adrenal autotransplants. Am J Physiol. 1981;240:E363–E366. doi: 10.1152/ajpendo.1981.240.4.E363. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Cornelius MD, et al. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 2004;26:521–532. doi: 10.1016/j.ntt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, et al. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Klippenstein V, Leweke FM, et al. Cannabinoid exposure in pubertal rats increases spontaneous ethanol consumption and NMDA receptor associated protein levels. Int J Neuropsychopharmacol. 2011;14:505–517. doi: 10.1017/S1461145710001562. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Gonzalez-Cuevas G, Moreno G, et al. The pharmacology of the endocannabinoid system: Functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol. 2008;13:160–187. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Lopez-Jimenez A, Gorriti MA, et al. Functional interactions between endogenous cannabinoid and opioid systems: Focus on alcohol, genetics and drug-addicted behaviors. Curr Drug Targets. 2010;11:406–428. doi: 10.2174/138945010790980312. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, et al. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fa M, Ferraro L, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100:4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, et al. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Monnazzi P, Scontrini A, et al. Behavioral sensitization to heroin by cannabinoid pretreatment in the rat. Eur J Pharmacol. 2001;421:R1–R3. doi: 10.1016/s0014-2999(01)01056-1. [DOI] [PubMed] [Google Scholar]
- Ramos JA, Gomez M, de Miguel R. Handb Exp Pharmacology; Cannabinoids. Springer; Germany: 2005. Effects on development; pp. 643–656. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, et al. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rubio P, Rodriguez de Fonseca F, Martin-Calderon JL, et al. Maternal exposure to low doses of delta9-tetrahydrocannabinol facilitates morphine-induced place conditioning in adult male offspring. Pharmacol Biochem Behav. 1998;61:229–238. doi: 10.1016/s0091-3057(98)00099-9. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD, USA: 2011. 2011. NSDUH Series H-41 HHS Publication No. (SMA) 11–4658. [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider M. Cannabis use in pregnancy and early life and its consequences: Animal models. Eur Arch Psychiatry Clin Neurosci. 2009;259:383–393. doi: 10.1007/s00406-009-0026-0. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Singh ME, Verty AN, McGregor IS, et al. A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004;1026:244–253. doi: 10.1016/j.brainres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fadda P, Fratta W, et al. Cannabinoid-opioid interactions in drug discrimination and self-administration: Effect of maternal, postnatal, adolescent and adult exposure to the drugs. Curr Drug Targets. 2010;11:450–461. doi: 10.2174/138945010790980295. [DOI] [PubMed] [Google Scholar]
- Steiner H, Bonner TI, Zimmer AM, et al. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G, Fuentes JA, Bonnin A, et al. Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioidrelated behavioral patterns in rats. Brain Res. 1995;680:142–147. doi: 10.1016/0006-8993(95)00255-o. [DOI] [PubMed] [Google Scholar]
- Vela G, Martin S, Garcia-Gil L, et al. Maternal exposure to delta9tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998;807:101–109. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- Wenger T, Gerendai I, Fezza F, et al. The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci. 2002;70:1407–1414. doi: 10.1016/s0024-3205(01)01516-8. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, et al. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]