Abstract
Background:
A relation has been established between infection with high-risk types of human papilloma virus (HPV) and development of cervical cancer. To estimate the risk of HPV infection for cervical malignancies, we conducted a case-control study in northeast Iran.
Materials and Methods:
This study was carried out on 123 paraffin embedded blocks with exact diagnosis of squamous cell carcinoma (SCC). A total of 100 cervical tissue specimens with normal histopathology product of hysterectomy were also used as control. Both groups were tested for the presence of HPV DNA and HPV 16/18 subtypes using PCR assay.
Results:
Large non-keratinising subtype of cervical carcinoma was the most frequent one (62.6%), followed by keratinising and small cell subtypes (27% and 10%, respectively). Overall prevalence of HPV infection in SCC of cervix was 34.2% (42 out of 123 cases). HPV 16 was the most common type in this group (21 cases, 17.1%), followed by HPV 18 (16 cases, 13%) and other subtypes (5 cases, 4.1%). In this study, overall prevalence of HPV infection in control group was 12% (including 3% HPV 16; 5% HPV 18 and 4% other subtypes).
Conclusion:
Although association of HPV 16/18 and SCC of cervix was relatively higher than control group, compared with the previous study, the association between cervical SCC and HPV infection was significantly lower in our study; and possibly, the other risk factors play a major role in carcinogenesis of cervical carcinoma in this region.
Keywords: HPV 16/18, HPV, PCR, SCC of cervix
INTRODUCTION
Squamous cell carcinoma (SCC) of cervix is one of the most frequent malignancies in women, and causes considerable morbidity. Indeed, cervical malignancies are the second most common cancer in women worldwide and the fifth most prevalent malignancy among Iranian females.1,2,3 It is predicted that 607,402 new cases of cervical cancer and 320,832 attributable deaths will occur among women during 2015 worldwide.4 Based on World Health Organisation (WHO) annual report on 2010, Iran has a population of about 25 millions women aged above 15 years and who are at risk of developing cervical cancer. Current estimates indicate that every year 643 women are diagnosed with cervical cancer and 286 die from the disease in Iran. Unfortunately, there is no exact data about the human papilloma virus (HPV) burden in the general population of Iran. However, rough estimations about cervical HPV infection in general population, based on the incidence of infection in the countries of this region (Southern Asia), is about 7.9% at a given time.5
So far, multiple factors have been evaluated to define the effect and role of them in cervical carcinogenesis. Factors including smoking, use of oral contraceptives, high parity, certain human leukocyte antigen (HLA) subtypes, immunosuppression, multiple partners and sexually transmitted diseases have been introduced as predisposing or risk factor of cervical carcinoma.6 HPV infection is the most notorious among them; as some studies have demonstrated that the persistence of HPV DNA is a necessary factor for the development of cervical SCCs. The association between certain HPVs, so-called high risk HPVs, and cervical cancer is well documented. HPV 16 and HPV 18 are the most important in cervical pathology. HPV 16 is detected in about 60% of cervical cancer cases, and HPV 18 accounts for 10% of cases.7,8 It seems there is no geographic variation in the distribution of HPV types.9
Currently, the most sensitive and reliable method for detection of HPV infection is PCR assay. Most laboratories use consensus primers, directed to a conserved L1 gene, and hence able to detect all mucosal HPV types. These primers includes GP5-GP6 set and MY09-MY11 set, which detect a region of L1 gene.10,11
The objective of the present study was to evaluate the prevalence of HPV infection in cervical SCCs and to estimate the relative risk according to HPV 16/18 infection and cervical invasive SCC.
MATERIALS AND METHODS
This descriptive cross-sectional case-control study was performed on 123 formalin fixed paraffin embedded (FFPE) blocks of cervical tissue specimens with histopathologic diagnosis of cervical invasive SCC in molecular pathology laboratory, Ghaem Hospital, Mashhad, Iran. The FFPE blocks were retrieved from Mashhad educational hospitals (Qaem, Imam reza, and Omid's pathology department) archive from five recent years. Two histopathologists reviewed slides for confirming diagnosis and selecting the best paraffin blocks for DNA extraction and for assurance of consistency and quality of our materials. In parallel, 100 blocks of paraffin embedded cervical tissue specimens with normal histopathology and no tumoral tissue was used as the control group.
Cervical tissue specimens cut in 6 micro sections using separate disposable items such as gloves, blades and tubes to avoid any cross contamination between samples. To isolate DNA, at least 3 sections were deparaffinised by Xylene and dried tissue was incubated overnight at 56°C in a solution composed of 50 mM Tris–HCl (pH 7.5), 10 mM EDTA, 0.5% sodium dodecyl sulphate, 50 mM NaCl and 300 μg/mL of proteinase K. Proteinase K was inactivated at 95°C for 10 min. The samples were centrifuged for 3 min at 12,000 rpm and finally the supernatant was undergone extensive extraction by phenol/chloroform (1:1 ratio) to use for PCR. The reaction was performed in a 1.5 ml microtubes with re-suspended DNA, 20 mM Tris–HCl (pH 8.3), 8 mM MgCl2, 7.5 mM DTT, 200 μM of each dNTP, 20 pmol of each primer and 0.25 U of Taq DNA polymerase. DNA concentrations were measured by nanodrop.
First, the extracts were checked for DNA quality by amplification of the human β-Actin gene. In all samples, HPV detection was performed by GP5+/GP6+ primers [Table 1]; and eventually, positive cases for common HPV DNA assay were tested for identification of HPV 16 and HPV 18 subtypes using primers for HPV 16 and HPV 18 specific target sequences.11,12,13 General HPV positive cases with HPV 16/18 negative results were of low risk type, as confirmed by Genpack®.
Table 1.
Characteristics of primers used in this study

Forty cycles of amplification were done using Applied Biosystems thermocycler. Standard PCR protocol consisted of a denaturation step at 94°C for 30 s, followed by a primer annealing step at 50°C for 1 min, and a chain elongation step at 72°C for 30 s for each cycle. Eventually, the amplification product was analysed by 2% agarose gel electrophoresis.
RESULTS
A total of 123 patients with SCC of cervix were included in this study; mean age was 49.8 years (ranging from 25 to 80 years) with a peak in the 40-50 years age group (35.8%). The most frequent SCC subtype was large non-keratinising type (77 cases, 62.6%) followed by keratinising (33 cases, 27%) and small cell type (13 cases, 10.4%). HPV DNA was found in 42 out of 123 patients (34.1%) and 12 out of 100 cases (12%) in controls [Table 2].
Table 2.
Frequencies of HPV 16 and 18 in squamous cell carcinoma of cervix
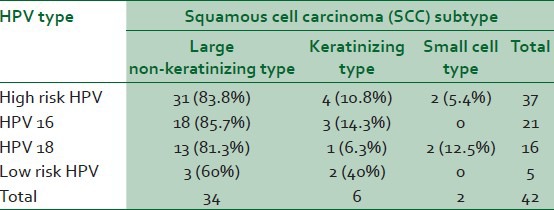
High risk HPV infection was significantly higher in SCC cases than controls (odd ratio = 4.9; 95% CI =2.18–11.22) including 21 HPV 16 positive in cases compared with 3 in controls (odd ratio = 6.65; 95% CI = 1.92–22.03) and 16 HPV 18 positive in cases compared with 5 in controls (odd ratio = 2.84; 95% CI = 1.00-8.05).
Low risk HPV DNA was found in 5 of 123 cases of SCC compared with 4 of 100 in controls. There was no significant difference between cases and controls in low risk HPV infection (odd ratio = 1.01; 95% CI = 0.26-2.89).
DISCUSSION
It has been shown that some viruses associate with the pathogenesis of various benign lesions and some malignant neoplasms such as cervical cancer.14,15,16,17 Currently, there are increasing evidences that confirm the multi-factorial nature of carcinogenesis through different molecular biologic pathways. Many studies overemphasise on the role of high risk HPV infection in the development and progression of cervical carcinoma, considering the existence of this entity without HPV infection impossible or exceptional.18,19,20
The overall frequency of HPV infection in our study group was 34.2% (30.1% considering high-risk type HPVs). This figure is much lower than what was reported in Mazandaran province, Iran, (78.6%),21 Brazil (76%),22 South Korea (90.3%)23 and other countries ranging over at least 65% of cases.11,24,25,26,27
This significant discrepancy may be, at least to some extent, due to formaldehyde fixation effect on PCR product result, especially for products less than 200 bp (including GP5/GP6), and eventually false negative results.11,22 Therefore, it is possible that if fresh specimens had been examined, HPV positive cases might have been higher. Moreover, recent studies have suggested the possibility of false negative results mainly in high grade squamous intraepithelial neoplasia and invasive SCC, and detection of HPV DNA by multiple methods or multiple primers may be conclusive and lead to a higher rate results.7,23
In contrast, multiparity and especially the age at first intercourse are common risk factors of cervical carcinoma and HPV infection simultaneously. Therefore, HPV positive cases with cervical SCC would be seen more frequent in regions with this high risk sexual habit. The other possible reason is the lower frequency of HPV infection in this region, which may cause overriding of the other risk factors effect over the HPV infection effect in carcinogenesis statistically.
CONCLUSION
Although association of HPV 16/18 and SCC of cervix was relatively higher than control group (odd ratio = 4.9; 95% CI = 2.18-11.22); compared with the previous study, the association between cervical SCC and HPV infection was significantly lower in this study; and other risk factors may play a major role in carcinogenesis of cervical carcinoma in this region.
ACKNOWLEDGEMENTS
This study was the result of a residency thesis and financially supported by the research Vice Chancellor of Mashhad University of Medical Sciences. The authors would like to thank them and also Mr. Mahmoud Bagheri for performing PCR.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hassumi-Fukasawa MK, Miranda-Camargo FA, Zanetti BR, Galano DF, Ribeiro-Silva A, Soares EG. Expression of BAG-1 and PARP-1 in precursor lesions and invasive cervical cancer associated with human papillomavirus (HPV) Pathol Oncol Res. 2012;18:929–37. doi: 10.1007/s12253-012-9523-y. [DOI] [PubMed] [Google Scholar]
- 2.Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–7. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 4.de Oliveira CM, Fregnani JH, Carvalho JP, Longatto-Filho A, Levi JE. Human papillomavirus genotypes distribution in 175 invasive cervical cancer cases from Brazil. BMC Cancer. 2013;13:357. doi: 10.1186/1471-2407-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summary Report Update. 3rd ed 2010. Human Papillomavirus and Related Cancers. WHO/ICO HPV information center. [Google Scholar]
- 6.Hedrick-Ellenson L, Pirog EC. The female genital tract. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Pennsylvania: Elsevier Saunders; 2010. p. 1019. [Google Scholar]
- 7.Salvia PN, Bergo SM, Bonesso-Sabadini PI, Tagliarini EB, Hackel C, De Angelo Andrade LA. Correlation between histological criteria and human papillomavirus presence based on PCR assay in cervical biopsies. Int J Gynecol Cancer. 2004;14:126–32. doi: 10.1111/j.1048-891x.2004.014030.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosai J. 10th ed. Vol. 2. Edinburgh, New York: Mosby; 2011. Rosai and Ackerman's Surgical Pathology; p. 1447. [Google Scholar]
- 9.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Tabrizi S. Methods for HPV detection: Polymerase chain reaction assays. In: Monsonego J, editor. Emerging Issues on HPV Infections: From Science to Practice. Basel: Karger; 2006. pp. 63–72. [Google Scholar]
- 11.Baay MF, Quint WG, Koudstaal J, Hollema H, Duk JM, Burger MP, et al. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J Clin Microbiol. 1996;34:745–7. doi: 10.1128/jcm.34.3.745-747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin NR, Bevan IS, Lewis FA, Wells M, Young LS. Demonstration of multiple HPV types in normal cervix and in cervical squamous cell carcinoma using the polymerase chain reaction on paraffin wax embedded material. J Clin Pathol. 1990;43:52–6. doi: 10.1136/jcp.43.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong D, Lu W, Ye F, Hu Y, Xie X. Gene silencing of HPV16 E6/E7 induced by promoter-targeting siRNA in SiHa cells. Br J Cancer. 2009;101:1798–804. doi: 10.1038/sj.bjc.6605344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezeshkpoor F, Jafarian AH, Ghazvini K, Yazdanpanah MJ, Sadeghian A, Esmaili H, et al. An association of human papillomaviruses low risk and high risk subtypes with skin tag. Iran J Basic Med Sci. 2012;15:840–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeghian MH, Ayatollahi H, Keramati MR, Memar B, Jamedar SA, Avval MM, et al. The association of Epstein-Barr virus infection with multiple myeloma. Indian J Pathol Microbiol. 2011;54:720–4. doi: 10.4103/0377-4929.91504. [DOI] [PubMed] [Google Scholar]
- 16.Keramati MR, Sadeghian MH, Ayatollahi H. Clinical and laboratory features in adult T-cell leukemia/lymphoma in Khorasan, Iran. Leuk Lymphoma. 2010;51:727–9. doi: 10.3109/10428191003611436. [DOI] [PubMed] [Google Scholar]
- 17.Nayereh KG, Khadem G. Preventive and therapeutic vaccines against human papillomaviruses associated cervical cancers. Iran J Basic Med Sci. 2012;15:585–601. [PMC free article] [PubMed] [Google Scholar]
- 18.Walboomers JM, Meijer CJ. Do HPV-negative cervical carcinomas exist? J Pathol. 1997;181:253–4. doi: 10.1002/(SICI)1096-9896(199703)181:3<253::AID-PATH755>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Isaakidis P, Pimple S, Varghese B, Khan S, Mansoor H, Ladomirska J, et al. HPV infection, cervical abnormalities, and cancer in HIV-infected women in Mumbai, India: 12-month follow-up. Int J Womens Health. 2013;5:487–94. doi: 10.2147/IJWH.S47710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conisation. Gynecol Oncol. 2008;108:549–54. doi: 10.1016/j.ygyno.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Hamkar R, Azad TM, Mahmoodi M, Seyedirashti S, Severini A, Nategh R. Prevalence of human papilloma virus in Mazandaran Province, Islamic Republic of Iran. East Mediterr Health J. 2002;8:805–11. [PubMed] [Google Scholar]
- 22.Rabelo-Santos SH, Zeferino L, Villa LL, Sobrinho JP, Amaral RG, Magalhães AV. Human papilloma virus prevalence among women with cervical intraepithelial neoplasia III and invasive cervical cancer from Goiania, Brazil. Mem Inst Oswaldo Cruz. 2003;198:181–4. doi: 10.1590/s0074-02762003000200003. [DOI] [PubMed] [Google Scholar]
- 23.Hwang TS, Jeong JK, Park M, Han HS, Choi HK, Park TS. Detection and typing of HPV genotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol Oncol. 2003;90:51–6. doi: 10.1016/s0090-8258(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 24.Torroella-Kouri M, Morsberger S, Carrillo A, Mohar A, Meneses A, Ibarra M, et al. HPV prevalence among Mexican women with neoplastic and normal cervixes. Gynecol Oncol. 1998;70:115–20. doi: 10.1006/gyno.1998.5055. [DOI] [PubMed] [Google Scholar]
- 25.Hindryckx P, Garcia A, Claeys P, Gonzalez C, Velasquez R, Bogers J, et al. Prevalence of high risk human papillomavirus types among Nicaraguan women with histological proved pre-neoplastic and neoplastic lesions of the cervix. Sex Transm Infect. 2006;82:334–6. doi: 10.1136/sti.2006.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger ER, Vernon SD, Lee DR, Miller DL, Reeves WC. Detection of human papillomavirus in archival tissues. Comparsion of in situ] hybridization and polymerase chain reaction. J Histochem Cytochem. 1998;46:535–40. doi: 10.1177/002215549804600414. [DOI] [PubMed] [Google Scholar]
- 27.Noronha V, Mello W, Villa L, Brito A, Macêdo R, Bisi F, et al. Human papillomavirus associated with uterine cervix lesions. Rev Soc Bras Med Trop. 1999;32:235–40. [PubMed] [Google Scholar]


