Abstract
Background:
Following the high rate of consumption of Cola nitida (cola nut) among the male population in Nigeria, this study seeks to determine the effects of consumption of Cola nitida on serum reproductive hormones and sperm count, which are major determinants of male fertility.
Materials and Methods:
Thirty-two male albino wistar rats weighing 180-220 g were used for this study and were divided into 4 groups of eight animals each. Group 1 served as control, group 2 received 2 mg/kg Cola nitida extract (Test 1), group 3 received 6 mg/kg Cola nitida extract (Test 2) and group 4 received 10 mg/kg Cola nitida extract (Test 3). After 6 weeks of treatment, reproductive hormonal assay was carried out using the rat serum. Epididymal spermatozoa were collected and sperm count determined.
Results:
Serum concentrations of luteinizing hormone (LH) and testosterone were significantly (P < 0.05) reduced in test 2 and 3, compared with control. Sperm count was significantly lower in test group 1 (P < 0.05), 2 (P < 0.001) and 3 (P < 0.001) compared with control, with test 3 significantly (P < 0.05) lower compared with test 1. There was no significant difference in testicular and epididymis weight in the different experimental groups studied.
Conclusion:
Aqueous seed extract of Cola nitida rubra resulted in reduced serum reproductive hormone concentrations and sperm count in male wistar rats, and may therefore be detrimental to reproductive health, hence the need for regulation of its consumption.
Keywords: Cola nitida, luteinizing hormone, sperm count, testosterone
INTRODUCTION
Cola nitida is a large tree grown and cultivated in tropical countries. It is one of the over 120 species belonging to family Sterculiaceae. A number of sub-species within Cola nitida were described as alba, rubra, mixta and pallida, all of which are cultivated in Nigeria.1 In west Africa and Sudan, especially among the Muslim population, kola nuts are popular consumables.2 They are important in various religious and social customs and may also be used to counteract hunger and thirst. In Nigeria, for example, the rate of consumption of cola nut especially by students is very high, as it has been named the principal stimulants to keep awake and withstand fatigue.3 Cola nitida has been reported to possess the ability of delaying or eliminating fatigue, undoubtedly due to its high caffeine content.4 It has also been reported beneficial in easing migraine headaches due to its caffeine content.5 Human studies show that cola has positive chronotropic and weak diuretic effects. Autonomic changes include increased body temperature, increased blood pressure and increased respiratory rate.6 Extracts of Cola nitida have been reported to increase acid secretion.7
Alterations of serum concentrations of reproductive hormones are implicative of disordered spermatogenesis. Serum testosterone, luteinizing hormone (LH) and sperm count are undoubtedly major determinants of male fertility.
Considering the conflicting reports on the effects of cola nut on male reproductive health, and following the high rate of consumption of cola nut among the male population in Nigeria, it became necessary to ascertain its effect on serum reproductive hormones and sperm count, which are major determinants of male fertility.
MATERIALS AND METHODS
The nuts of Cola nitida were obtained from Enugu and authenticated in the botanical unit of the Department of Biological Sciences, Madona University, Nigeria. The nuts were chopped to pieces, and dried in Astell Hearson oven at a 45°C. The dried sample was ground to powder using an electric blender and stock solution prepared using appropriate methods. The median lethal dose of the plant extract was determined by the method of Lorke8 and found to be non-toxic at the highest tested dose of 60 mg/kg.
Thirty-two adult male albino wistar rats weighing 180-220 g were obtained from the animal house of the Department of Physiology, Madona University, Rivers State, Nigeria, and were kept in well ventilated cages and exposed to 12/12 h light/dark cycle. Animals were randomly divided into 4 groups, thus; control, test group 1, 2 and 3. The test groups were given aqueous extract of Cola nitida at a daily oral dose of 2, 6 and 10 mg/kg for test group 1, 2 and 3, respectively, for 6 weeks. All animals had access to food and drinking water ad libitum.
Extract administration commenced after 2 weeks of habituation. The extract was orally administered to test group 1, 2 and 3 at a dose of 2, 6 and 10 mg/kg, respectively. The extract was administered once daily, for 6 weeks. Administration was facilitated by the use of a syringe and orogastric tube. All experiments regarding the animals and their care were in line with ethical standards laid down in the 1964 Declaration of Helsinki.
The animals were anaesthetised using chloroform anaesthesia. Blood sample was then collected via cardiac puncture. The samples were introduced into plain capped bottles and allowed to stand for 2 h, after which they were centrifuged at 10,000 rpm for 10 min and the serum collected for reproductive hormonal assay.
The serum testosterone and LH concentrations were determined using the enzyme linked immunosorbent assay (ELISA).
The epididymis and testes were carefully removed, rinsed in normal saline solution and weighed using an electronic weighing balance.
Epididymal spermatozoa were collected and sperm count was done by method of Freud and Carol.9
Statistical analysis
Results are presented as mean ± standard error of mean. The one-way analysis of variance (ANOVA) was used to analyse differences between means, followed by the least significant difference procedure. P = 0.05 was considered significant. Computer software, SPSS version 17.0 and Excel analyser were used for the analysis.
RESULTS
The mean serum testosterone concentration was 0.59 ± 0.002, 0.58 ± 0.04, 0.55 ± 0.003 and 0.51 ± 0.01 μmol/L for control, test group 1, 2 and 3, respectively. The mean serum testosterone concentration was significantly (P < 0.05) reduced in test groups 2 and 3, compared with control [Figure 1].
Figure 1.
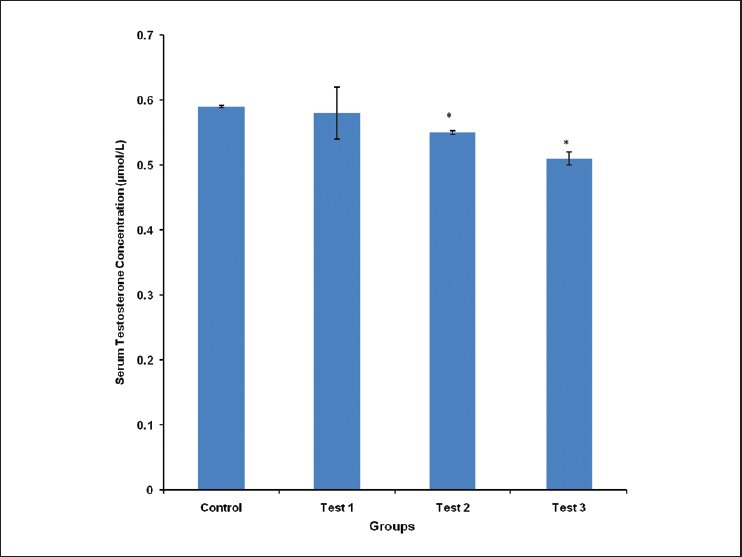
Comparison of serum testosterone concentration in the different experimental groups. Values are mean ± SEM, n = 8. *P < 0.05 vs control
The mean serum LH concentration was 0.78 ± 0.00, 0.77 ± 0.02, 0.75 ± 0.00 and 0.72 ± 0.00 μmol/L for control, test group 1, 2 and 3, respectively. Test groups 2 and 3 had their serum LH concentration significantly (P < 0.05) lower, compared with control [Figure 2].
Figure 2.
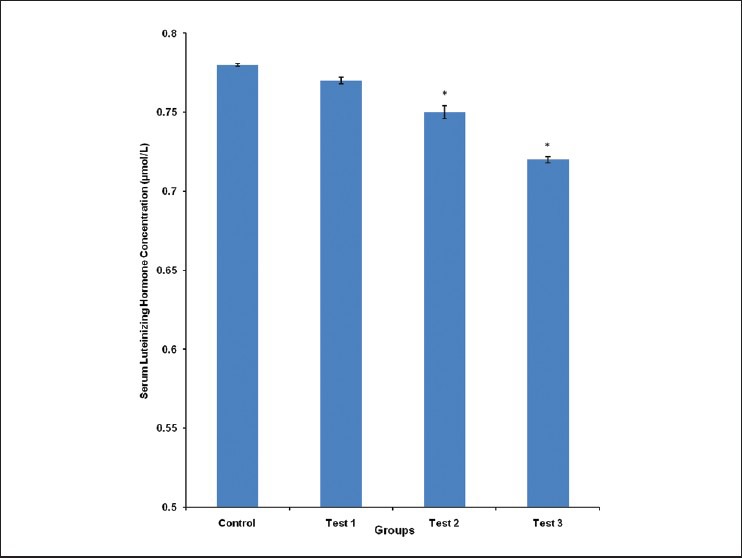
Comparison of serum luteinizing hormone concentration in the different experimental groups. Values are mean ± SEM, n = 8. *P < 0.05 vs control
Epididymal sperm count was 92 ± 3.05, 68 ± 6.11, 56 ± 2.30 and 52 ± 3.21 million/ml for control, test group 1, 2 and 3, respectively. Epididymal sperm count was significantly (P < 0.05) reduced in test group 1, compared with control. It was also significantly (P < 0.001) reduced in test groups 2 and 3, compared with control. Epididymal sperm count was significantly (P < 0.05) reduced in test group 3, compared with test group 1 [Figure 3].
Figure 3.
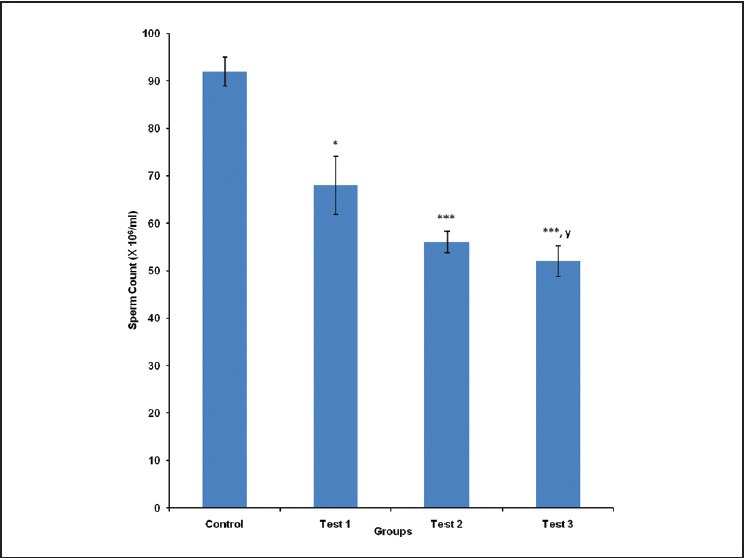
Comparison of sperm count in the different experimental groups. Values are mean ± SEM, n = 8. ***P < 0.001,*P < 0.05 vs control; y = P < 0.05 vs test 1
The epididymal weight for control, test group 1, 2 and 3 was 0.47 ± 0.02, 0.47 ± 0.02, 0.46 ± 0.02 and 0.45 ± 0.02 g, respectively. There was no statistically significant difference in the epididymal weight of animals in the different groups studied [Figure 4].
Figure 4.
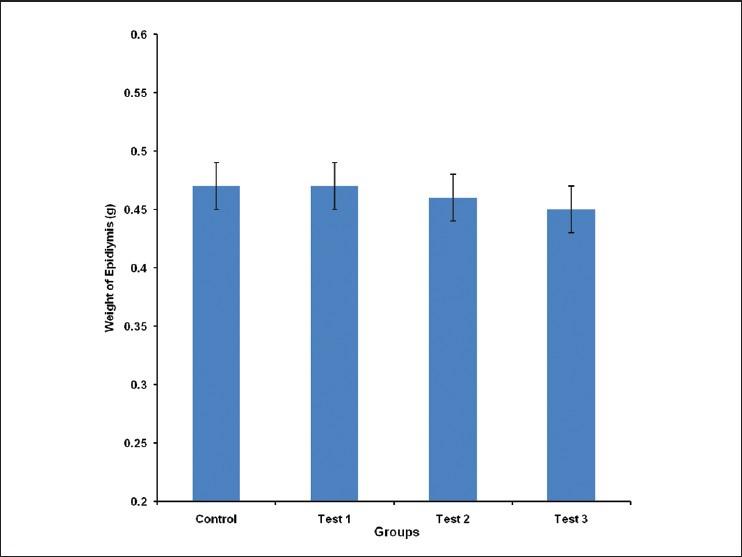
Comparison of Epididymis Weight in the different experimental groups. Values are mean ± SEM, n = 8
The testicular weight was 1.49 ± 0.02, 1.49 ± 0.02, 1.35 ± 0.12 and 1.21 ± 0.15 g for control, test group 1, 2 and 3, respectively. There was no statistically significant difference in testicular weight of animals in the different groups studied [Figure 5].
Figure 5.
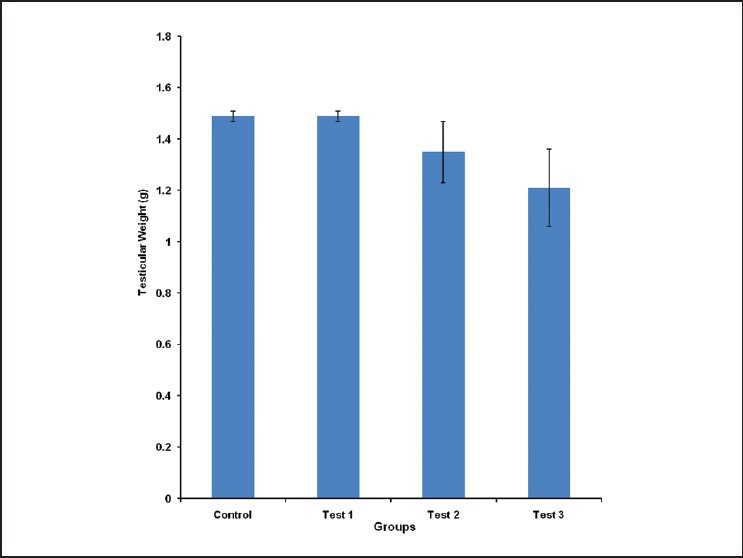
Comparison of Testicular Weight in the different experimental groups. Values are mean ± SEM, n = 8
DISCUSSION
Male fertility depends on but not limited to serum testosterone concentration, LH concentration, sperm count and sperm quality. Altered levels of male sex hormones are indicative of male reproductive dysfunction. In our study, the concentration of serum testosterone, which is produced primarily in the testicles, was significantly (P < 0.05) reduced in test groups 2 and 3, compared with control, [Figure 1]. The testosterone concentration was inversely proportional to the dose of the aqueous seed extract of Cola nitida, with test group 3 (10 mg/kg: Highest tested dose) having the lowest concentration. This is contrary to the work of Adisa et al., who reported an increase in serum testosterone concentration following Cola nitida administration.10
Serum concentration of LH produced in the anterior pituitary gland was significantly (P < 0.05) reduced in test groups 2 and 3, compared with control. The pattern of decrease was similar to that of testosterone concentration, with test group 3 (highest tested dose) having the lowest LH concentration [Figure 2]. This correlates well with other reports that Cola nitida extract reduces serum LH levels.10,11 Reduced levels of LH is implicated in reduced secretion of sex steroids and atrophy of interstitial cells.
Sperm count was significantly reduced in test groups 1, 2 and 3, compared with control. Sperm count was lowest in test group 3 (highest tested dose). This is possibly due to the observed decrease in serum levels of reproductive hormones. This is, however, contrary to some studies that reported that Cola nitida extract did not significantly alter sperm count.10,12
Although the weight of the epididymis was reduced in a dose-dependent pattern in the extract treated groups (test groups 1, 2 and 3), the decrease was not significantly different from control [Figure 3]. A similar non-significant dose-dependent decrease was observed in the testicular weight measurement [Figure 4]. These suggest that the reduced serum testosterone and LH concentrations may not necessarily be influenced by reduced epididymal and testicular mass alone.
Phytochemical screening of aqueous seed extract of Cola nitida revealed that it contains some active chemical constituents like caffeine, glucoside, theobromine and kolatin, which are stimulants.13,14 Others include methylxanthines, theopilline, d-catechin, lepicatechin, kolanin, glucose, starch, fatty matter, tannins, anthocyanin pigment, betaine and protein.7,15 Parkhurst et al.,16 reported that 50 ml oral dose of methylxanthines appeared to be detrimental to the sperm. Also, caffeine, a major methylxanthine constituent of Cola nitida seed extract inhibits androgen binding protein (ABP) resulting in reduced caudal epididymal sperm reserve, seminiferous tubular fluid volume, resulting in low sperm production and infertility.17 The decreased sperm count observed in this study may also be an implication of the reduced testosterone and LH concentration, which are major regulators of spermatogenesis.18
CONCLUSION
Aqueous extract of Cola nitida rubra seed resulted in reduced serum reproductive hormone concentrations in male wistar rats, and a dose-dependent reduction in sperm count and may therefore be detrimental to reproductive health, hence the need for regulation of its consumption.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lowor ST, Aculey PC, Assuah MK. Analysis of some quality indicators in cured Cola nitida (Vent) Agri Bio J North Am. 2010;1:1206–14. [Google Scholar]
- 2.Russel TA. The kola of nigeria and the cameroons. Tropical agriculture, Trinidad. 1995;32:210–40. [Google Scholar]
- 3.Purseglove JW. London: Dicotyledons Longmans Green & Co Ltd; 1968. Tropical crops. [Google Scholar]
- 4.Somorin O. Spectrophotometric determination of caffeine in Nigerian Kola Nuts. J Food Sci. 1973;38:911–2. [Google Scholar]
- 5.Irvine FR. Oxford: Oxford University Press; 1961. Woody plants of Ghana. [Google Scholar]
- 6.Fereday N, Gordon A, Oji G. Chatham: Natural Institute (NRI); 1997. Domestic market potential for tree products from farms and rural communities: Experience from Cameroon. NRI Socio-Economic Services Report No.13. [Google Scholar]
- 7.Tende JA, Ezekiel I, Dare SS, Okpanachi AO, Kemuma SO, Goji AD. Study of the effect of aqueous extract of kola nut (Cola nitida) on gastric acid secretion and ulcer in white wistar rats. Br J Pharmacol Toxicol. 2011;2:132–4. [Google Scholar]
- 8.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 9.Freud M, Carol B. Factors affecting haemocytometer counts of sperm concentration in human semen. J Reprod Fertil. 1964;8:149–55. doi: 10.1530/jrf.0.0080149. [DOI] [PubMed] [Google Scholar]
- 10.Adisa WA, Otamere HO, Osifo CU, Idonije OB, Nwoke EO. Effects of aqueous extract of Kola nut (Cola Nitida Rubra) on reproductive hormones in rats. Niger J Physiol Sci. 2010;25:121–3. [PubMed] [Google Scholar]
- 11.Benie T, Thieulant ML. Interaction of some traditional plant extracts with uterine oestrogen or progesting receptors. Phytother Res. 2003;17:756–60. doi: 10.1002/ptr.1208. [DOI] [PubMed] [Google Scholar]
- 12.Lopez A. Assessessment of motility, acrosomal integrity and viability of giant panda (Ailuropoda melanoleuca) sperm following short term storagpe at 4ºC. Zoo Biol. 2000;22:529–44. [Google Scholar]
- 13.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82:85–7. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infante-Rivard C, Fernandez A, Gauthier R, David M, Rivard GE. Fetal loss associated with caffeine intake before and during pregnancy. JAMA. 1993;270:2940–3. [PubMed] [Google Scholar]
- 15.Smith A. Effects of caffeine on human behaviour. Food Chem Toxicol. 2002;40:1243–55. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 16.Parkhurst AM, Korn N, Thurston RJ. The effects of methylxanthines on the mobility of stored turkey sperm. Poult Sci. 2000;79:1803–9. doi: 10.1093/ps/79.12.1803. [DOI] [PubMed] [Google Scholar]
- 17.Eteng MU, Eyong EU, Akpanyung EO, Agiang MA, Aremu CY. Recent advances in caffeine and theobromine toxicities: A review. Plant Foods Hum Nutr. 1997;51:231–43. doi: 10.1023/a:1007976831684. [DOI] [PubMed] [Google Scholar]
- 18.Seeley R, Stephens T, Tate P. 6th ed. Mc Graw: Hill Publishers; Anatomy and Physiology; pp. 1017–30. [Google Scholar]


