INTRODUCTION
Sickle cell disease (SCD) is the name for a group of genetic blood disorders caused by sickle hemoglobin (Hb S). The 2 key features of SCD are chronic hemolytic anemia and vaso-occlusion. Although it is fundamentally a blood disease, SCD affects the entire body, and the pathophysiology begins in very early infancy. Pediatricians and family practitioners are crucial partners in the multidisciplinary team that is required to manage children with SCD. This article provides a broad overview of SCD in childhood, focusing on common complications and current treatments. Special attention is given to the results of important clinical trials that have changed the management of SCD.
HEMOGLOBIN, GENETICS, AND BASIC PATHOPHYSIOLOGY
Hb is the oxygen-carrying protein in blood. It is a tetramer of 4 proteins, 2 α-globins and 2 β-globins. Each globin has an associated oxygen-binding heme group. The α-globins and β-globins are encoded by genes on different chromosomes. The Hb S mutation (βS) is a single nucleotide substitution in the sixth codon of the β-globin gene (HBB). This yields a protein with a hydrophobic valine residue, instead of the normal hydrophilic glutamic acid at the sixth position, that is prone to polymerization on deoxygenation.
Heterozygosity for βS, called sickle cell trait, occurs frequently in individuals of African ancestry (sub-Saharan, equatorial Africa), but it also occurs commonly in the eastern provinces of Saudi Arabia, central India, and parts of the Mediterranean. It is now found throughout the world due to migration. Heterozygosity for βS provides some protection against severe malarial infection, which is the generally accepted explanation for the maintenance of this balanced polymorphism. The inheritance of SCD is often referred to as autosomal recessive. However, one can consider βS to be inherited in an autosomal codominant fashion, because even a single βS gene is expressed and produces phenotypic changes in the Hb profile (and rare clinical complications). Moreover, homozygous inheritance (βS/βS) or compound heterozygosity with certain other mutant β-globins, such as Hb C (βS/βC), produce different types of SCD (Table 1).
Table 1.
Common types of sickle cell disease
| Genotype | Abbreviation | Name | Typical Peripheral Blood Findings in Untreated SCD | Severitya | |||
|---|---|---|---|---|---|---|---|
| Main Hbs Present | Hb (g/dL) | MCVb (fL) | Reticulocytes (%) | ||||
| βS/βS | Hb SS | Sickle cell anemiac | S | 6–9 | Normal | 10–25 | +++ |
| βS/β0 | Hb Sβ0 | Sickle-β0-thalassemia | S | 6–9 | Decreased | 10–25 | +++ |
| βS/βC | Hb SC | Sickle-Hb C disease | S, C | 9–12 | Usually normal | 5–10 | ++ |
| βS/β+ | Hb Sβ+ | Sickle-β+-thalassemia | S, A | 10–13 | Decreased | 2–10 | + |
Abbreviations: Hb, hemoglobin; Hbs, hemoglobins; MCV, mean cell volume; SCD, sickle cell disease.
A population-based generalization that may not apply to the individual (the number of plus signs corresponds to the overall degree of severity of the disease).
Coinheritance of α-thalassemia trait, which is common, will produce microcytosis in Hb SS and Hb SC.
Because they may be clinically indistinguishable, some use the term sickle cell anemia to apply to both Hb SS and Hb Sβ0.
The polymerization of Hb S within red blood cells (RBCs) (“sickling”) on deoxygenation underlies all the pathophysiology of SCD. As Hb S-containing RBCs traverse the circulation undergoing cycles of oxygenation and deoxygenation, rigid polymers of Hb S repeatedly form and damage the RBC membrane, drastically shortening the RBC life span. RBCs also become dehydrated, relatively inflexible, and abnormally adhesive. Consequently, they are prone to adhere to the endothelium of blood vessels, in concert with leukocytes and platelets, impeding the flow of blood. This microvascular obstruction, called vaso-occlusion, leads to ischemia, infarction, and ischemia-reperfusion injury of multiple organs and tissues. This pathophysiology produces an ongoing inflammatory response and endothelial dysfunction. Some complications of SCD can be considered to be primarily a consequence of either hemolysis or vaso-occlusion. For example, chronic hemolysis predisposes to bilirubinate cholelithiasis, whereas vaso-occlusive ischemia and infarction of bone marrow is thought to cause the acute painful event (“crisis”), the hallmark of SCD. The pathophysiology of SCD is more complex than a simple “log jam” model of vaso-occlusion by irreversibly sickled RBCs (Fig. 1).
Fig. 1.
The initiating factor of SCD pathophysiology is RBC sickling, which leads to hemolysis and vascular stasis. Ischemia-reperfusion injury is the end result of multiple, complex pathophysiologic interactions. (Modified from Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol 2011;86(2):123–54; with permission.)
FORMS OF SCD AND DIAGNOSIS
The most common and severe form of SCD is the homozygous state for βS, called sickle cell anemia (Hb SS). Other forms of SCD result from coinheritance of βS with one of several other abnormal β-globin genes. The most common interacting variants include Hb C and several β+-thalassemia and β0-thalassemia mutations. These compound heterozygous states produce types of SCD called sickle-hemoglobin C disease (Hb SC), sickle-β+-thalassemia (Hb Sβ+), and sickle-β0-thalassemia (Hb Sβ0). In general, Hb SS and Hb Sβ0 are the most severe forms of SCD, and they may be clinically indistinguishable. In comparison, Hb SC and Hb Sβ+ are usually less severe. Table 1 provides an overview of these common forms of SCD.
In the United States and many other developed nations, SCD is usually diagnosed by universal newborn screening for hemoglobinopathies, which involves Hb separation techniques on extracted material from dried blood spots. Initial newborn screening test results should be confirmed by follow-up studies. Genetic testing is increasingly used for this purpose. Beyond the newborn period, the diagnosis of SCD is made by careful consideration of the complete blood count, peripheral blood morphology, and some combination of Hb separation techniques, family studies, and genetic testing.
The typical blood counts in the common types of SCD are shown in Table 1. The presence of irreversibly sickled cells (ISCs) is pathognomonic for SCD. ISCs are commonly seen in Hb SS and Hb Sβ0 but are rare or absent in Hb SC and Hb Sβ+. So, the absence of ISCs does not exclude the diagnosis of SCD. Target cells are common in Hb SC, Hb Sβ0, and Hb Sβ+. Microcytosis is a feature of Hb Sβ0 and Hb Sβ+ (and sometimes Hb SC), but it also occurs when α-thalassemia is independently coinherited with Hb SS or Hb SC.
The mainstay of the diagnosis of SCD, whether at birth or later in life, is an Hb separation technique, such as high-pressure liquid chromatography (HPLC), isoelectric focusing (IEF), or citrate agar electrophoresis. These techniques determine the presence and relative proportions of the different types of Hb present in RBC hemolysates (see Table 1). Two different techniques, such as HPLC followed by IEF, should be used to confirm the presence of an abnormal Hb, because different Hb molecules may comigrate in one but not another technique. Hb F will also be present in variable amounts in SCD, as well as small amounts of Hb A2. Sickle Hb solubility testing (eg, Sickledex) is an inadequate diagnostic test because it cannot distinguish sickle trait from SCD. Simple electrophoresis (in cellulose acetate) is usually no longer performed.
Genetic testing is increasingly used to make the initial diagnosis or to confirm a diagnosis of SCD. Different techniques are used to detect point mutations, small deletions or insertions, and large deletions or rearrangements. It is necessary to know which particular technique is being used for the genetic diagnosis of a patient, because each technique has limitations that affect the reliability of the findings.
CLINICAL SCENARIOS AND COMPLICATIONS OF SCD
The Newborn and Infant
The first few months of life are asymptomatic, because affected newborns and very young infants still make a significant amount of fetal Hb (Hb F), which inhibits the polymerization of Hb S and protects against SCD. As Hb F declines over the first few months, there is a commensurate rise in Hb S (instead of the normal Hb A). This period of protection afforded by Hb F usually lasts about 3 months, allowing for newborn screening and early intervention. Universal newborn screening for hemoglobinopathies is performed in all US states (it is important to remember that immigrants may not have had newborn screening). Infants identified to have SCD, or at least those with Hb SS and Hb Sβ0, should be prescribed prophylactic penicillin to prevent early fatal pneumococcal sepsis (Box 1). Splenic dysfunction (hyposplenism) begins as early as 3 months of age, so prophylactic penicillin should be started by then. Children with SCD require extra vaccinations as well (see Box 1). Anemia is usually seen by 6 months of age, and it becomes more severe over the next several months to years. Dactylitis and splenic sequestration can occur in the first year of life, but most other overt complications tend to begin after 1 year of age.
Box 1 Penicillin prophylaxis and immunizations.
Pencillin Prophylaxis
- For children with Hb SS and Hb Sβ0
- Begin at 1–2 months of age
- Age <3 years: penicillin V potassium 125 mg by mouth twice a day
- Age 3–5 years: penicillin V potassium 250 mg by mouth twice a day
- Continue until at least 5 years of age
- May continue past 5 years for pneumococcal sepsis, surgical splenectomy, or parental preference
- For children with Hb SC and Hb Sβ+
- Hyposplenism occurs years later than in Hb SS
- Practice varies by center
- Consider starting at age 4–5 years of age or for a history of pneumococcal sepsis or surgical splenectomy
Immunizations
- For all forms of SCD
- The 23-valent pneumococcal polysaccharide vaccine (PPV-23): Ages 2 and 5 years (consider reimmunization at 5-year intervals)
- 4-valent meningococcal conjugate vaccine (MCV-4): Age 2 years (consider re-immunization at 5-year intervals)
- Influenza vaccine: yearly
- The normal vaccine series of childhood that includes the 13-valent pneumococcal conjugate vaccine (PCV-13), the H. influenzae type b and hepatitis B virus vaccines
Hemolytic Anemia
The rate of hemolysis in SCD usually exceeds the rate at which new RBCs can be produced by the bone marrow, resulting in anemia despite ongoing reticulocytosis. RBC life span in Hb SS may be as short as 12 days (compared with the normal 120 days). The degree of anemia varies by the genotype of SCD. Table 1 shows the typical “baseline” or “steady-state” Hb concentrations; that is, when the patient is not experiencing an acute illness or complication. Because it is chronic, most children are reasonably well compensated physiologically for their anemia, even if severe (eg, 6–7 g/dL). Chronic scleral icterus and a systolic ejection murmur are common and expected findings in patients with moderate to severe anemia, especially Hb SS and Hb Sβ0. Unconjugated hyperbilirubinemia and an elevated lactate dehydrogenase concentration are also expected. Generally, the degree of chronic anemia itself is not an indication for transfusion in children. A minority of children have symptomatic chronic anemia that interferes with daily activities or quality of life, for whom hydroxyurea therapy or chronic transfusions should be considered. Folic acid supplements are often recommended to prevent depletion of folate stores and megaloblastic “crises,” but this is probably unnecessary for most children with SCD in developed countries where many foods are fortified with folate. The 2 classic causes of acute, severe exacerbations of chronic anemia are the transient aplastic crisis and acute splenic sequestration (Table 2), discussed individually later in this article. Less severe acute exacerbations of chronic anemia occur during other complications or with concurrent illnesses.
Table 2.
Acute severe anemia in sickle cell disease: splenic sequestration versus transient aplastic crisis
| Acute Splenic Sequestrationa | Transient Aplastic Crisisa | |
|---|---|---|
| Spleen | Acutely enlarged | Not palpable or not acutely enlarged |
| Reticulocytes | Increased from baseline | Inappropriately lowb |
| NRBCs | Present | Absentb |
| Platelets | Decreased (Hypersplenism) | Normal or increasedc |
| Transfusion | May need rapid transfusion for hypovolemic shock | May need slow/small volume transfusion to prevent fluid overload and heart failure |
Abbreviation: NRBC, nucleated red blood cell.
Parvovirus infection can trigger acute splenic sequestration, so a patient can have simultaneous sequestration and aplasia. Thus, the typical features listed may not always be present or discriminative.
If a patient presents in the recovery phase of aplastic crisis, then the reticulocyte and NRBC counts will be increased (see text).
Parvovirus can sometimes cause multilineage cytopenias, so the platelet count may be decreased (see text).
Hyposplenism, Fever, and Sepsis
Children with SCD are at very high risk of invasive pneumococcal disease (300–500 times higher than the general population) because of loss of splenic filtrative function due to infarction (resulting in functional hyposplenism). Typical forms of pneumococcal disease in SCD include bacteremia, sepsis, meningitis, and pulmonary infection. Hyposplenism is detectable by 3 months of age in Hb SS and Hb Sβ0, so it is necessary to begin prophylactic penicillin before then to prevent fatal pneumococcal sepsis (see Box 1).1 The standard recommendation is to stop prophylactic penicillin at age 5 years in Hb SS and Hb Sβ0, based on the results of a randomized clinical trial.2 The routine administration of prophylactic penicillin to infants and young children with Hb SC disease may not be necessary, because hyposplenism does not begin until after 4 years of age.3 Immunizations are especially important for children with SCD (see Box 1). Fatal pneumococcal sepsis is now rare in children with SCD in the United States and United Kingdom (and other developed nations),4,5 but pneumococcal disease has not been eradicated. Vigilance is still required for all febrile illnesses, especially because of the recent emergence of nonvaccine serotypes of Streptococcus pneumoniae, as well as sepsis in children older than 5 years with SCD.6,7
Accordingly, high fever (eg, >101–101.5°F) is a medical emergency in patients with SCD, because it can be the first sign of bacteremia. Patients and caregivers need to present promptly to medical attention. Febrile patients should be promptly evaluated with careful attention to cardiopulmonary status and identification of possible sites of infection. After obtaining a complete blood count and a blood culture, a broad-spectrum parenteral antibiotic (eg, ceftriaxone) should be given without delay. Most children with SCD and fever do not need to be hospitalized.8 Inpatient management is needed for septic or toxic-appearing children and should be strongly considered for those who have high-risk features, such as very young age (<6 months), concomitant pulmonary disease, blood counts that are significantly different from baseline, missed doses of prophylactic penicillin, or uncertainty of follow-up. Follow-up of both the patient and the results of the blood culture are required for outpatient management.
Splenic Sequestration
Before involution is complete, the spleen is prone to sequestration (trapping of RBCs within the splenic sinusoids). Splenic sequestration typically occurs between 1 and 4 years of age, but much earlier presentation is possible. Splenic involution is usually complete by 5 years of age in Hb SS and Hb Sβ0, so sequestration is uncommon thereafter. In acute splenic sequestration, the spleen becomes acutely engorged with blood sequestered from the systemic circulation. This leads to potentially severe anemia, hypovolemia, and possibly shock. Sequestration usually develops without warning, so it is important for both parents and health care professionals to be able to detect splenomegaly and the signs and symptoms of acute severe anemia to prevent death. Not all sequestration is severe and life threatening; transfusion is usually reserved for symptomatic or severe anemia. Acute splenic sequestration needs to be differentiated from the transient aplastic crisis, because the approach to transfusion can differ (see Table 2). In severe sequestration, the initial transfusion of packed RBCs (PRBCs) may need to be given rapidly to correct hypovolemic shock. Subsequent transfusions, if needed, should be given cautiously and in smaller volumes because of the potential for autotransfusion of sequestered blood that could lead to hyperviscosity. Splenectomy should be considered for severe or recurrent acute splenic sequestration.
In Hb SC and Hb Sβ+, splenic involution is delayed, and sequestration occurs in older children and adults. Splenic sequestration in older individuals is often quite painful because of concomitant infarction. The use of hydroxyurea and chronic transfusions can delay the course of splenic involution, so preservation or “regrowth” of splenic tissue (but not proper splenic immune function) and sequestration can also occur in older children with Hb SS and Hb Sβ0.
Transient Aplastic Crisis
Human parvovirus (B19) infects RBC precursors in the bone marrow and temporarily impairs the production of new RBCs (erythroblastopenia and reticulocytopenia). In the healthy host, parvovirus infection does not cause anemia because of the long life span of normal RBCs. However, in SCD and other forms of chronic hemolytic anemia (ie, conditions with a shortened RBC life span), parvovirus causes anemia that can be severe. This transient anemia is called the aplastic crisis. Jaundice decreases during aplastic crisis, because the red cell mass has greatly decreased, providing a clue from the history. Spontaneous recovery begins about 1 week after the onset of reticulocytopenia due to antibody-mediated clearance of the virus, heralded by an outpouring of nucleated RBCs followed shortly by reticulocytosis. If a patient presents in this early recovery phase, rather than the reticulocytopenic phase, the anemia and reticulocytosis can be confused with splenic sequestration or an acutely increased hemolytic rate. The need for RBC transfusion depends on the severity of anemia and the clinical status of the patient. Parvovirus aplastic crisis does not recur due to long-lasting humoral immunity.
Parvovirus infection of immunocompetent individuals classically produces isolated RBC aplasia. However, it may cause transient hypoplasia of multiple blood cell lines, including pancytopenia, which may be confusing and lead to unnecessary workup.9 The acute anemia of the aplastic crisis also needs to be differentiated from acute splenic sequestration, because transfusion therapy can differ (see Table 2). The anemia of aplastic crisis develops progressively over the course of a week, so there is physiologic compensation, including increased blood volume and cardiac output. As such, patients with aplastic crisis may be euvolemic or mildly hypervolemic, and rapid transfusion of PRBCs or intravenous fluid boluses may precipitate heart failure. Strong consideration should be given to slow transfusion of PRBCs in small, sequentially administered aliquots (eg, each over 4 hours).
Painful Events and Bony Complications
Dactylitis, or hand-foot syndrome, is one of the earliest physical manifestations of SCD. Vaso-occlusive ischemia and infarction of the metacarpals and phalanges produces painful and often symmetric swelling of the hands, feet, or both. About 30% of children with Hb SS will have dactylitis in the first 3 years of life. Two-thirds of cases occur between 6 months and 2 years of age, and it is unusual beyond 5 years.
Acute SCD pain is typically multifocal and often regional or bilaterally symmetric. It has both nociceptive and neuropathic characteristics. Abdominal pain, the cause of which is unclear, occurs in about one-third of painful events. Fever, especially low grade, occurs in 40% of episodes of uncomplicated pain. With the exception of dactylitis, painful events often have no associated physical signs, like edema or erythema, or overt evidence of inflammation, like joint effusions or leukocytosis. Objective signs occur only 15% of the time, so it is important to believe a patient’s report of pain despite a nonspecific history and physical examination. Antecedents of pain, some of which can be avoided, include infection, dehydration, and cooling of the skin. However, most painful episodes are unpredictable and occur without known triggers. Most children with SCD do not have frequent hospitalizations for pain (on average <1 per year). Mild to moderate pain is often managed at home with prompt use of oral analgesics and hydration.
The treatment of painful events is primarily supportive (Box 2). A combination of a nonsteroidal anti-inflammatory drug (NSAID) and opiate analgesics, titrated to effect, can usually provide adequate relief. Once the pain begins to resolve, analgesia can usually be decreased quickly. The complete resolution of a painful event may take as long as a week or two, with complete convalescence occurring at home. Transfusion is not indicated for uncomplicated painful events. There is still no abortive therapy for painful events; however, a number of trials of different drugs that target blood rheology, blood cell adhesion, or inflammation are currently under way.
Box 2 Principles of supportive care for hospitalized children with sickle cell disease.
Acute Painful Episodes
Pharmacologic analgesia (nonsteroidal anti-inflammatory drugs [NSAIDs] and opiates) that is individualized to the patient and the degree of pain. Avoid overtreatment and undertreatment of pain.
Nonpharmacological methods to decrease pain, such as warm compresses, relaxation, and massage.
Correct any dehydration and maintain normal hydration. Avoid overhydration, which can precipitate acute chest syndrome (ACS).
Incentive spirometry to prevent ACS.
Vigilance for the development of fever and ACS.
Laxative therapy for opiate-related constipation.
Anti-pruritic therapy for opiate-related pruritus.
ACS
Supplemental oxygen for hypoxemia or increased work of breathing.
Correct any dehydration and maintain normal hydration. Avoid overhydration, which can cause pulmonary edema.
Adequate analgesia to prevent respiratory splinting. Inadequate or excessive analgesia can lead to hypoventilation and atelectasis.
Empiric antibiotic therapy for S pneumoniae, Mycoplasma, and Chlamydia—commonly a cephalosporin and a macrolide.
Incentive spirometry.
Bronchodilator therapy for asthma or asthmalike signs and symptoms.
Surgery and Anesthesia
Preoperative: For elective surgeries and procedures, the patient should be in the baseline state of health, well hydrated, and free of infection and acute pulmonary disease.
Intraoperative: careful attention to the physiologic status of the patient and prevention of hypoxemia, acidemia, hypotension, hypovolemia, and hypothermia.
Post-operative: Adequate analgesia to prevent respiratory splinting. Inadequate or excessive analgesia can lead to hypoventilation/atelectasis and ACS.
Incentive spirometry to prevent ACS.
Maintain normal hydration. Avoid overhydration, which can cause pulmonary edema and ACS.
Supplemental oxygen until the patient is fully awake and breathing normally.
SCD predisposes to osteomyelitis, which is thought to result from secondary infection of ischemic or avascular bone. Differentiating osteomyelitis from painful events is sometimes challenging, because both can cause bony tenderness and joint effusions. Imaging studies may not be discriminative, because sterile bony infarction and osteomyelitis often produce similar findings on radiographs, radionuclide scans, and magnetic resonance imaging (MRI). Clinical features that increase the likelihood of osteomyelitis over an uncomplicated painful event are a single focus of pain, fever, and bacteremia.10 However, painful events are far more common (50 times more common) than osteomyelitis in SCD, so the pretest probability of osteomyelitis always needs to be considered. Special imaging and biopsy of bone are best reserved for patients for whom there is high clinical suspicion of osteomyelitis. Empiric therapy should be directed against Salmonella and Staphylococcus, which are the most common causes of osteomyelitis in SCD. Specific therapy is given once an organism is identified.
Avascular necrosis (AVN) can occur as early as 5 years of age, but is most commonly diagnosed in the third decade of life in the United States. The most common site of AVN is the femoral heads, but it also occurs in the proximal humerus and other bones. Pain in the hips or knees should prompt the consideration of AVN. Plain radiographs are often sufficient to make the diagnosis, but MRI may be needed to detect early disease or to plan surgery. No therapy is needed for incidentally detected, asymptomatic AVN. Symptomatic AVN is managed with long-acting NSAIDs (eg, naproxen) and physical therapy. Some patients may require surgery.
Pulmonary Complications
Acute chest syndrome (ACS) refers to a spectrum of acute pulmonary illness in a person with SCD.11 ACS is the second most common cause of hospitalization in SCD, and it is now the most common cause of death due to SCD. Diagnostic criteria vary, but most definitions specify a new radiographic pulmonary infiltrate and some combination of fever, chest pain, and signs or symptoms of pulmonary disease, such as tachypnea, dyspnea, cough, or hemoptysis. ACS has many antecedents or triggers, including infection, pulmonary fat embolism or thromboembolism, hypoventilation/atelectasis, bronchospasm, and inflammation of any cause. ACS is common in young children in whom it is associated with viral respiratory infections and is often self-limited. The death rate is lower in children than adults. ACS will not be apparent in 30% to 60% of patients at the time of hospitalization, yet ACS often develops in children hospitalized for treatment of other conditions, such as a painful event, or after surgery. Therefore, ACS should be an anticipated complication and prevented when possible (see Box 2).
Management of ACS is primarily supportive (see Box 2). Unfortunately, there are no randomized controlled trials that compare upfront transfusion (yes or no) or mode of transfusion (simple vs exchange) for established ACS. With this important caveat in mind, simple transfusion of RBCs should be considered for hypoxemia or acute exacerbation of anemia, whereas exchange transfusion should be performed for hypoxemia despite oxygen supplementation, widespread (bilateral, multilobar) pulmonary infiltrates, or rapid clinical deterioration. Corticosteroids can shorten the duration and severity of ACS but can also precipitate “rebound” painful events.12,13 It is reasonable to use corticosteroids for patients with asthma when it is otherwise indicated (eg, an exacerbation of asthma with ACS) or for rapidly progressive or severe ACS with the intent of preventing or limiting mechanical ventilation.
Asthma is a common diagnosis in children and adults with SCD. This additional diagnosis is associated with an increased frequency of ACS and painful events and a higher risk of death. There is phenotypic overlap between ACS and acute exacerbation of asthma, so clearly differentiating the two is not always possible. There is evidence that asthma and SCD can be separate, comorbid conditions and also evidence that SCD may have asthmalike features.14 It is prudent to evaluate for asthma when suspected, based on respiratory signs or symptoms, but also when a patient has frequent episodes of pain or ACS. Although there are no appropriate randomized trials to best inform management, good control of asthma or asthmalike pathology might decrease the frequency of ACS and the severity of SCD in general.14
Pulmonary hypertension in SCD has been the focus of much recent research and debate. Echocardiography is used as a screening test to estimate pulmonary artery pressure, and a tricuspid regurgitant jet velocity (TRJV) greater than 2.5 m/s is consistent with elevated pressure. A high TRJV is associated with early mortality in adults15 but not in children.16 The benefit of screening for elevated pulmonary artery pressures has not been established for children.
Neurologic Complications
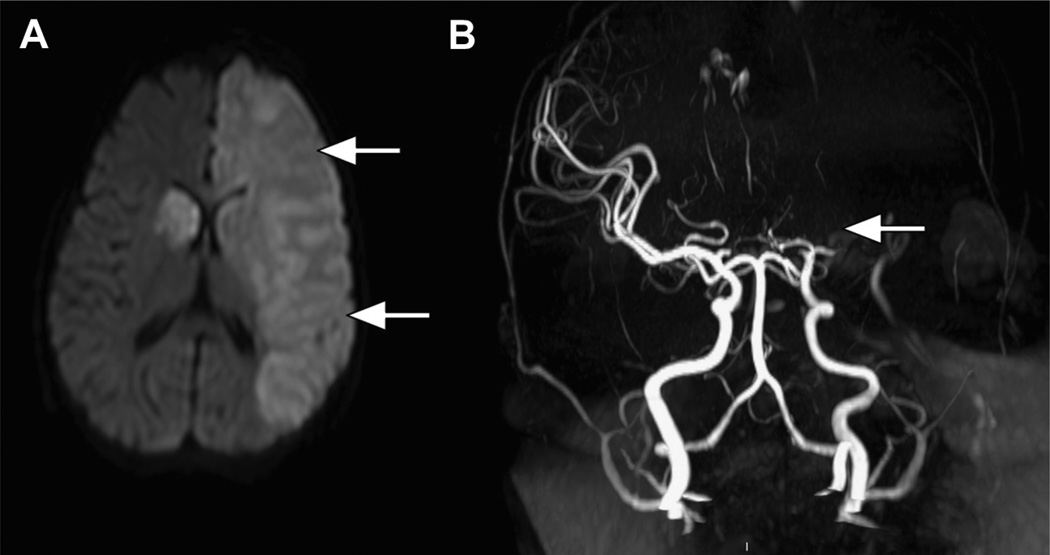
The brain is affected in a number of ways in SCD, both structurally and functionally. Without primary prevention (discussed later in this article), overt stroke occurs in 11% of children with Hb SS by 18 years of age (Fig. 2), with a peak yearly incidence of about 1% between 2 and 9 years of age. Stroke is much less common in Hb SC and Hb Sβ+. Transient ischemic attacks (TIAs) may be premonitory. Stroke may be an isolated event or occur in conjunction with another complication of SCD, such as ACS, aplastic crisis, or priapism. Most overt strokes in children are ischemic and are associated with occlusive cerebral arterial vasculopathy in large intracranial vessels (see Fig. 2B). Hemorrhagic strokes occur with increasing frequency in young adulthood. Suspicion of a neurologic event (overt stroke or TIA) requires emergent neuroimaging. Initial computed tomography to assess for hemorrhage can be considered, especially for patients with severe headache, prior stroke, or known vasculopathy (eg, moya moya vessels), but MRI and MR angiography (MRI/MRA) are needed to define the timing and location of any ischemia or infarction and assess the cerebral arteries. A critical component of the management of acute overt stroke is transfusion. Initial exchange transfusion is associated with a lower risk of recurrent stroke than initial simple transfusion. Patients who have an associated medical event, such as ACS, at the time of stroke also seem to have a lower risk of recurrent stroke. Chronic transfusion therapy to maintain the Hb S lower than 30% decreases the chance of recurrent overt stroke from 60% to 90% to about 20% (see the section on Chronic Transfusions later in this article).
Fig. 2.
Overt stroke. (A) A diffusion-weighted image that shows extensive acute ischemia in the left cerebral hemisphere (arrows) as well as in the right basal ganglia in a child with Hb SS. (B) MRA showing complete occlusion of the proximal middle cerebral artery (arrow).
Abnormally increased transcranial Doppler (TCD) blood flow velocities identify children with Hb SS at highest risk of overt stroke. TCD detects dynamic or fixed cerebral arterial stenosis that can be the antecedent of overt stroke (see Fig. 2). An abnormal TCD status confers about a 10% risk of stroke per year for 3 years after the test. The Stroke Prevention Trial in sickle cell anemia (STOP trial) showed that chronic transfusions decreased the rate of first stroke in children with abnormal TCD by 92% compared with observation.17 Therefore, screening (TCD) should be performed at least annually for children of age 2 to 16 years with Hb SS or Hb Sβ0 to direct the initiation of chronic transfusion therapy for primary stroke prophylaxis. A follow-up study (STOP 2) showed that discontinuation of transfusions after 30 months resulted in a high rate of reversion to abnormal TCD velocities and stroke.18 So, outside of a clinical trial, transfusions still need to be continued indefinitely. In the decade since the publication of the STOP study (1999–2009), the mean annual incidence of hospitalization for overt stroke in children with SCD in the United States has decreased by 45%.19 Although this is clearly an important advance in the care of children with SCD, most children with abnormal TCD velocities will not actually have a stroke (if untreated) and chronic transfusion therapy is burdensome (see section on chronic transfusions later in this article), so further progress is needed.
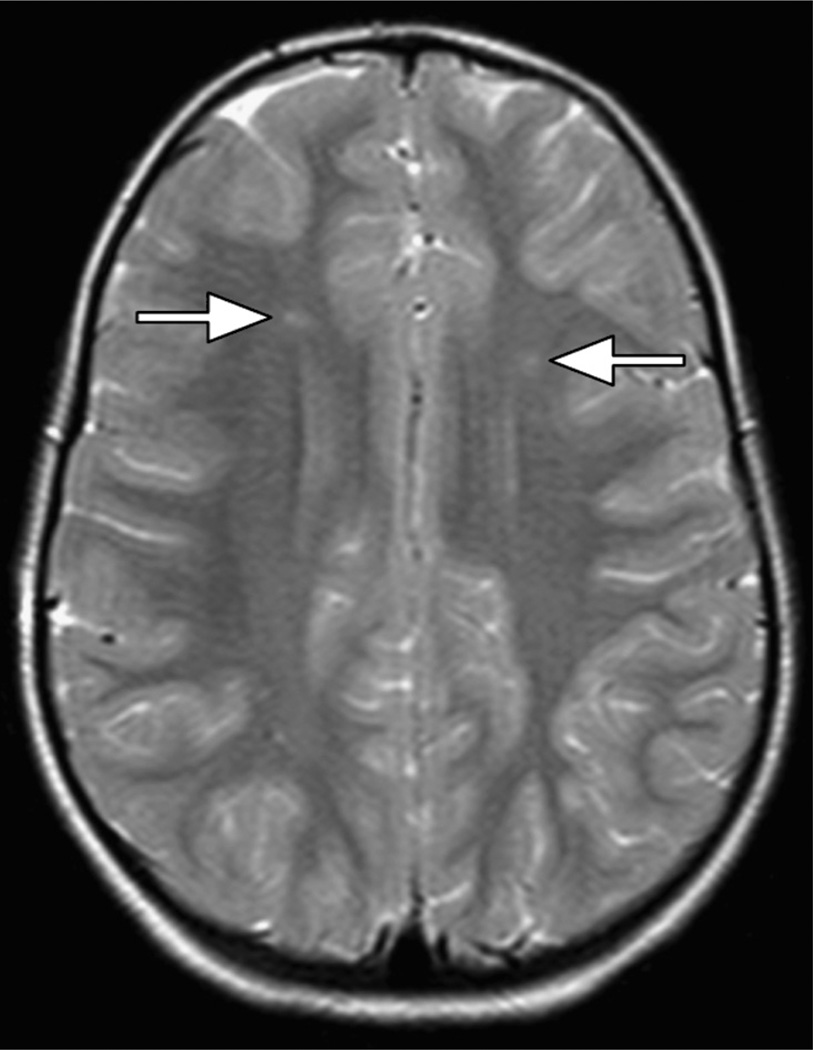
Silent cerebral infarction (SCI) is more common than overt stroke, occurring in up to 37% of children with Hb SS or Hb Sβ0 by 18 years of age (Fig. 3).20 By definition, SCI produces no motor or sensory deficits, so it must be identified by screening MRI of the brain. SCI is associated with neurocognitive impairment, poor school performance, and increased risk for subsequent overt stroke. Low Hb concentration and high (relative) systolic blood pressure are risk factors for SCI.21 The medical management of SCI is not yet defined. A newly recognized covert brain lesion, the acute silent cerebral ischemic event (ASCIE), occurs 40 times more frequently than initial SCI.22,23 Acute anemic events appear to dramatically increase the risk of ASCIE.23 A fraction of ASCIEs appear to be transient; others evolve into typical, permanent SCI. Anemia itself also affects neuropsychological function, and children commonly have academic and behavioral difficulties that cannot readily be explained by brain lesions alone. Neuropsychiatric testing, psychological counseling and support, and school intervention programs are key components of comprehensive SCD care.
Fig. 3.
SCI. Bilateral SCI is seen in the frontal white matter (arrows) in a child with Hb SS.
Renal and Genitourinary Complications
The hypoxic, acidic, and hyperosmolar environment of the renal medulla promotes RBC sickling and vaso-occlusion. Repeated vaso-occlusion eventually destroys the vasa recta, leading to inability to maximally concentrate the urine. This is the first renal manifestation of SCD, often occurring before 1 year of age. The urinary concentrating defect results in fixed urinary output (polyuria) and predisposes to dehydration and nocturia or enuresis. Renal ischemia also causes papillary necrosis and microscopic or gross hematuria. Ischemic medullary interstitial fibrosis is thought to promote glomerular hyperfiltration and hypertrophy, which can eventually result in focal segmental glomerular sclerosis, renal insufficiency, and even renal failure. Proteinuria is a marker of glomerular disease, and patients are increasingly prescribed angiotensin-converting enzyme inhibitors and angiotensin receptor blockers to decrease proteinuria with the hope of preventing progression to renal failure. Definitive clinical trial results are lacking for this indication, but a multicenter study of losartan is ongoing (clinicaltrials.gov: NCT01479439).
Priapism is an unwanted, painful erection of the penis. It occurs in all SCD genotypes but is far more common in Hb SS. It can occur as young as 3 years of age, and 90% of males with Hb SS will have at least one episode by 20 years. At the onset of priapism, patients should urinate, drink water, and take a warm shower to promote detumescence. Immediate-release oral pseudoephedrine can be given at home to terminate priapism along with oral analgesics for pain. For prolonged priapism (≥4 hours), patients should seek urgent medical attention. Intravenous hydration and pseudoephedrine (or another vasoactive agent) should be administered if not taken recently by the patient. Aspiration and irrigation of the corpora cavernosa, which can be performed at the bedside, produces rapid detumescence and is the treatment of choice for prolonged priapism.24 Acute transfusion for priapism is likely ineffective, and exchange transfusion has been associated with stroke. Surgical shunts should be avoided, if possible, to preserve erectile function.
Hepatobiliary Complications
SCD predisposes to cholelithiasis with bilirubinate or pigment gallstones. The prevalence of gallstones is about 10% in children 2 to 4 years of age, increasing to more than 50% in adults. Cholelithiasis and cholecystitis should be considered in the differential diagnosis of abdominal pain in patients with SCD. Symptomatic cholelithiasis and cholecystitis are managed the same way in SCD as in other patients. The caveat is that the patient with SCD must be properly prepared and carefully managed to minimize surgical and anesthetic complications, such as ACS (see Box 2). The liver, itself, can be affected in a number of ways by SCD, but consistent terminology has not been established. Acute complications include acute hepatic sequestration, right upper quadrant syndrome, hepatocellular necrosis with hepatic failure, and intrahepatic cholestasis. Cholestasis, even extreme, may appear “benign” and self-limited, but it may be associated with hepatic dysfunction or multiorgan failure. Biliary obstruction should always be excluded.
Surgery and Anesthesia
Patients with SCD are prone to complications after general anesthesia, especially ACS and painful events. This has been attributed to alterations in pH, oxygenation, blood flow, blood volume, and temperature that may promote polymerization of deoxyhemoglobin, blood cell adhesion, and vaso-occlusion. Postoperative respiratory splinting and atelectasis also contribute. ACS may occur in 10% of patients after surgery. Risk factors include the type of surgery, such as thoracic or intra-abdominal operations, and a history of pulmonary disease, such as ACS or asthma. Principles of perioperative management, including adequate analgesia and aggressive pulmonary support, are shown in Box 2.
Two randomized trials showed that preoperative transfusion can prevent postoperative complications. The first trial showed that a single simple transfusion to a total Hb concentration of 10 g/dL was as effective as an aggressive regimen to decrease the Hb S to 30% or less (by exchange transfusion or multiple simple transfusions).25 However, there was not a randomized, no-transfusion study arm. The second, recent trial randomized patients with SCD undergoing low-risk or medium-risk surgery to transfusion or not.26 The trial was stopped early because of an excess of complications in the no-transfusion arm. Mostly Hb SS patients who had medium-risk surgery were included. A reasonable conclusion is that preoperative simple transfusion should be offered to patients with Hb SS having medium-risk surgery, and considered for other genotypes and low-risk surgery. Of note, exchange transfusion may be better for prolonged surgery, procedures in which regional blood flow is compromised, or when hypothermia is used. Patients with existing pulmonary or cardiac disease may also fare better following exchange transfusion. Patients with Hb SC or Hb Sβ+ may need exchange transfusion to prevent hyperviscosity because of their relatively high baseline Hb concentration. Lower-risk procedures, such as myringotomy, herniorrhaphy, and circumcision, may not require preoperative transfusion.27 Transfusion may also not be needed for imaging under anesthesia.
DISEASE-MODIFYING THERAPIES
Hydroxyurea
Hydroxyurea has multiple beneficial effects for patients with SCD, and it is still the only approved medication for prevention of complications in SCD (for adults). Hydroxyurea increases the production of Hb F, which inhibits the polymerization of Hb S, and this is believed to be the principal mechanism of action of the drug. Hydroxyurea also lowers the leukocyte and platelet counts and improves blood rheology, thereby decreasing the propensity for vaso-occlusion. Hydroxyurea reduces the frequency of painful events, ACS, and transfusions by about 50% in adults. Smaller, mostly nonrandomized studies in children have shown similar effects. There is now suggestive evidence that hydroxyurea therapy decreases mortality in adults28 and children.29 Side effects are mostly mild, and include dose-related, reversible leukopenia and thrombocytopenia. Hydroxyurea does not appear to increase the risk of malignancy or impair growth.
When first introduced into clinical practice, clinicians waited to prescribe hydroxyurea until a patient manifested some arbitrary degree of disease “severity.” Hydroxyurea is now being used increasingly for less rigidly defined indications. Indeed, some hematologists now advocate that a diagnosis of Hb SS or Hb Sβ0 itself is an indication for hydroxyurea therapy and recommend it be offered to children with Hb SS or Hb Sβ0 as young as 9 months of age, regardless of clinical severity, to reduce SCD-related complications (eg, pain, dactylitis, ACS, and anemia).
Hydroxyurea might provide primary prevention of SCD-related organ injury if the medication is started in very early life, but no high-quality clinical evidence yet supports this. The Pediatric Hydroxyurea Phase III Clinical Trial (BABY HUG) trial randomized young children (9–18 months) to a fixed dose of hydroxyurea or placebo for 2 years with the goal of preservation of splenic and renal function.30 The study failed on its co-primary end points; that is, no differences were observed between the hydroxyurea and placebo arms. Secondary analyses suggested that hydroxyurea did decrease the frequency of dactylitis, other painful events, ACS, and the need for transfusion. Another primary prevention trial is now under way, the Hydroxyurea to Prevent Brain Injury in Sickle Cell Disease (clinicaltrials.gov: NCT01389024) trial, which randomizes children with Hb SS or Hb Sβ0, 12 to 48 months of age, who have no evidence of overt or covert cerebrovascular disease to hydroxyurea or placebo. The primary outcome is a composite of abnormally increased cerebral arterial blood flow velocity (measured by TCD), SCI, or overt stroke. This critically important trial should be completed before hydroxyurea is routinely offered clinically for primary prevention of central nervous system injury.
There is also hope that hydroxyurea can be given instead of chronic transfusions for the prevention of stroke in high-risk patients. A randomized controlled trial (the SWiTCH study; clinicaltrials.gov: NCT00122980) of continued chronic transfusions versus hydroxyurea for long-term, secondary stroke prevention was stopped early due to futility, and there was an excess of recurrent strokes in the hydroxyurea arm compared with continued transfusions (7 vs 0).31 The advanced baseline cerebral vasculopathy in many SWiTCH patients may have reduced the effectiveness of hydroxyurea, but the conclusion of the study is that chronic transfusions and chelation remain the best therapy for secondary stroke prevention. A follow-up trial (the TWiTCH study, clinicaltrials.gov: NCT01425307) is currently under way to compare hydroxyurea and chronic transfusions for primary stroke prevention in children with abnormal TCD velocities (and no overt stroke or severe cerebral vasculopathy on MRA).
Hydroxyurea has been used to treat patients with other SCD genotypes, such as Hb SC, but the published experience is limited to case series that provide modest evidence for efficacy of hydroxyurea. There is a theoretical concern that hydroxyurea might increase the frequency of painful events in some patients with Hb SC, perhaps because of increased blood viscosity due to a higher Hb concentration (in patients without baseline severe anemia), but this could be offset by the decrease in RBC adhesion and improvement in blood rheology.
Chronic Transfusions
Chronic transfusions are a prophylactic, disease-modifying therapy that involve regular, usually monthly, transfusions of PRBCs to suppress substantially the percentage of Hb S in peripheral blood and minimize the degree of chronic anemia. The most common indications are primary and secondary prophylaxis of overt stroke, for which the duration of chronic transfusion therapy is indefinite. The role of chronic transfusions for the prevention progressive SCI is currently being studied in the Silent Infarct Transfusion Trial (clinicaltrials.gov: NCT00072761). Chronic transfusions are also offered for other recurrent and severe SCD-related complications, either for a short period (eg, 6 months to 1 year) or indefinitely.
The usual goal of chronic transfusions is to maintain the pretransfusion Hb S <30% with a nadir pretransfusion Hb concentration of 9 to 10 g/dL. After 3 to 5 years of chronic transfusions for prophylaxis of stroke without recurrent neurologic events, some physicians “liberalize” the transfusion regimen to maintain the Hb S lower than 50%. Complications of transfusions include iron overload (and the need for chelation therapy), alloimmunization, autoantibody formation, and transfusion-transmitted infections. Blood may be administered by simple transfusion, partial exchange transfusion, or automated erythrocytapheresis. Exchange techniques may offer the long-term advantage of delaying the accumulation of iron. Up to 30% of patients with SCD who are repeatedly transfused will become alloimmunized to RBC antigens (especially C, E, and Kell). Extended antigen matching can decrease the frequency of alloimmunization.
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HCST) is the only cure for SCD, and more than 500 transplants for SCD have been reported to international registries. In North America, the most common indication is stroke or cerebrovascular disease, but transplantation is also offered for other recurrent and severe SCD-related complications. Widespread use of HSCT is limited by the lack of suitable related donors and concerns about the toxicities of the procedure. HSCT is safest with an HLA-matched sibling donor (without SCD), but only 10% will have such a donor. Regimen-related mortality for myeloablative HSCT using an HLA-matched sibling donor is about 5%, with a concomitant 5% to 10% risk of graft rejection and 5% to 10% risk of chronic graft versus host disease. There may be additional late effects of transplantation (eg, infertility, cardiovascular, endocrine). Of course, all these risks need to be considered in the context of the lifelong risk of SCD itself. All patients and families with SCD should be aware of the potential of HSCT. It is also reasonable to perform tissue typing on all full siblings to know if there is a matched sibling donor. The procedure should be performed only in centers with experience in HSCT for SCD and, ideally, as part of a clinical research study. The use of alternative donors and reduced intensity conditioning is an area of ongoing study to expand access to HSCT, but these procedures should be performed only on a prospective research protocol.
DISEASE SEVERITY AND PROGNOSTICATION
Hb SS and Hb Sβ0 are more severe forms of SCD than Hb SC and Hb Sβ+ (see Table 1). However, even within individual genotypes, there is a broad range of disease severity. The concentration of Hb F is the main (but not the only) determinant of this variability. Hb F modulates disease severity by inhibiting the polymerization of Hb S in a “dose-dependent” manner. The coinheritance of α-thalassemia can modify the phenotype of Hb SS by decreasing hemolytic rate and risk of stroke. However, there is some evidence that α-thalassemia may increase the frequency of painful episodes and AVN. Thus, α-thalassemia can modify the phenotype of Hb SS in both favorable and unfavorable ways.
Except for the successful prediction and prevention of overt stroke using screening TCD programs, it remains difficult to identify young children with Hb SS who are at highest risk of adverse outcomes before irreversible organ damage occurs. A promising predictive model was developed based on the occurrence of dactylitis, severe anemia, and leukocytosis in very young children,32 but it was not validated in an independent cohort.33 Ongoing research using broad genetic approaches and sophisticated statistical modeling might benefit future children.
SURVIVAL AND TRANSITION TO ADULT MEDICAL CARE
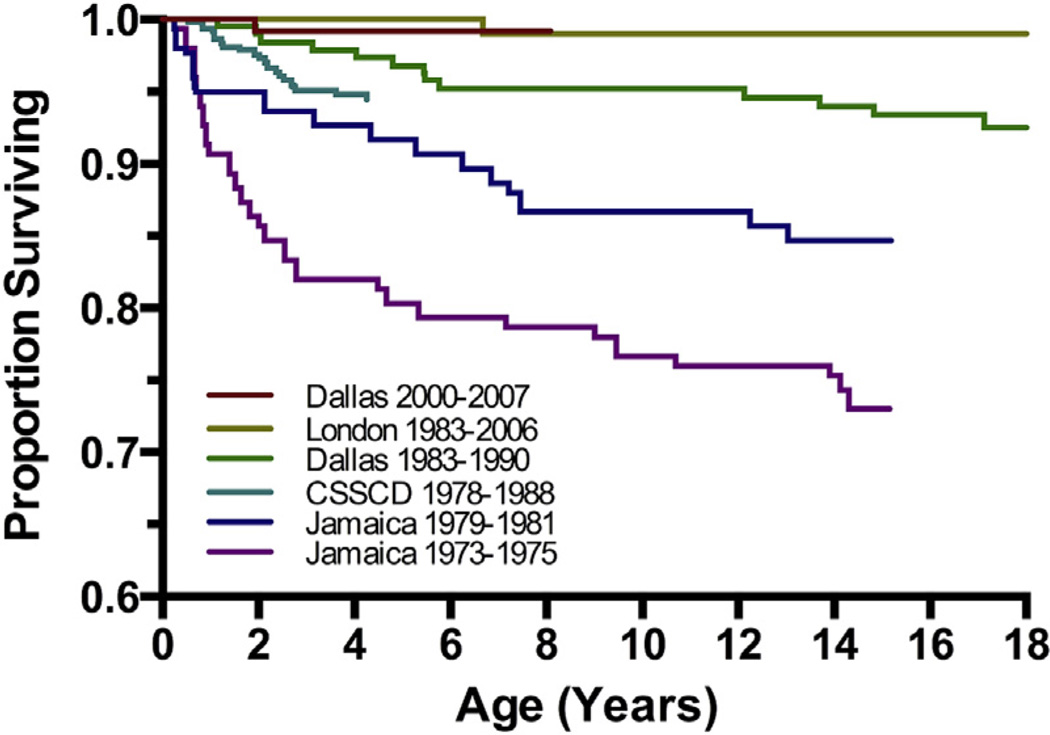
With current multidisciplinary care in an experienced SCD center, almost all children (>95%) born with SCD in developed nations now survive to adulthood.4,5 The remarkable improvement in survival over the past 4 decades (Fig. 4) is the additive result of a variety of interventions, including newborn screening, prophylactic penicillin, immunizations against Haemophilus influenzae type b and S pneumoniae, advances in supportive care, and the increased use of disease-modifying treatments (hydroxyurea, chronic transfusions, and stem cell transplantation).
Fig. 4.
Improvements in survival for children with Hb SS and Hb Sβ0. Overall survival curves spanning 4 decades are shown for large SCD cohorts in the United States, United Kingdom, and Jamaica. (Data from Quinn CT, Rogers ZR, McCavit TL, et al. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.)
The burden of mortality in SCD has now shifted to adults, and the transition to adult medical care is a high-risk period for death.4 There are a number of possible explanations for this vulnerability, including a flawed interface between pediatric and adult medical care in general and the gradual accumulation of SCD-related chronic organ injury during childhood that becomes manifest in young adulthood. Long-term survival estimates (beyond childhood) are less accurately known, but are estimated to be more than 50 years. Individuals with Hb SC and Hb Sβ+ have survival estimates that approximate the general population.
SUMMARY
SCD affects the entire body, beginning in very early infancy. A multidisciplinary team is required to manage children with SCD. Thanks to prognostic and therapeutic advancements, some forms of SCD-related morbidity in childhood, such as overt stroke, are decreasing. Primary prevention of organ injury is an important focus of current research. Fortunately, almost all children now born with SCD in developed nations survive to adulthood, but the transition to adult medical care is a high-risk period for death.
KEY POINTS.
SCD affects the entire body, beginning in very early infancy, and a multidisciplinary team is needed to care for children with SCD.
Some forms of SCD-related morbidity, such as overt stroke, are decreasing owing to prognostic and therapeutic advancements.
Primary prevention of organ injury is an important focus of current research.
Almost all children born with SCD in developed nations now survive to adulthood, but the transition to adult medical care is a high-risk period for death.
Acknowledgments
Funding Sources: Research funding from NHLBI, Eli Lilly and Co, MAST Therapeutics, Inc.
Footnotes
Conflict of Interest: Former advisory board member for Apotex Corporation.
REFERENCES
- 1.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 2.Falletta JM, Woods GM, Verter JI, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995;127(5):685–690. doi: 10.1016/s0022-3476(95)70154-0. [DOI] [PubMed] [Google Scholar]
- 3.Lane PA, O’Connell JL, Lear JL, et al. Functional asplenia in hemoglobin SC disease. Blood. 1995;85(8):2238–2244. [PubMed] [Google Scholar]
- 4.Quinn CT, Rogers ZR, McCavit TL, et al. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telfer P, Coen P, Chakravorty S, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica. 2007;92(7):905–912. doi: 10.3324/haematol.10937. [DOI] [PubMed] [Google Scholar]
- 6.McCavit TL, Quinn CT, Techasaensiri C, et al. Increase in invasive Streptococcus pneumoniae infections in children with sickle cell disease since pneumococcal conjugate vaccine licensure. J Pediatr. 2011;158(3):505–507. doi: 10.1016/j.jpeds.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCavit TL, Xuan L, Zhang S, et al. Hospitalization for invasive pneumococcal disease in a national sample of children with sickle cell disease before and after PCV7 licensure. Pediatr Blood Cancer. 2012;58(6):945–949. doi: 10.1002/pbc.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilimas JA, Flynn PM, Harris S, et al. A randomized study of outpatient treatment with ceftriaxone for selected febrile children with sickle cell disease. N Engl J Med. 1993;329(7):472–476. doi: 10.1056/NEJM199308123290705. [DOI] [PubMed] [Google Scholar]
- 9.Cauff BE, Quinn CT. Transient parvovirus-associated hypoplasia of multiple peripheral blood cell lines in children with chronic hemolytic anemia. Pediatr Blood Cancer. 2008;50(4):861–864. doi: 10.1002/pbc.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger E, Saunders N, Wang L, et al. Sickle cell disease in children: differentiating osteomyelitis from vaso-occlusive crisis. Arch Pediatr Adolesc Med. 2009;163(3):251–255. doi: 10.1001/archpediatrics.2008.545. [DOI] [PubMed] [Google Scholar]
- 11.Quinn CT, Buchanan GR. The acute chest syndrome of sickle cell disease. J Pediatr. 1999;135(4):416–422. doi: 10.1016/s0022-3476(99)70162-9. [DOI] [PubMed] [Google Scholar]
- 12.Bernini JC, Rogers ZR, Sandler ES, et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92(9):3082–3089. [PubMed] [Google Scholar]
- 13.Quinn CT, Stuart MJ, Kesler K, et al. Tapered oral dexamethasone for the acute chest syndrome of sickle cell disease. Br J Haematol. 2011;155(2):263–267. doi: 10.1111/j.1365-2141.2011.08827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematology Am Soc Hematol Educ Program. 2009;2009(1):45–53. doi: 10.1182/asheducation-2009.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 16.Lee MT, Small T, Khan MA, et al. Doppler-defined pulmonary hypertension and the risk of death in children with sickle cell disease followed for a mean of three years. Br J Haematol. 2009;146(4):437–441. doi: 10.1111/j.1365-2141.2009.07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 18.Adams RJ, Brambilla D Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353(26):2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 19.McCavit TL, Xuan L, Zhang S, et al. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(5):823–827. doi: 10.1002/pbc.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117(4):1130–1140. doi: 10.1182/blood-2010-06-293514. [DOI] [PubMed] [Google Scholar]
- 21.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn CT, McKinstry RC, Dowling MM, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol. 2013;70(1):58–65. doi: 10.1001/jamaneurol.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowling MM, Quinn CT, Plumb P, et al. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood. 2012;120:3891–3897. doi: 10.1182/blood-2012-01-406314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers ZR. Priapism in sickle cell disease. Hematol Oncol Clin North Am. 2005;19:917–928. doi: 10.1016/j.hoc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. N Engl J Med. 1995;333(4):206–213. doi: 10.1056/NEJM199507273330402. [DOI] [PubMed] [Google Scholar]
- 26.Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet. 2013;381(9870):930–938. doi: 10.1016/S0140-6736(12)61726-7. [DOI] [PubMed] [Google Scholar]
- 27.Fu T, Corrigan NJ, Quinn CT, et al. Minor elective surgical procedures using general anesthesia in children with sickle cell anemia without pre-operative blood transfusion. Pediatr Blood Cancer. 2005;45(1):43–47. doi: 10.1002/pbc.20283. [DOI] [PubMed] [Google Scholar]
- 28.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115(12):2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 29.Lopes de Castro Lobo C, Pinto JF, Nascimento EM, et al. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161(6):852–860. doi: 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 30.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware RE, Helms RW SWiTCH Investigators. Stroke with transfusions changing to hydroxyurea (SWiTCH) Blood. 2012;119(17):3925–3932. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 33.Quinn CT, Lee NJ, Shull EP, et al. Prediction of adverse outcomes in children with sickle cell anemia: a study of the Dallas Newborn Cohort. Blood. 2008;111(2):544–548. doi: 10.1182/blood-2007-07-100719. [DOI] [PMC free article] [PubMed] [Google Scholar]