Abstract
Objective:
To assess and describe the call services delivered by drug and poison information call center (DPIC) of 13-Aban pharmacy, which is closely operated by the Department of Clinical Pharmacy, College of Pharmacy affiliated to Tehran University of Medical Sciences.
Methods:
All calls services including counseled and follow-up calls provided by 13-Aban DPIC to health care professionals and public were collected, documented, and evaluated in a 2 years period from July 2010 to June 2012 using the designed software. Data analysis was done by SPSS version 16.0.
Findings:
Totally 110,310 calls services delivered during a 2 years period. Among healthcare professionals, pharmacists, general physicians, and nurses requested more call services respectively (P = 0.001). DPIC could detect 585 potential cases of adverse drug reactions (ADRs) and 420 cases of major drug-drug interactions (DDIs).
Conclusion:
This study by analyzing and reporting the two-years activities of one of the major DPICs in Iran, showed that DPICs can offer drug consultation for healthcare professional and public as well as detect and prevent ADRs and DDIs, and therefore can promote patients’ health regarding drug therapy.
Keywords: Adverse drug reaction, clinical pharmacy, drug information call center, drug interaction, Iran
INTRODUCTION
After establishment of the first drug and poison information center (DPIC) at the University of Kentucky in 1962,[1] the number of DPICs has increased in the United States and other countries.[2,3,4] DPICs provide easy access, valid and evidence-based drug information for health care professionals and general population.[5,6,7,8] According to available data, these services can help to detect and prevent adverse drug reactions (ADRs), medication errors and promote rational use of drugs.[9,10,11] Therefore, these centers can positively improve the outcome of therapy.[11,12,13,14,15]
Drug and poison information call centers generally are located in hospitals, medical centers, or in pharmacy faculties.[6,7,16,17] In our country Iran, the first DPIC was established in the Ministry of Health and Medical Education under supervision of the Deputy of Food and Drug Organization (FDO). The second center was started its activities in Loghman-Hakim hospital affiliated to Shahid Beheshti University of Medical Sciences, Tehran. In March 2008, the third DPIC was established as “13-Aban DPIC,” one of the largest DPICs in Iran which is linked to 13-Aban pharmacy at Tehran. This center is the first pharmacist-operated DPIC closely operated and managing by the Department of Clinical Pharmacy, College of Pharmacy affiliated to Tehran University of Medial Sciences (the first ranked and the largest medical university of Iran) with 23 large institutional community and hospital pharmacies. Moreover, our country includes more than 30 DPICs, which are locally active in other provinces under the supervision of FDO. However, they are not active like the three of them in Tehran that was mentioned above.
The 13-Aban DPIC has a telephone lines system which enables answering 30 calls simultaneously. There is an especial line devoted to healthcare professionals. Currently, this center provides a 12-h service (8 am to 8 pm) in two morning and afternoon working shifts every day except for official holidays. In each shift, two board certified clinical pharmacists and four staffs including pharmacists, physicians, and/or pharmacy interns answer the calls. The board certified clinical pharmacists are working as supervisors and are responsible for answering healthcare professionals’ inquiries as well as consulting staffs in offering valid and updated responses. They also randomly listen to recorded calls to evaluate the accuracy and quality of staff answers. The performance of supervisors evaluates by a senior board certified pharmacists. In addition, educational and professional seminars and continuing medical education programs are holding by our center especially for pharmacists and general practitioners. We also have a monthly newsletter including general information regarding to pharmacotherapy updates, introduction of new drugs or new labeling and educational cases of DPIC, which was sent to pharmacists who working in community and hospital pharmacies of Tehran University of Medical Sciences and is accessible in our website (www.dpic13aban.com).
Finally, the main goal of this study was to assess health services of 13-Aban DPIC to public and healthcare professionals during a 2 years period. To date, this is the first official report from the DPIC affiliated to Tehran University of Medical Sciences, the largest medical university in Iran.
METHODS
This retrospective study was performed on all calls of 13-Aban DPIC during a 2 years period from July 2010 to June 2012.
The designed software (Microsoft Office Outlook, 2007) for data documentation included the caller's demographic data, caller identification (as patient or his/her relevant or friend), type of client (public or health-care professionals), phone number, time and date of dialogue, type of requested drug information (e.g. indication, interactions, side effects, contraindications, use in pregnancy and lactation), summary of question and answer, references used to find the answer, and device used to deliver the answer (e.g. telephone, fax, E-mail).
In additions, a structured data collection form was designed for registering educational or challenging cases to train pharmacy students and staffs. The items of this form include demographic characteristics of the caller, date and time of the call, duration of dialogue, consultants name, summary of past medical, habitual, family and drug history, present illness, finally formulated question regarding to patient history, answer, final recommendation, reference(s) used to answer the question, and determining whether the call need follow-up. The ethical considerations of all patients were reserved by the center.
All answered calls were registered and documented by staffs in the special software. During this quality control process, ADRs were reviewed by a clinical pharmacist specialist and reported to the national ADR center. Delivery of answers was mainly performed through telephone device. In some situations if necessary, complete responses were offered to clients (public or health-care professionals) through E-mail or fax. All drug information consults were performed according to a validated systematic approach,[18] the principals of evidence-based medicine, and an ethical protocol that has been designed by our center.
The data were analyzed using the SPSS, version 16 (SPSS Inc., Chicago, IL, USA). The results were showed as mean ± standard deviation. The Chi-squire test was used to compare frequencies. P < 0.05 was considered to be statistically significant.
RESULTS
Demographic features
Totally 110,310 calls included 46,734 and 63,576 calls were offered to health care professionals and public during the first and second study years respectively. This 16,842 increase in the number of calls during 1 year was statistically significant (P = 0.001). Regarding gender, majority of callers in both years were female (P = 0.001). Eighteen to 60 years was identified as the most common age group of the callers (P = 0.001). Demographic features of callers were demonstrated in Table 1.
Table 1.
Consumers’ demographic data, total calls and resource types of drug information
Composition of health-care professionals
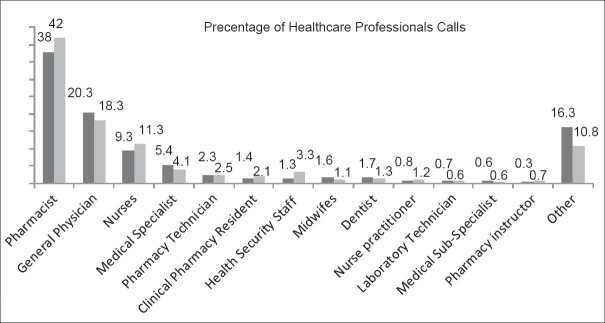
Among health-care professionals, clinical data were provided more frequently to pharmacists, general practitioners, and nurses respectively (P = 0.001). In this regard, totally 10,118 (9.2%) calls services were rendered to health care professionals included 4025 (39.8%) calls services for pharmacists, 1923 (19%) for general physicians, 1144 (11.3%) for nurses, 470 (4.6%) for specialists, 56 (0.5%) for sub-specialists and 2400 (24.8%) calls for other health care professionals.
Types of inquiries received by drug and poison information call center
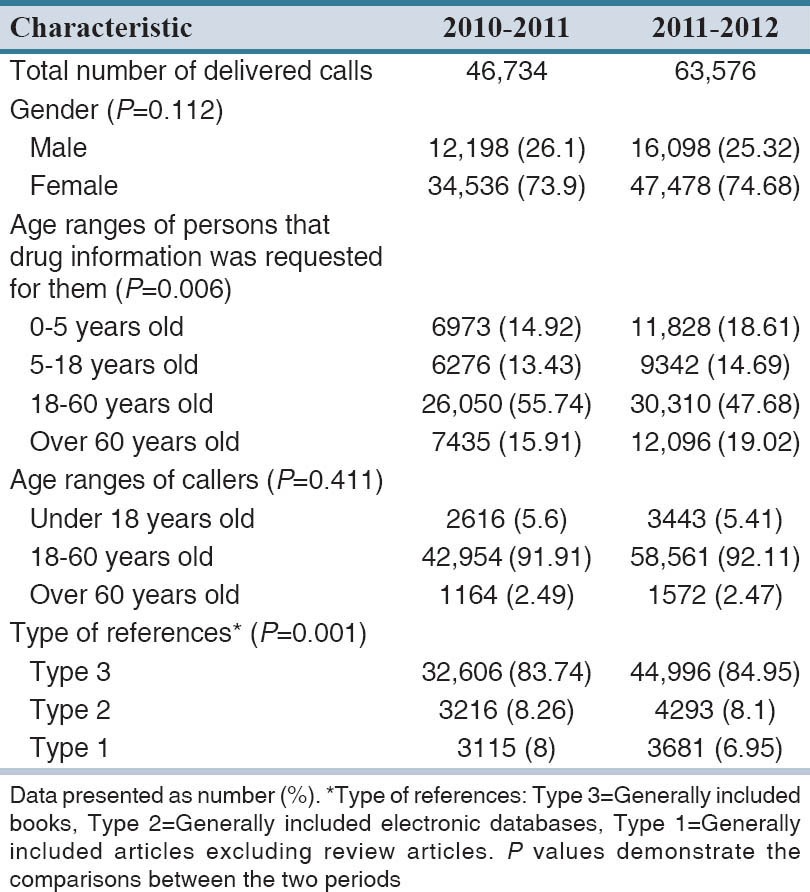
The most five drug information services offered to these groups included therapeutic uses of drugs (19.32%), drug identification (18.74%), drug availability (15.87%), side effects (15.05%), and drug administration (7.58%). The characteristics of delivered information in the 2 years period are shown in the Figure 1.
Figure 1.
Question Type of callers. Black columns 2010–2011; white columns 2011–2012
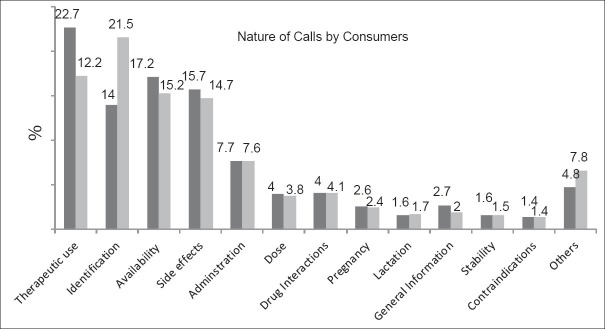
For more detail, drug identification is the most common information requested by pharmacists, and nurses (P = 0.001). Moreover, the most common types of questions (requested) by physicians, dentists, and clinical pharmacy specialists included information regarding contraindications of drugs, ADRs, and drugs interactions, respectively (P = 0.001). The percentage (proportion) of calls by each group of health-care professionals is shown in Figure 2.
Figure 2.
Proportion of healthcare professionals’ calls. Black columns 2010–2011; white columns 2011–2012
Types of references used to answer questions
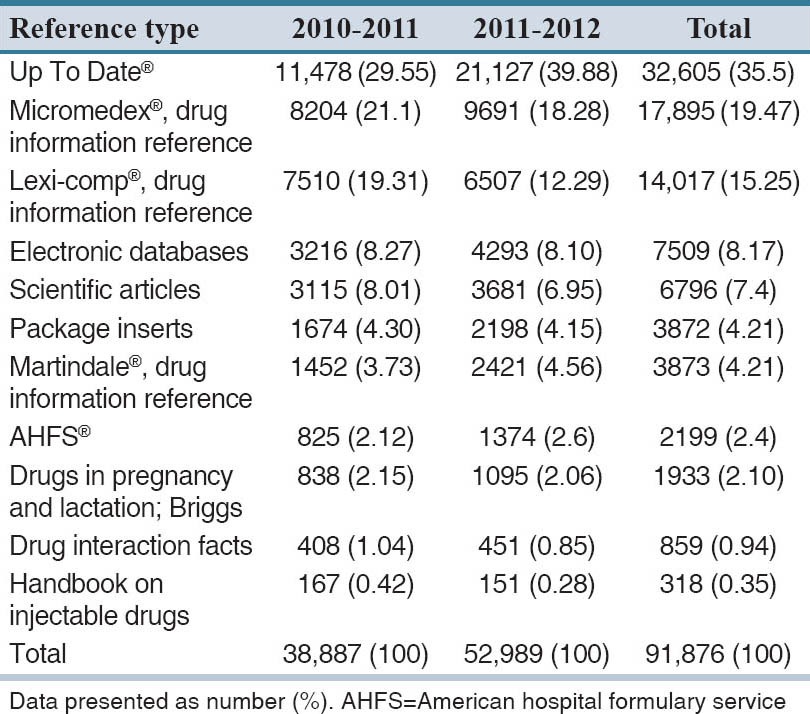
The references used to answer questions are presented in Table 2. The UptoDate® was the most commonly used reference (35.5%) (P = 0.001). Furthermore, our analysis revealed that tertiary references was the most common drug information sources used in both periods (P = 0.001).
Table 2.
References used to answer the questions
Detection of potential adverse drug reactions and drug interactions
We had 16,700 calls about ADRs. The majority of these calls were related to mild, common, and known ADRs, which were managed by the center. Of which, 585 cases those were uncommon and major ADRs were reported to ADR National Center. Furthermore, among 4525 calls regarding drug-drug interactions (DDIs) or drug-food interactions, approximately 432 cases of potential drug interactions were detected using Drug Interaction Facts® and Lexi® drug interaction checker. Of these, 12 cases were the serious and major DDI included clopidogrel-omeprazole (n = 3), pimozide-selective serotonin reuptake inhibitors (SSRIs) (n = 3), sildenafil-nitrate (n = 2), SSRIs-amphetamines (n = 2), monoamine oxidase inhibitors-SSRIs (simultaneously use) (n = 2). Other 420 detected DDIs were mild-moderate type of DDIs. Finally 208 and 232 individuals that needed more follow-up regarding their drug therapy were monitored in 2010–2011 and 2011–2012, respectively.
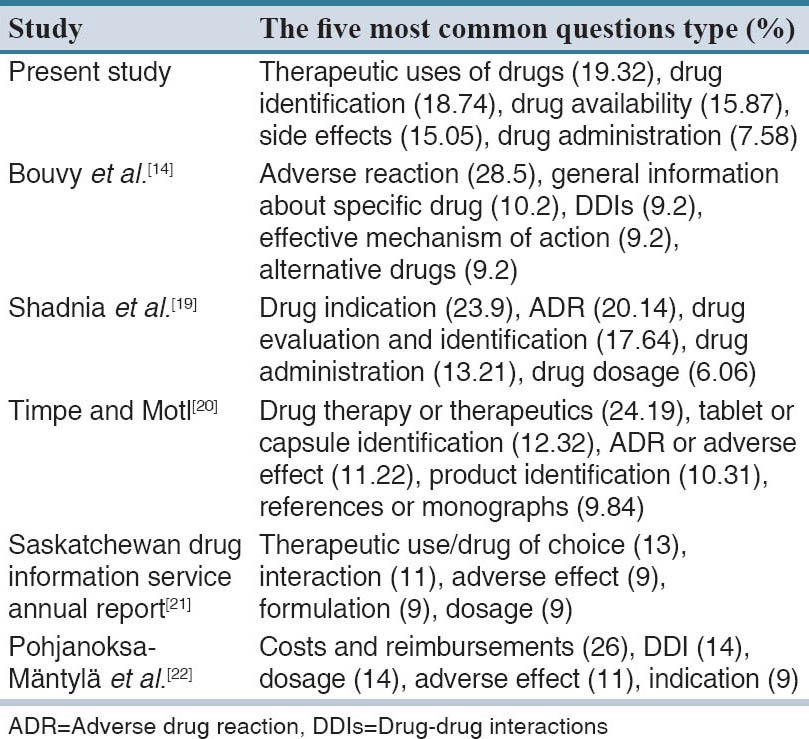
The five most common questions types requested from 13-Aban DPIC in comparison with other reports are shown in Table 3.
Table 3.
Comparison of the most five questions requested for answers from drug information centers reported in previous studies
The telephone was the most frequently used instrument (about 98%) to offer the answers, (P = 0.001). The remaining 2% of information was delivered by fax or E-mail.
DISCUSSION
In this study, we tried to evaluate the call services and clinical data providing of 13-Aban DPIC during 2 years, which is the first report of utilization of pharmacist-operated drug information call center affiliated to the largest medical university of Iran.
Our analyses demonstrated that drug information requests obtained from our center have increased significantly since the establishment of the center. Number of annual calls to our center are more than those from Loghman-Hakim hospital DPIC in Iran (2671, 2576 and 4447 calls in 2006, 2007 and 2008 respectively), University of Tennessee DPIC in the United States (3502 in 1995, 4463 in 1997, and 1549 in 2004), and Saskatchewan DPIC in Canada (2789 in 2003–2004 and 2426 in 2009–2010).[19,20,21] The five most common questions types requested from 13-Aban DPIC in comparison with other reports are shown in Table 3. This pattern is similar to the other reports[14,19,20,21,22] and it may be showed that therapeutic drug information is one of the main requirements of public in their health matter. In line with previous studies, clients requested the most inquiries were at the age of 18–60 years.[11,14,19,22] The majority of callers were female that is in agreement with the reports from other parts of the world.[11,14,19,22]
Moreover, among health care professionals contacted to our center, pharmacists, general physicians, and nurses were the most engaged groups. Importantly, this pattern significantly increased in the second year of study and is consistent with other reports.[20,21,23] These findings may also show the interest of health care providers from using of health services of drug information call center.
Providing evidence-based and reliable data using the valid references is one of the main activities of our center. The majority of references used by our center to deliver the answers were similar with the sources using by the U.S. drug information call centers.[9] Moreover, DPIC could help pharmacy students training evidence-base practice.[24]
In agreement with the previous reports, detecting and preventing of ADRs were the other main health services delivered by our DPIC.[9,10,11] In this line, Sarkar et al. also reported the detection of ADRs in ambulatory diabetes patients using a telephone-based self-management program.[25] These findings become more important when concerning to this point that it has been estimated that ADRs are the fourth to sixth leading cause of death in the United States with annually cost of $1.5–$4 billion to health care system.[26] Moreover, ADRs are responsible to about 2–5% of hospital admissions[27] and more than 100,000 deaths per year.[28,29,30]
Recognition and prevention of major DDIs was the other important findings of this study. DDIs cause to increase length of hospitalization and increase treatment cost.[31,32] It is also linked with increased hospital mortality.[33] In the last part, the result of this study revealed that DPIC can provide reliable, precise, and evidence-based drug information for health care professionals and public and can detect and prevent potential ADRs and drug interactions.
This study showed that 13-Aban DPIC affiliated to the Tehran University of Medical Sciences, as an area for offering the services of clinical pharmacy could provide relevant, evidence-based clinical data about drug therapy for healthcare professionals and public. Furthermore, our center can detect and prevent ADRs and DDIs, and therefore can promote patients’ health regarding drug therapy.
The present study has a number of limitations. First, due to the procedure of the center, the real clinical and economical impacts of our services on patients and healthcare system cannot be determined. Second, satisfaction level of callers with our center service was not assessed. In this regards, development (designing) a feedback system for callers that enables them to give their opinions about the performance of our center at the end of the call is warranted.
AUTHORS’ CONTRIBUTION
TEM designed the study, analyzed data and wrote the manuscript. MT, ME, IK, and KG supervised the study and wrote the manuscript. MRJ supervised and supported the study. MHH and KE designed the software and collected data.
ACKNOWLEDGMENTS
We would like to thank especially to all staffs, supervisors, and students collaborate us in data collection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Burkholder D. Some experiences in the establishment and operation of a drug information center. Am J Hosp Pharm. 1963;20:506–13. [Google Scholar]
- 2.Beaird SL, Coley RM, Crea KA. Current status of drug information centers. Am J Hosp Pharm. 1992;49:103–6. [PubMed] [Google Scholar]
- 3.Calder G, Davies JS, McNulty H, Smith JC. Drug information network in the United Kingdom National Health Service. Am J Hosp Pharm. 1981;38:663–6. [PubMed] [Google Scholar]
- 4.Maguire ME, D’Arcy PF. Drug information services in four capital cities in the United Kingdom. ‘A tale of four cities’ – London (North East Thames), Cardiff, Belfast, Edinburgh. J Clin Pharm Ther. 1988;13:207–12. doi: 10.1111/j.1365-2710.1988.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 5.The Council of Europe Expert Group on Safe Medication Practices, Creation of a better medication safety culture in Europe: Building up safe medication practices. Council of Europe. 2007. [Last accessed on 2007 May 08]. pp. 1–l275. Available from: http://www.coe.int/vt/e/social_cohesion/socsp/Medication%20Safety%20Report.pdf .
- 6.Markind JE, Stachnik JM. European drug information centers. J Hum Lact. 1996;12:239–42. doi: 10.1177/089033449601200324. [DOI] [PubMed] [Google Scholar]
- 7.Hall V, Gomez C, Fernandez-Llimos F. Situation of drug information centers and services in Costa Rica. Pharm Pract (Granada) 2006;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Narhi U. Drug information for consumers and patients review of the research. Publ Nat Agency Med. 2006;1:1–40. [Google Scholar]
- 9.Rosenberg JM, Koumis T, Nathan JP, Cicero LA, McGuire H. Current status of pharmacist-operated drug information centers in the United States. Am J Health Syst Pharm. 2004;61:2023–32. doi: 10.1093/ajhp/61.19.2023. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg JM, Schilit S, Nathan JP, Zerilli T, McGuire H. Update on the status of 89 drug information centers in the United States. Am J Health Syst Pharm. 2009;66:1718–22. doi: 10.2146/ajhp080563. [DOI] [PubMed] [Google Scholar]
- 11.Vassilev ZP, Chu AF, Ruck B, Adams EH, Marcus SM. Evaluation of adverse drug reactions reported to a poison control center between 2000 and 2007. Am J Health Syst Pharm. 2009;66:481–7. doi: 10.2146/ajhp080267. [DOI] [PubMed] [Google Scholar]
- 12.Angaran DM. Telemedicine and telepharmacy: Current status and future implications. Am J Health Syst Pharm. 1999;56:1405–26. doi: 10.1093/ajhp/56.14.1405. [DOI] [PubMed] [Google Scholar]
- 13.Melnyk PS, Shevchuk YM, Remillard AJ. Impact of the dial access drug information service on patient outcome. Ann Pharmacother. 2000;34:585–92. doi: 10.1345/aph.19173. [DOI] [PubMed] [Google Scholar]
- 14.Bouvy ML, van Berkel J, de Roos-Huisman CM, Meijboom RH. Patients’ drug-information needs: A brief view on questions asked by telephone and on the Internet. Pharm World Sci. 2002;24:43–5. doi: 10.1023/a:1015574315469. [DOI] [PubMed] [Google Scholar]
- 15.Hands D, Stephens M, Brown D. A systematic review of the clinical and economic impact of drug information services on patient outcome. Pharm World Sci. 2002;24:132–8. doi: 10.1023/a:1019573118419. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JM, Fuentes RJ, Starr CH, Kirschenbaum HL, McGuire H. Pharmacist-operated drug information centers in the United States. Am J Health Syst Pharm. 1995;52:991–6. doi: 10.1093/ajhp/52.9.991. [DOI] [PubMed] [Google Scholar]
- 17.Müllerová H, Vlcek J. European drug information centres – Survey of activities. Pharm World Sci. 1998;20:131–5. doi: 10.1023/a:1008672311674. [DOI] [PubMed] [Google Scholar]
- 18.Malone PM, Kier KL, Stanovich JE. 3th ed. New York: The McGraw-Hill Companies; 2007. Drug Information: A Guide for Pharmacists. [Google Scholar]
- 19.Shadnia S, Soltaninejad K, Sohrabi F, Rezvani M, Barari B, Abdollahi M. The performance of Loghman-Hakim drug and poison information center from 2006 to 2008. Iran J Pharm Res. 2011;10:647–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Timpe EM, Motl SE. Frequency and complexity of queries to an academic drug information center, 1995-2004. Am J Health Syst Pharm. 2005;62:2511–4. doi: 10.2146/ajhp050063. [DOI] [PubMed] [Google Scholar]
- 21.Saskatoon SK: College of Pharmacy and Nutrition, University of Saskatchewan 110 Science Place; 2010. [Last accessed date 2014 June]. Saskatchewan Drug Information Service Annual Report April 1, 2009 – March 31. S7N 5C9. Available from: http://medsask.usask.ca/documents/annual-reports/2009-2010_SDIS_Annual_Report.pdf . [Google Scholar]
- 22.Pohjanoksa-Mäntylä MK, Antila J, Eerikäinen S, Enäkoski M, Hannuksela O, Pietilä K, et al. Utilization of a community pharmacy-operated national drug information call center in Finland. Res Social Adm Pharm. 2008;4:144–52. doi: 10.1016/j.sapharm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Churi S, Abraham L, Ramesh M, Narahari MG. Evaluation of poison information services provided by a new poison information center. Indian J Pharmacol. 2013;45:496–501. doi: 10.4103/0253-7613.117781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Sousa IC, de Lima David JP, Noblat Lde A. A drug information center module to train pharmacy students in evidence-based practice. Am J Pharm Educ. 2013;77:80. doi: 10.5688/ajpe77480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar U, Handley MA, Gupta R, Tang A, Murphy E, Seligman HK, et al. Use of an interactive, telephone-based self-management support program to identify adverse events among ambulatory diabetes patients. J Gen Intern Med. 2008;23:459–65. doi: 10.1007/s11606-007-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 27.Kavitha D. Adverse drug reaction (ADR) monitoring and pharmacovigilance. J Pharm Res Health Care. 2010;2:127–34. [Google Scholar]
- 28.Ann. Guidance for Industry – Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. US Food and Drug Administration. 2005. [Last accessed on 2010 Mar 01]. Available from: http://www.fda.gov/downloads/Regulatory Information/Guidances/UCM126834.pdf .
- 29.Riedl MA, Casillas AM. Adverse drug reactions: Types and treatment options. Am Fam Physician. 2003;68:1781–90. [PubMed] [Google Scholar]
- 30.Oberg KC. Adverse drug reactions. Am J Pharm Educ. 1999;63:199–204. [Google Scholar]
- 31.Moura CS, Acurcio FA, Belo NO. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12:266–72. doi: 10.18433/j35c7z. [DOI] [PubMed] [Google Scholar]
- 32.Moura C, Prado N, Acurcio F. Potential drug-drug interactions associated with prolonged stays in the intensive care unit: A retrospective cohort study. Clin Drug Investig. 2011;31:309–16. doi: 10.1007/BF03256929. [DOI] [PubMed] [Google Scholar]
- 33.Buajordet I, Ebbesen J, Erikssen J, Brørs O, Hilberg T. Fatal adverse drug events: The paradox of drug treatment. J Intern Med. 2001;250:327–41. doi: 10.1046/j.1365-2796.2001.00892.x. [DOI] [PubMed] [Google Scholar]


