Abstract
We describe the production of collagen fibre bundles through a multi-strand, semi-continuous extrusion process. Cross-linking using an EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide), NHS (N-hydroxysuccinimide) combination was considered. Atomic Force Microscopy (AFM) and Raman spectroscopy focused on how cross-linking affected the collagen fibrillar structure.
In the cross-linked fibres, a clear fibrillar structure comparable to native collagen was observed which was not observed in the non-cross-linked fibre. The amide III doublet in the Raman spectra provided additional evidence of alignment in the cross-linked fibres. Raman spectroscopy also indicated no residual polyethylene glycol (from the fibre forming buffer) or water in any of the fibres.
In 1989 Kato and Silver first described the extrusion of an acid swollen collagen gel under aqueous conditions on a laboratory scale[1]. Since then there have been several studies of this process of self-assembly [2], with production parameters and cross-linking treatments [3-6] considered in order to optimise mechanical and biological characteristics. The acidic collagen suspension is typically extruded into a bath containing neutral fibre forming buffer, the collagen gels on contact with neutral pH as the fibrils, reconstitute and the macroscopic fibres form. Along the length of the bath the thread dehydrates due to the osmotic gradient between the collagen and the fibre forming buffer[7].
When collagen is generated in vivo, cross-linking occurs enzymatically and covalent inter and intramolecular bonds are formed that provide sufficient mechanical strength and proteolytic resistance [3]. These same cross-links are not formed during the self-assembly of collagen in neutral pH and as such cross-linking routes are a particular area of consideration in the production of artificial collagen fibre based structures. Cross-linking approaches include chemical (glutaraldehyde, isocyanates or carbodiimide based) [6], physical (dehydrothermal) [8, 9] and enzymatic [10]. The authors have previously settled upon the use of the zero length cross-linker carbodiimide 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) in combination with N-hydroxysuccinimide (NHS) as an effective route for the cross-linking of extruded collagen fibres [11-13]. This route results in a lower cross-linking density than other chemical and physical cross-linking routes but it has been shown to exhibit a favourable biological response [14] unlike more aggressive techniques that have been associated with inflammatory response and reduced bioactivity [15].
Whilst the mechanical properties [3, 4, 6, 16], degradative characteristics [14,17,18] and surface microstructure [3, 6] of extruded fibres have been considered quite extensively, any rigorous investigation into the fibrillar structure and the effect of cross-linking is more limited. Polarised light microscopy has been carried out to provide a qualitative indication of alignment [11, 19] and Transmission Electron Microscopy (TEM) [6] and Small Angle X-Ray Scattering (SAXS) [11, 19] have also been applied to a limited extent, however not with a view of investigating the effect of cross-linking on collagen structure. Atomic force microscopy (AFM) has been considered quite extensively for the characterisation of native collagen [20-24] but can also provide evidence as to the effect of cross-linking on the collagen order and alignment in extruded collagen fibres. Raman spectroscopy may also provide a useful route for investigating orientation and alignment within the collagen structure [25-27]. A recent study investigating bundles of collagen fibres from bovine achilles tendon demonstrated that even non-polarized Raman measurements (i.e. where a polarisation analyser is not present between the sample and detector) are sensitive to the laser polarisation direction [28]. The amide III doublet at 1245 and approximately 1270 cm−1 was shown to be highly sensitive to the orientation of the fibre [28].
The aim of the work was to study the effects of cross-linking of collagen fibres extruded through a semi-continuous process [13] using AFM and Raman Spectroscopy. These techniques allowed the alignment of the collagen fibres as well as the banding and chemistry to be analysed. This understanding of the basic structure is key to better understand fibre mechanics, degradation and ultimately the biological response to the material.
Collagen fibre ‘bundles’ were extruded using a method applied previously by the authors [13] and based upon the route of Silver et al [29]. The collagen source was an acid swollen gel type I collagen derived from bovine dermis (Devro Medical, Scotland). Briefly, the collagen was added to 2mM HCl in a concentration of 6mg/ml and left to swell overnight before blending and degassing. 6 channels of the collagen slurry were then extruded into a long trough containing a fibre forming buffer of 20% by weight polyethylene glycol (PEG) (molecular weight 8000 Sigma Aldrich UK) in 0.01M phosphate buffered saline (PBS) solution. A peristaltic pump provided a constant flow rate of the fibre forming buffer, the 6 strands were brought together at the end of the trough and wound onto a horizontally moving spool in the form of a 6 ply fibre. After winding onto the spools, the fibres were air-dried overnight before washing or cross-linking.
Where bundles were cross-linked, fibres still on the spools were soaked in a cross-linking solution of 25 mM EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) (Sigma-Aldrich UK) and 12.5 mM NHS (N-hydroxysuccinimide) (Sigma-Aldrich UK) in an 80/20 acetone/PBS solution for 2 hours. After cross-linking, fibres underwent a multi-stage washing process, with 2 half hour washes in 0.01M PBS solution followed by 2 half hour washes in deionised water. Bundles where cross-linking was not carried out underwent the multi-stage washing process in order to remove any residual PEG. All samples were then dried overnight before being removed from the spools and stored.
Scanning Electron Microscopy (SEM) was performed on sections of fibre secured onto a metal stub with carbon tape. Samples were sputter-coated with gold and viewed with a JEOL 820 SEM in the secondary electron mode.
For AFM imaging, fibres were sectioned and secured onto microscope slides with silver-dag. Viewing was carried out in tapping mode on a Digital Instruments multimode AFM (VEECO) with Nanoscope III control. ImageJ [30] was used in order to measure the periodic D-spacing.
Raman spectroscopy was applied to understand the effect of the cross-linking process on the chemical and structural characteristics of the collagen fibre bundles and to give qualitative indications of any residual PEG or water within the fibre structure. Raman Spectroscopy was carried out using a Renishaw Ramascope 1000 Raman spectrometer with a 633 nm He-Ne laser in combination with a Leica DM-LM microscope. An acquisition time of 10 seconds with 10 acquisitions was applied across a wavenumber range of 800-3050 cm−1 on fibre samples with the fibre axis both parallel and perpendicular to the direction of polarization of the incident laser. Particular regions of interest were between wavenumbers of 1150 and 1350cm−1 (for the amide III doublet [28]) and at the higher wavenumbers (2800-3050 cm−1) where the highest intensity PEG peaks are present [31]. Additionally literature suggests the δ and ν modes of the OH absorption within H20 to be present at 1640 and 3280cm−1 so these regions were considered to investigate any water presence.
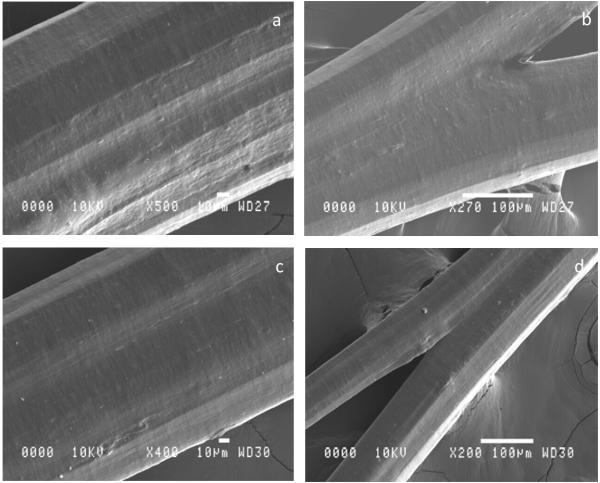
The SEM images of Figure 1, suggest that cross-linking had no effect on the surface structure of the collagen fibres, with all images showing a comparatively smooth surface, with minimal and regular surface artifacts at the microstructural level. Despite the 6 ply nature of these fibres, a dense homogeneous fibre resulted, with the individual ply clearly adhering well during the winding process. Some evidence of the separately extruded collagen channels is evident across both cross-linked and non-cross-linked fibres, in the contrast variations observed, probably a result of variations in the tension applied to the collagen during the winding process. The horizontal motion of the spool throughout the winding process was designed to minimize overlapping of the collagen fibre, however as clearly demonstrated in figure 1(b) and (d), fibres, whilst separated over the spool rods could bind together, away from them, resulting in the creation of a thicker fibre in places.
Figure 1. SEM imaging of extruded collagen fibres: (a,b) with no cross-linking, (c,d) with EDC-NHS cross-linking.
Figure 2 shows images of regions of collagen fibre taken in the tapping mode, without cross-linking (a,b) and with the EDC-NHS treatment (c,d). In the cross-linked material the characteristic banding pattern of collagen fibrils, such as that found in native collagenous tissue is clearly observed. Measurement of a d-spacing of 66.1± 1.29 nm was also consistent with native tissue. Individual collagen fibrils showed a relatively high degree of alignment within the structure. Slight tangling or clumping of collagen fibrils was evident and this is likely to explain surface defects observed during electron microscopy. Where fibres were not cross-linked, the degree of order was much less significant and only in very localized regions was the characteristic banding evident. It is possible that the non-cross-linked fibres are more swollen and hydrated (cross-linking in acetone-based solution could further dehydrate the fibres). Water content was investigated using Raman spectroscopy.
Figure 2. AFM image of: (a, b) fibres without cross-linking, (c, d) with the EDC-NHS treatment.
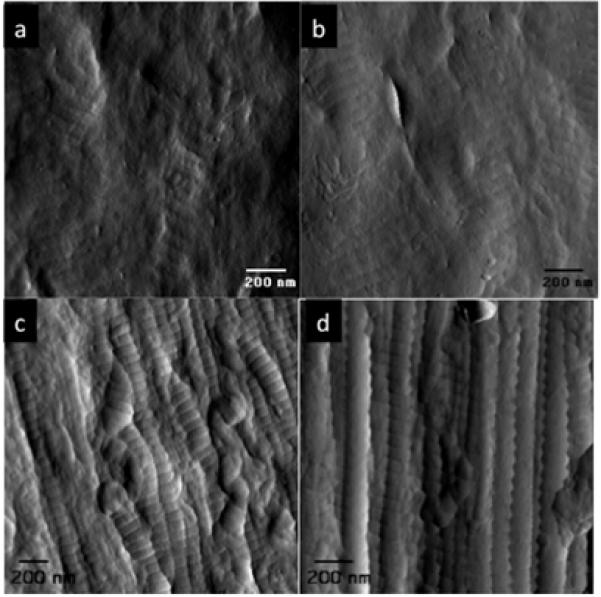
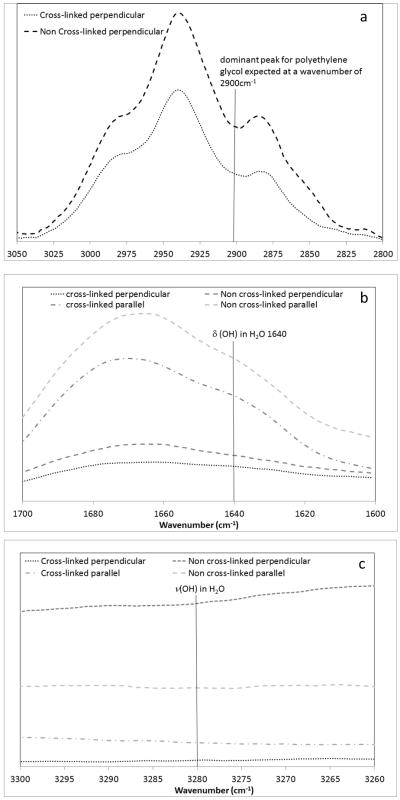
Figure 3 shows the Raman spectra for fibres oriented parallel and perpendicular to the direction of polarization of the incident laser between wavenumbers of 1150 and 1350 cm−1 and Figure 4 investigates the presence of PEG and water within the samples.
Figure 3. Raman spectra of the collagen fibres.
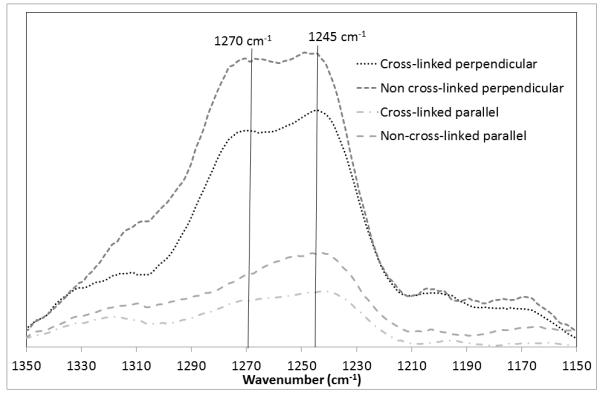
The amide doublet is clearly marked. The doublet appears more prominent where cross-linking has been applied and the peak at approximately 1270cm−1 absent where the fibre axis is parallel to the laser polaristion direction.
Figure 4. Investigation of residual PEG (a) and significant water content using Raman spectroscopy ((b) and (c)).
None of the fibres demonstrated the characteristic PEG peak at a wavenumber of around 2900 cm−1 [31]. There were also not significant peaks present attributable to the δ or ν modes of water absorption at 1640 or 3280 cm−1 [32].
Anisotropic Raman scattering has previously been observed in collagen bundles from bovine tendon [26,28] and human skin [26] with differences particularly observed in the amide doublet at around 1250 cm−1. Consistent with the work of Janko et al [26] only a single peak was observed at around 1245cm−1 with alignment parallel to the laser alignment (Figure 3) whilst a clear doublet was observed particularly in the case of the cross-linked fibres where alignment was perpendicular to the laser polarisation. The second peak of the doublet at 1270 cm−1 was less well defined in the case of the non-cross-linked fibre bundle, suggesting a lesser degree of alignment in this instance.
The dominant peak for polyethylene glycol occurs at a wavenumber of 2900 cm−1 [31] and as can be observed from Figure 4, no significant variation was observed between the spectra of cross-linked and non-cross-linked material in this region. This clearly indicates both that the cross-linking process did not offer additional advantage in terms of PEG removal and that the structure of the non-cross-linked collagen fibres was not a result of residual PEG presence. Whilst the presence of adsorbed water in the samples was not rigorously investigated, the absence of significant peaks at either 1640cm-1 or 3280cm-1 [32], suggests water content even in the non-cross-linked fibres was not significant and thus differences in structure as observed with AFM are unlikely to be attributable to water content.
This study builds on a body of literature that has focused on the extrusion of collagen fibres and the effect of cross-linking, not least the previous work by Kew et al which applied almost the same approach to fibre production [11]. Whilst previous papers have considered the effect of cross-linking agents on surface structure, mechanics and biological response [2, 3, 6, 11, 16, 33, 34], this paper additionally considers an investigation of fibrillar arrangement using AFM and Raman spectroscopy and for the first time clearly shows that cross-linking brings about alignment of the collagen and shows characteristic banding similar to native collagen with a d-spacing of 67 nm.
Although a multi-strand approach was taken to the extrusion process, the resultant fibres were dense and homogeneous contrary to the earlier work by Kew et al [11]. Surfaces were relatively smooth with the different strands identified only by a change in contrast both with and without cross-linking. Figure 5 taken from Kew’s paper shows a fibre section cross-linked with the same EDC/NHS chemistry and in this image the individual strands that make up the fibre are very clearly differentiated. Whilst the early fibres were produced by overlaying once on the spool, in this current study 6 individual strands were brought together before being placed onto the spool and wound. It appears likely that the tension during winding brings the strands together forming a better integrated structure.
Figure 5. SEM image of a section of Kew et al’s synthetic collagen fascicle cross-linked with EDC/NHS.
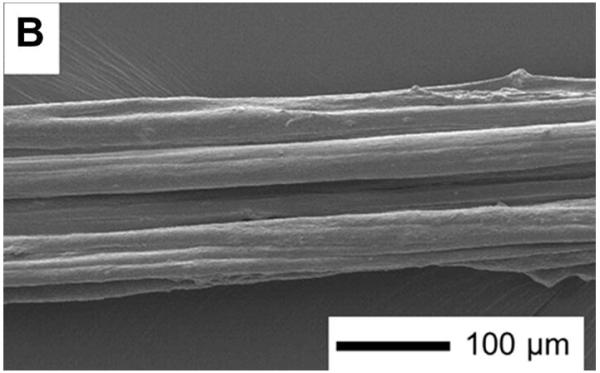
This single fibre length was produced by a multiple fibre overlayer process where the individual strands are clearly differentiated. Reproduced from [11]
Whilst a banding structure similar to that of native tendon has been previously observed in extruded collagen fibres, this has been largely through TEM (for example [6]). The AFM images of cross-linked fibres (Figure 4 (c) and (d)) provide a much clearer similarity to the native banding structure [24]. This banding is observed due to the alternating overlap and gap zones produced by the specific packing of the 300nm long and 1.5nm wide collagen molecules [35]. The ability of type I collagen to form striated fibrils is believed to involve specific charge–charge and hydrophobic interactions and it could be hypothesized this that explains the much more limited banding observed in the non-cross-linked material compared to the cross-linked. Cross-linking was carried out in an acetone/PBS mixture meaning any residual water within the fibres after extrusion would have been removed during this process. Any water presence could reduce interactions between adjacent fibrils. The whole premise of fibre extrusion is based upon the dehydration of the acid swollen collagen as it passes through the neutral fibre forming buffer and it is suggestive that Raman does not identify significant water presence in either cross-linked or non-cross-linked fibre bundles. Significant PEG presence in either of the fibre types is also ruled out by Raman spectroscopy. However, what is not known is the effect of the soaking in the acetone/PBS mixture independent of the cross-linking and in future efforts this should be considered.
There is a level of disagreement regarding the identification of peaks within the collagen Raman spectrum. For example the amide III doublet of interest at around 1250 cm−1 [28] has been assigned to: distinct amide III vibrations of different secondary structures (random coil at 1244 cm−1 and triple helix at 1265 cm−1) [36]; to two regions of the polypeptide strands having different polarities [37]; or to different positions of the proline residues in the structural motif typical of the collagen triple helix [38]. Bonifacio and Sergio suggest that because of the sensitivity to orientation, the 2 modes must arise from vibrations taking place along different directions within the same molecular structure. They found the intensity of the 1270 cm−1 band to be weaker when the fiber axis is oriented parallel to the laser polarisation, suggesting that this mode involves vibrations along a direction perpendicular to the fibril axis [28]. This finding is supported in the current work with the band at 1270 cm−1 being largely absent in the perpendicular direction. The intensity of this band also appears reduced in the case of the non-cross-linked material supporting the less aligned structure observed for these fibres with AFM.
While a significant body of literature has investigated the effect of EDC/NHS cross-linking on fibre mechanics, this is the first work to consider in any real detail the effect of this cross-linking on the fibrillar alignment. The more aligned structure in the case of the cross-linked fibre, as demonstrated by both Raman Spectroscopy and AFM is likely to support the superior mechanics [3, 4, 6], reduced swelling [3, 4, 14] and increased proteolytic resistance observed in previous studies of extruded collagen fibres [14,17,18].
Homogeneous and dense collagen fibre bundles have been successfully produced through a multi-strand, broadly continuous extrusion process. Cross-linking with an EDC/NHS solution resulted in a highly aligned fibrillar structure with banding similar to that observed in native collagen. The non-cross-linked fibres on the other hand exhibited a more swollen structure with only isolated regions of banding evident. The difference in order and alignment between the two fibre types was also evident in the amide III doublet in the Raman spectra. It is suggested that it is this difference in structure, in combination with the increased cross-linking concentration that explains frequently reported increases in mechanics and proteolytic resistance associated with cross-linking.
Acknowledgments
The authors would like to acknowledge the support of the Engineering and Physical Sciences Research Council (EPSRC), UK through a Knowledge Transfer Secondment (KTS) (to JHS), The National Institute for Health Research (NIHR) through their i4i grant to Tigenix Ltd and the TSB grant TP/8/BIO/6/I/Q0052.
References
- [1].Kato YP, Christiansen DL, Hahn RA, Shieh S-J, Goldstein JD, Silver FH. Mechanical Properties of collagen fibres: a comparison of reconstituted and rat tail tendon fibres. Biomaterials. 1989;10:38–42. doi: 10.1016/0142-9612(89)90007-0. [DOI] [PubMed] [Google Scholar]
- [2].Pins GD, Christiansen DL, Patel R, Silver H. Self-Assembly of Collagen Fibers. Influence of Fibrillar alignment and Decorin on Mechanical Properties. Biophysical Journal. 1997;73:2164–72. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zeugolis DI, Paul GR, Attenburrow G. Cross-linking of extruded collagen fibers--A biomimetic three-dimensional scaffold for tissue engineering applications. J Biomed Mater Res A. 2009;89:895–908. doi: 10.1002/jbm.a.32031. [DOI] [PubMed] [Google Scholar]
- [4].Pins GD, Silver FH. A self-assembled collagen scaffold suitable for use in soft and hard tissue replacement. Materials Science and Engineering: C. 1995;3:101–7. [Google Scholar]
- [5].Zeugolis DI, Paul RG, Attenburrow G. The influence of a natural cross-linking agent (Myrica rubra) on the properties of extruded collagen fibres for tissue engineering applications. Materials Science & Engineering C-Materials for Biological Applications. 2010;30:190–5. [Google Scholar]
- [6].Zeugolis DI, Paul RG, Attenburrow G. Post-self-assembly experimentation on extruded collagen fibres for tissue engineering applications. Acta Biomaterialia. 2008;4:1646–56. doi: 10.1016/j.actbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- [7].Seo Y-K, Youn H-H, Park C-S, Song K-Y, Park J-K. Reinforced bioartificial dermis constructed with collagen threads. Biotechnology and Bioprocess Engineering. 2008;13:745–51. [Google Scholar]
- [8].Haugh MG, Jaasma MJ, O’Brien FJ. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J Biomed Mater Res A. 2009;89:363–9. doi: 10.1002/jbm.a.31955. [DOI] [PubMed] [Google Scholar]
- [9].Ellis DL, Yannas IV. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials. 1996;17:291–9. doi: 10.1016/0142-9612(96)85567-0. [DOI] [PubMed] [Google Scholar]
- [10].Chau DYS, Collighan RJ, Verderio EAM, Addy VL, Griffin M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials. 2005;26:6518–29. doi: 10.1016/j.biomaterials.2005.04.017. [DOI] [PubMed] [Google Scholar]
- [11].Kew SJ, Gwynne JH, Enea D, Brookes R, Rushton N, Best SM, et al. Synthetic collagen fascicles for the regeneration of tendon tissue. Acta Biomaterialia. 2012;8:3723–31. doi: 10.1016/j.actbio.2012.06.018. [DOI] [PubMed] [Google Scholar]
- [12].Enea D, Gwynne J, Kew S, Arumugam M, Shepherd J, Brooks R, et al. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surgery, Sports Traumatology, Arthroscopy. 2013;21:1783–1793. doi: 10.1007/s00167-012-2102-7. [DOI] [PubMed] [Google Scholar]
- [13].Shepherd JH, Ghose S, Kew SJ, Moavenian A, Best SM, Cameron RE. Effect of fiber crosslinking on collagen-fiber reinforced collagen–chondroitin-6-sulfate materials for regenerating load-bearing soft tissues. J Biomed Mater Res A. 2013;101:176–84. doi: 10.1002/jbm.a.34317. [DOI] [PubMed] [Google Scholar]
- [14].Cornwell KG, Lei P, Andreadis ST, Pins GD. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell–matrix interactions. J Biomed Mater Res A. 2007;80:362–71. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- [15].Chvapil M, Speer D, Mora W, Eskelson E. Effect of tanning agent on tissue reaction to tissue implanted collagen sponge. Journal of Surgical Research. 1983;35:402–9. doi: 10.1016/0022-4804(83)90029-x. [DOI] [PubMed] [Google Scholar]
- [16].Wang MC, Pins GD, Silver FH. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials. 1994;15:507–12. doi: 10.1016/0142-9612(94)90016-7. [DOI] [PubMed] [Google Scholar]
- [17].Weadock KS, Miller EJ, Keuffel EL, Dunn MG. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. Journal of Biomedical Materials Research. 1996;32:221–6. doi: 10.1002/(SICI)1097-4636(199610)32:2<221::AID-JBM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [18].Caruso AB, Dunn MG. Functional evaluation of collagen fiber scaffolds for ACL reconstruction: Cyclic loading in proteolytic enzyme solutions. J Biomed Mater Res A. 2004;69:164–71. doi: 10.1002/jbm.a.20136. [DOI] [PubMed] [Google Scholar]
- [19].Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278–88. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- [20].Rele S, Song Y, Apkarian RP, Qu Z, Conticello VP, Chaikof EL. D-Periodic Collagen-Mimetic Microfibers. Journal of the American Chemical Society. 2007;129:14780–7. doi: 10.1021/ja0758990. [DOI] [PubMed] [Google Scholar]
- [21].Jastrzebska M, Barwinski B, Mroz I, Turek A, Zalewska-Rejdak J, Cwalina B. Atomic Force Microscopy Investigation of chemically stabilized pericardium tissue. Eur Phys J E Soft Matter. 2005;16:381–8. doi: 10.1140/epje/i2004-10093-1. [DOI] [PubMed] [Google Scholar]
- [22].Revenko I, Sommer F, Minh DT, Garrone R, Franc J-M. Atomic force microscopy study of the collagen fibre structure. Biology of the Cell. 1994;80:67–9. doi: 10.1016/0248-4900(94)90019-1. [DOI] [PubMed] [Google Scholar]
- [23].Rigozzi S, Stemmer A, Müller R, Snedeker JG. Mechanical response of individual collagen fibrils in loaded tendon as measured by atomic force microscopy. Journal of Structural Biology. 2011;176:9–15. doi: 10.1016/j.jsb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- [24].Baselt DR, Revel JP, Baldeschwieler JD. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophysical journal. 1993;65:2644–55. doi: 10.1016/S0006-3495(93)81329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Masic A, Bertinetti L, Schuetz R, Galvis L, Timofeeva N, Dunlop JWC, et al. Observations of Multiscale, Stress-Induced Changes of Collagen Orientation in Tendon by Polarized Raman Spectroscopy. Biomacromolecules. 2011;12:3989–96. doi: 10.1021/bm201008b. [DOI] [PubMed] [Google Scholar]
- [26].Janko M, Davydovskaya P, Bauer M, Zink A, Stark RW. Anisotropic Raman scattering in collagen bundles. Opt Lett. 2010;35:2765–7. doi: 10.1364/OL.35.002765. [DOI] [PubMed] [Google Scholar]
- [27].Wang YN, Galiotis C, Bader DL. Determination of molecular changes in soft tissues under strain using laser Raman microscopy. Journal of Biomechanics. 2000;33:483–6. doi: 10.1016/s0021-9290(99)00194-3. [DOI] [PubMed] [Google Scholar]
- [28].Bonifacio A, Sergo V. Effects of sample orientation in Raman microspectroscopy of collagen fibers and their impact on the interpretation of the amide III band. Vibrational Spectroscopy. 2010;53:314–7. [Google Scholar]
- [29].Silver FH, Kato YP. Synthetic collagen orthopaedic structures such as grafts, tendons and other structures. US: Office UP; 1992. [Google Scholar]
- [30].Rasband WS. Image J. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2011. [Google Scholar]
- [31].Docoslis A, Huszarik K, Papageorgiou G, Bikiaris D, Stergiou A, Georgarakis E. Characterization of the distribution, polymorphism, and stability of nimodipine in its solid dispersions in polyethylene glycol by micro-Raman spectroscopy and powder x-ray diffraction. The AAPS Journal. 2007;9:E361–E70. doi: 10.1208/aapsj0903043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chrit L, Hadjur C, Morel S, Sockalingum G, Lebourdon G, Leroy F, et al. In vivo chemical investigation of human skin using a confocal Raman fiber optic microprobe. J Biomed Opt. 2005;10:44007. doi: 10.1117/1.2003747. [DOI] [PubMed] [Google Scholar]
- [33].Wang M-C, Pins GD, Silver FH. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials. 1994;15:507–12. doi: 10.1016/0142-9612(94)90016-7. [DOI] [PubMed] [Google Scholar]
- [34].Cheng V, Screen H. The micro-structural strain response of tendon. Journal of Materials Science. 2007;42:8957–65. [Google Scholar]
- [35].Hulmes DJS, Miller A, Parry DAD, Piez KA, Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. Journal of Molecular Biology. 1973;79:137–48. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- [36].Wisniewski M, Sionkowska A, Kaczmarek H, Lazare S, Tokarev V, Belin C. Spectroscopic study of a KrF excimer laser treated surface of the thin collagen films. Journal of Photochemistry and Photobiology A: Chemistry. 2007;188:192–9. [Google Scholar]
- [37].Frushour BG, Koenig JL. Raman scattering of collagen, gelatin, and elastin. Biopolymers. 1975;14:379–91. doi: 10.1002/bip.1975.360140211. [DOI] [PubMed] [Google Scholar]
- [38].Merlino A, Sica F, Mazzarella L, Zagari A, Vergara A. Correlation between Raman and X-ray crystallography data of (Pro-Pro-Gly)10. Biophysical Chemistry. 2008;137:24–7. doi: 10.1016/j.bpc.2008.06.008. [DOI] [PubMed] [Google Scholar]