Abstract
Objective:
The aim of the current study was to determine various aspects of methylphenidate adverse reactions in children with attention deficit-hyperactivity disorder (ADHD) in Iran.
Methods:
During the 6 months period, all children under methylphenidate treatment alone or along with other agents attending a university-affiliated psychology clinic were screened regarding all subjective and objective adverse drug reactions (ADRs) of methylphenidate. Causality and seriousness of detected ADRs were assessed by relevant World Health Organization definitions. The Schumock and Thornton questionnaire was used to determine preventability of ADRs.
Findings:
Seventy-one patients including 25 girls and 46 boys with ADHD under methylphenidate treatment were enrolled within the study period. All (100%) ADHD children under methylphenidate treatment developed at least one ADR. Anorexia (74.3%), irritability (57.1%), and insomnia (47.2%) were the most frequent methylphenidate-related adverse reactions. Except for one, all other detected ADRs were determined to be mild. In addition, no ADR was considered to be preventable and serious.
Conclusion:
Our data suggested that although methylphenidate related adverse reactions were common in children with ADHD, but they were mainly mild and nonserious.
Keywords: Adverse drug reactions, attention deficit-hyperactivity disorder, Methylphenidate
INTRODUCTION
Attention deficit and impulsivity is one of the prevalent disorders in school-aged children with a prevalence rate of about 3–5% in this age group.[1,2,3] Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) defines attention deficit-hyperactivity disorder (ADHD) as symptoms of attention deficit, high activity and impulsivity in daily actions and speaking too much.[2,4] Children with ADHD are at greater risk for self-injury, motor vehicle accidents, impaired academic functioning, and antisocial personality disorder in the future.[5]
Long-term treatment plan for children with ADHD, especially at school ages includes pharmacotherapy combined with behavioral/psychological interventions.[6] Among different pharmacologic interventions, stimulants are generally considered as a first-line agent for school-aged children or adolescents with ADHD. Several studies have demonstrated that stimulants such as methylphenidate and dextroamphetamine can help ADHD symptoms like agitation, inattention, and excitation. Although the effect of discontinuation of medication is short and it may go away.[1]
Among different stimulants that have been used for treatment of children with ADHD so far, methylphenidate is a popular one.[7,8] In therapeutic doses, methylphenidate can cause anorexia, insomnia, headache, abdominal pain, nausea and irritability.[9,10] Insomnia and loss of appetite are dose-related, but its other adverse effects appear not to be dose-dependent.[11] Weight loss and developmental delay are adverse effects, which may occur in long-term methylphenidate consumption.[4,12] Data also exist about cardiovascular adverse effects of methylphenidate such as tachycardia and hypertension especially in chronic users.[13] Methylphenidate, even in therapeutic dose range, may also lower seizure threshold.[14] These adverse reactions of methylphenidate may compromise the treatment course of ADHD in children and also raise parents’ concerns over them.
The Iranian Pharmacovigilance Center (IPC), as a full member of World Health Organization (WHO) International Drug Monitoring Program, was established in 1998 with the main goal of increasing drug safety and preventing drug-related morbidity and mortality. Currently, more than 80 countries are involved in this program as full or associate members.[15] Shalviri et al. have described the 10 years activities of IPC.[15] According to this report, a total number of 17,967 adverse drug events has been collected and evaluated by the IPC. Ceftriaxone, tramadol, and streptokinase are the three most common medicinal products suspected to cause adverse drug reactions (ADRs). Based on the WHO standards, countries with the best reporting rates generate over 200 reports per 1,000,000 inhabitants per year. Considering the population of Iran (over 60 million), it is expected to receive at least 12,000 reports per year.[16] However, this rate was only 2330 in 2006.[17] Hanafi et al. demonstrated that about 68% of the nurses in teaching university hospital in Tehran did not even know the correct definition of the term pharmacovigilance and only 2.2% of them had sent the reports to IPC.[16] These findings highlight the importance and prevalence of ADR under-reporting in our country. To obviate this problem, the IPC has been conducted several strategies such as developing spontaneous reporting system and training over 30,000 health care professionals through drug safety workshops. Other achievements of the IPC are as follows: Issuing 86 drug safety alerts to health care professionals, recalling 23 pharmaceutical products and changing the labeling of 30 others, suspending the distribution of 8 medicines, and withdrawing 4 different products from the national drug list.[15]
Regarding the fact that methylphenidate side effects can adversely affect adherence to treatment, clinical outcome and quality of life of its recipients, determining different features of methylphenidate side-effects are crucial for clinicians to develop optimal preventive as well as management strategies. Therefore, we conducted the current study to assess various aspects of methylphenidate adverse reactions, including incidence, causality relationship, preventability, and severity in children with ADHD in Iran.
METHODS
During the 6 months period from mid-September 2013 to mid-March 2014, a cross-sectional study was conducted on children and adolescents with ADHD under methylphenidate therapy attending the office of Children Medical Center psychology clinic affiliated to the Tehran University of Medical Sciences, Tehran, Iran. The diagnosis of ADHD was performed by an expert pediatric psychologist based on DSM-IV diagnostic criteria. The Medical Ethics Committee of the Tehran University of Medical Sciences approved the study, and informed consent was obtained from all patients. No specific inclusion-exclusion criteria regarding duration of methylphenidate treatment course, methylphenidate dose, and probable co-administered medications for management of ADHD were used for patient selection. The regimen of the study population included methylphenidate, alone or with an antipsychotic agent (including risperidone) or other relevant medications (e.g, clonidine).
All ADRs that occurred after starting methylphenidate therapy were recorded by a well-educated and qualified pharmacist. Detection of ADRs was performed by face-to-face interview with patients or his/her parents at regular follow-up office visits through a checklist of methylphenidate adverse reactions in relevant scientific literature and reviewing their brief office charts. Required data including patients’ age, sex, weight and height at the beginning of methylphenidate therapy, and at the present, comorbidities, ADHD treatment, drug regimen and co-administered medications (name, dosage, frequency, indication, and route of administration) and detected ADRs (clinical manifestation and the causative drug[s]) were registered in a predesigned form. ADRs reported by the patient daily (on a daily basis) and 2–3 times a week within the recent 1–2 weeks were classified as “always” and “sometimes,” respectively.
The WHO definition of ADR was used in the present study.[18] The WHO probability scale was used to assess the causality relation between reported ADRs and methylphenidate administration(s).[19] The seriousness of ADRs was determined by WHO definition. Based on this definition, any ADR resulting in death, life-threatening situation, persistent or significant disability/incapacity, hospital admission, or prolonged hospital stay was classified as serious.[20] The severity of ADRs was determined by Hartwig et al. definition.[21] The Schumock and Thornton questionnaire was used to evaluate the preventability of reported ADRs.[22]
Categorical variables were expressed as a percentage. Continuous variables were reported as mean ± standard deviation (SD). Frequency of methylphenidate-related adverse reactions was expressed as the number of patients developed a certain ADR. Descriptive analyses were performed using the SPSS version 15 software (SPSS, Inc. Chicago, IL, USA).
RESULTS
Demographic characteristics of the cohort are shown in Table 1. Within the study period, 71 patients including 25 girls and 46 boys with ADHD under methylphenidate treatment were enrolled. Table 2 lists the comorbidities of the cohort. Forty-four (61.9%) of patients had no underling diseases. Among comorbidities, neonatal jaundice (25.4%) and seizure (4.2%) were most common. Treatment regimens of ADHD are listed in Table 3. More than half of (56.3%) the study population received methylphenidate alone. About 63.1% of patients received methylphenidate for at least 6 months and in only 2.8%, the treatment duration was <1 month. The mean ± SD (range) of methylphenidate dose given to the study population was 20.5 ± 9.6 (5–40) mg.
Table 1.
Demographic properties of the study population
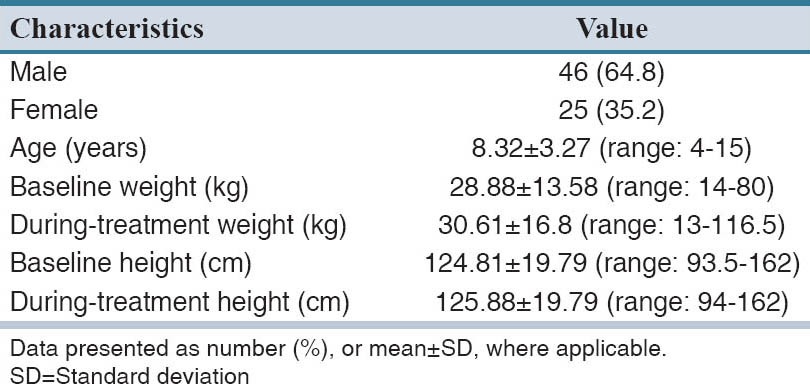
Table 2.
Comorbidities of the study population
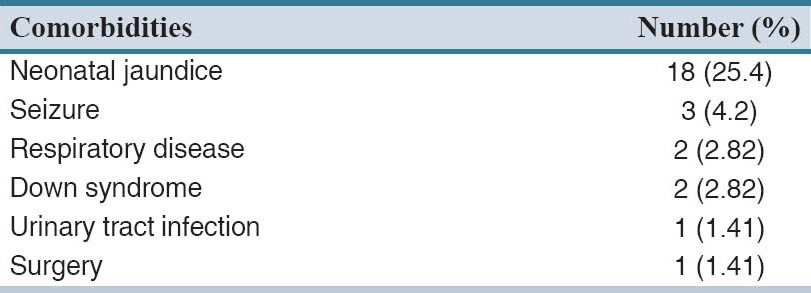
Table 3.
ADHD treatment regimen of the study population

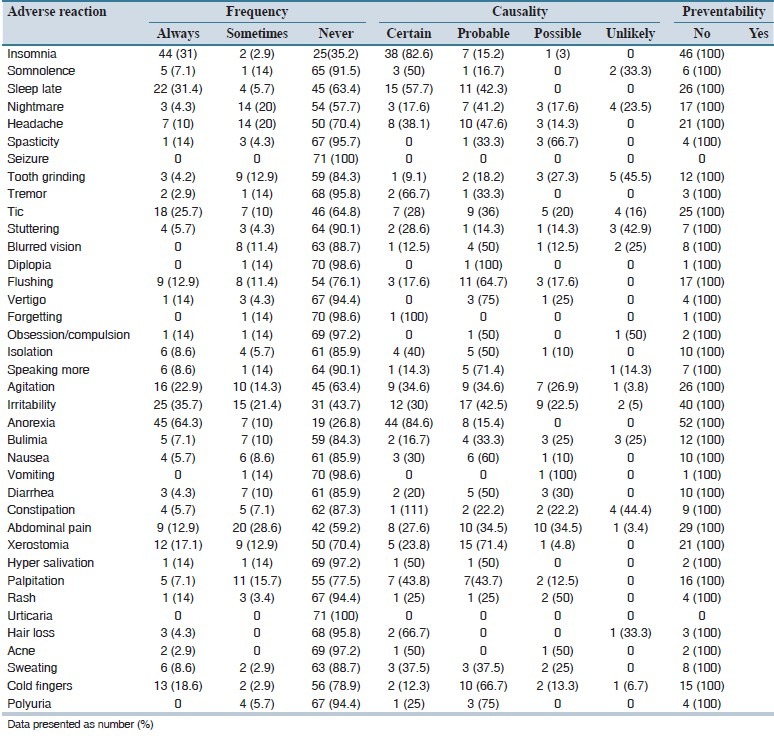
A total of 38 different types of methylphenidate adverse reactions were detected in the study population [Table 4]. All patients experienced at least 1 ADR. The three most frequent methylphenidate-related adverse reactions were anorexia (74.3%), irritability (57.1%), and insomnia (47.2%). Table 4 also provides causality and preventability data of detected ADRs. Most ADRs were recognized to be possible, and none was preventable. All but one methylphenidate related adverse reactions were classified as mild level 1 or 2. Based on the WHO definition, all detected ADRs were nonserious.
Table 4.
Frequency, causality assessment and preventability data of detected adverse reactions of methylphenidate
DISCUSSION
Decreased appetite was the most frequent detected methylphenidate adverse reaction in the current study. In this regards, near three-fourth (74.3%) of our cohort or their parents reported decreased appetite during methylphenidate treatment. In line with this, a meta-analysis of placebo-controlled randomized trials of short-acting methylphenidate in children implicated the lowest number-needed-to-harm for decreased appetite (4) followed by insomnia (7).[23] Probable variations in its frequency from different studies may be related to administered dose. Methylphenidate-induced decreased appetite is primarily mild, dose-dependent, and may resolve with time. It also could be managed by exploiting simple strategies such as taking the medication at or after a meal, encouraging foods with high caloric density, and offering evening/bedtime snack.[6,24]
Previous studies and reviews demonstrated sleep disturbances as a common adverse reaction of stimulants such as methylphenidate.[25,26] Based on polysomnography, various sleep abnormalities including delayed onset, shorter duration, and delayed rapid eye movement may be associated with methylphenidate. According to parental reports, sleep problems are about 3 times more frequent in children with ADHD receiving methylphenidate than untreated individuals.[27] On the other hand, sleep abnormalities may be an intrinsic part of childhood ADHD and its treatment with stimulants has been even associated with clinical improvement of sleep disorders. Distinguishing methylphenidate-induced sleep disturbances from intrinsic features of ADHD in this condition may be difficult.[28] Sleep disturbances due to methylphenidate are generally dose-related and transient. In this regards, for example, Efron et al. implicated that short- and long-term stimulant use are associated with sleep abnormalities in 70% and 29% of individuals, respectively.[29] The rate of sleep disorders in our study population was 47.2%. Difference in the frequency of methylphenidate-related sleep abnormalities may be justified by various causes including methylphenidate dose as well as duration of treatment and method of detecting adverse reaction (parent or self-reporting vs. polysomnography). Methylphenidate-related sleep disturbances can be managed by approaches such as giving doses earlier in the day, omit or reduce the afternoon/evening dose, and changing from long-acting to short-acting preparations.[30]
Despite 11.2% of the study, population had a history of epilepsy before starting methylphenidate, aggravation of their epilepsy during methylphenidate treatment was not documented by either electroencephalography (EEG) monitoring or history taking from patients or their parents. Methylphenidate has been shown to lower the seizure threshold, promote the onset of the seizure,[31] and the rate of seizure in children with ADHD under stimulant therapy is estimated to be twice more common than the general population.[32] On the other hand, between 6.1% and 30.1% of children with ADHD had abnormal EEG.[14] There is fair evidence that administration of stimulants is safe in ADHD patients with controlled epilepsy. In this regards, Gucuyener et al. reported that methylphenidate was effective in controlling ADHD without no seizure episode and no change in mean seizure frequency in patients with baseline EEG abnormalities and epilepsy, respectively.[33] Therefore, stimulants can be considered as an option for epileptic children with ADHD especially when the seizures are well-controlled.[28]
In the current survey, more than one-thirds (35.7%) of individuals experienced mild tic or repeated involuntary movements during methylphenidate treatment. None led to methylphenidate dose reduction, discontinuation, or adding an agent to relieve these movement disorders. Stimulants can potentially induce or worsen tics in a dose-dependent manner.[34] In about 65 reported cases, stimulants aggravate tic severity.[35] Retrospective case reviews supported this finding.[31] Nevertheless, similar to sleep abnormalities and epilepsy, about 20% of children with ADHD are also involved with underlying chronic tic disorders.[36] Furthermore, review of more recent studies suggests that most stimulant-associated tics and movement disorders are mild and transient, and benefits of ADHD treatment with stimulants considerably outweigh the potential risk of developing tics and repeated involuntary movements.[28] In severe or prolonged cases, methylphenidate should be replaced by a nonstimulant agent such as clonidine.[37]
The only cardiovascular-related adverse reaction of methylphenidate was mild palpitation observed in near one-fourth (22.9%) of our cohort. No documented episode of clinically significant arrhythmia or hypertension during methylphenidate treatment was recorded in their office charts. Noting that none of the study population had known underlying cardiovascular diseases. In compatible with our findings, most placebo-controlled, double-blind studies of methylphenidate demonstrate that the medication is safe from a cardiovascular perspective in children if there is no underlying cardiovascular pathology.[38] Recipients of methylphenidate may develop on average a modest increase of 1–6 bpm in pulse rate and 3–4 mmHg in blood pressure.[38,39] However, between January 1992 and February 2005, 18 cases of sudden death during methylphenidate treatment, 14 in children and 4 in adults, were reported to the Food and Drug Administration (FDA) Adverse Event Reporting System.[39] Therefore, FDA added a boxed warning about cardiac risk of methylphenidate and emphasized on taking a careful history of cardiovascular disease from both patient and his/her family (especially regarding sudden death and ventricular arrhythmia) before commencing the treatment. Screening baseline electrocardiograms is also suggested but not mandatory. Patients developed relevant symptoms of cardiovascular disease such as exertional angina pectoris during methylphenidate treatment should promptly undergo a careful cardiac evaluation.[28]
Dermatologic adverse effects of methylphenidate identified in our cohort were cold extremities (21.13%), increased sweating (11.27%), skin eruptions (rash without pruritus [5.63%]), hair loss (4.23%), and acne (1.41%). No measure such as dose reduction, medication discontinuation, or supportive therapy was implemented to manage these adverse reactions. Product monograph provided by the manufacturer list a number of cutaneous reactions such as skin rash and urticaria without defining their frequencies.[32] Four cases of peripheral vasculopathy suspected to methylphenidate were also described by Syed and Moore.[40] At least three cases of methylphenidate-related skin rash (two developed in the scrotum) have been reported in the literature.[41,42] These were managed by either medication withdrawal[41] or desensitization.[42] Reversible and temporary alopecia secondary to methylphenidate has been also reported.[43] To our knowledge, no case of methylphenidate-related acne has been cited in the relevant scientific literature. Despite persistence of acne in both patients, the physician preferred to continue methylphenidate to achieve optimal therapeutic response. Medications that affect norepinephrine and dopamine neurotransmitters can potentially cause sweating abnormalities. In this regards, for example, tricyclic antidepressants such as imipramine, nortriptyline, and amitriptyline can induce hyperhidrosis in up to 14% of their recipients probably through the stimulation of peripheral adrenergic receptors.[44] Although it is not mentioned specifically in the literature, this may also true for methylphenidate as a norepinephrine and dopamine reuptake inhibitor to cause hyperhidrosis. Regular monitoring of patients under methylphenidate treatment regarding dermatologic adverse reactions may be helpful in early diagnosis and more effective management of these ADRs.
All but one methylphenidate related adverse effects (headache) were categorized as either mild levels 1 and 2 regarding Hartwig scale. In addition, based on the WHO definition, no serious ADRs were detected. This is in accordance with that observed routinely in clinical practice. The risk of severe adverse reactions with methylphenidate is really rare.[28] Nevertheless, as noted by Merkel and Kuchibhatla in their review about safety of stimulant treatment in ADHD, serious and severe adverse reactions of methylphenidate such as cardiac complications may be under-reported and underestimated due to small sample sizes, short duration of follow-up, and drawbacks of detecting ADRs.[45] All the detected ADRs were determined to be nonpreventable because all seven inquiries of Schumock and Thornton questionnaire (e.g, giving inappropriate dose and interval, positive history of drug allergy or adverse reaction, and presence of potential drug-drug interactions) were given negative answers. No published clinical study has considered the preventability, severity, and seriousness of methylphenidate adverse reactions so far. These features of methylphenidate safety warrant more attention.
Results of the current study demonstrated that all (100%) ADHD children under methylphenidate treatment developed at least one ADR. Many organ-systems of methylphenidate recipients were involved with adverse reactions. Anorexia, irritability, and insomnia were the most frequent methylphenidate-related adverse reactions. Two cases of acne after taking methylphenidate were recorded that has not been previously reported in the relevant literature. All but one methylphenidate-related adverse reaction (headache) were categorized as mild. No preventable or serious ADRs were detected. Our preliminary data could be used to establish a database of methylphenidate safety especially for Iranian children with ADHD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:922–8. doi: 10.1097/00004583-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Suvarna BS, Kamath A. Prevalence of attention deficit disorder among preschool age children. Nepal Med Coll J. 2009;11:1–4. [PubMed] [Google Scholar]
- 3.Karapinar U, Saglam O, Dursun E, Cetin B, Salman N, Sahan M. Sudden hearing loss associated with methylphenidate therapy. Eur Arch Otorhinolaryngol. 2014;271:199–201. doi: 10.1007/s00405-013-2763-y. [DOI] [PubMed] [Google Scholar]
- 4.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 5.Krull KR. Attention deficit hyperactivity disorder in children and adolescents: Overview of treatment and prognosis. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2014. [Last updated: 2014, Jun 10]. Available from: http://www.uptodate.com/contents/attention-deficit-hyperactivitydisorder-in-children-and-adolescents-overview-oftreatment-and-prognosis . [Google Scholar]
- 6.Wolraich M, Brown L, Brown RT, DuPaul G, et al. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–22. doi: 10.1542/peds.2011-2654. Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HM, Reiff MI. Clinical practice. Attention deficit-hyperactivity disorder in children and adolescents. N Engl J Med. 2014;370:838–46. doi: 10.1056/NEJMcp1307215. [DOI] [PubMed] [Google Scholar]
- 9.Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1100–7. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 10.Marchei E, Farrè M, Pellegrini M, García-Algar O, Vall O, Pacifici R, et al. Pharmacokinetics of methylphenidate in oral fluid and sweat of a pediatric subject. Forensic Sci Int. 2010;196:59–63. doi: 10.1016/j.forsciint.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Tagaya H. Methylphenidate: Pharmacology, indication and potential of abuse. Nihon Rinsho. 2010;68:1550–5. [PubMed] [Google Scholar]
- 12.Scharman EJ, Erdman AR, Cobaugh DJ, Olson KR, Woolf AD, Caravati EM, et al. Methylphenidate poisoning: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2007;45:737–52. doi: 10.1080/15563650701665175. [DOI] [PubMed] [Google Scholar]
- 13.Olfson M, Huang C, Gerhard T, Winterstein AG, Crystal S, Allison PD, et al. Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:147–56. doi: 10.1016/j.jaac.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90:57–9. doi: 10.1136/adc.2003.048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalviri G, Valadkhani M, Dinarvand R. Ten years pharmacovigilance activities in Iran. Iran J Public Health. 2009;38:162–5. [Google Scholar]
- 16.Hanafi S, Torkamandi H, Hayatshahi A, Gholami K, Javadi M. Knowledge, attitudes and practice of nurse regarding adverse drug reaction reporting. Iran J Nurs Midwifery Res. 2012;17:21–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31:183–7. doi: 10.1007/s11096-008-9276-6. [DOI] [PubMed] [Google Scholar]
- 18.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 19.Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf. 1997;17:374–89. doi: 10.2165/00002018-199717060-00004. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Uppsala: Uppsala Monitoring Centre; 2000. Uppsala Monitoring Centre (the UMC). Safety Monitoring of Medicinal Products: Guidelines for Setting up and Running a Pharmacovigilancecentre. [Google Scholar]
- 21.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 22.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 23.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475–88. [PMC free article] [PubMed] [Google Scholar]
- 24.Wender EH. Managing stimulant medication for attention-deficit/hyperactivity disorder. Pediatr Rev. 2001;22:183–90. doi: 10.1542/pir.22-6-183. [DOI] [PubMed] [Google Scholar]
- 25.Lerner M, Wigal T. Long-term safety of stimulant medications used to treat children with ADHD. Pediatr Ann. 2008;37:37–45. doi: 10.3928/00904481-20080101-11. [DOI] [PubMed] [Google Scholar]
- 26.Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1294–303. doi: 10.1097/01.chi.0000235082.63156.27. [DOI] [PubMed] [Google Scholar]
- 27.Owens JA. The ADHD and sleep conundrum: A review. J Dev Behav Pediatr. 2005;26:312–22. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Merkel RL. Safety of stimulant treatment in attention deficit hyperactivity disorder: Part II. Expert Opin Drug Saf. 2010;9:917–35. doi: 10.1517/14740338.2010.503238. [DOI] [PubMed] [Google Scholar]
- 29.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: A double-blind, crossover trial. Pediatrics. 1997;100:662–6. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- 30.Corkum P, Davidson F, Macpherson M. A framework for the assessment and treatment of sleep problems in children with attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 2011;58:667–83. doi: 10.1016/j.pcl.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Wolraich ML, McGuinn L, Doffing M. Treatment of attention deficit hyperactivity disorder in children and adolescents: Safety considerations. Drug Saf. 2007;30:17–26. doi: 10.2165/00002018-200730010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: Epidemiology, prevention and management. CNS Drugs. 2008;22:213–37. doi: 10.2165/00023210-200822030-00003. [DOI] [PubMed] [Google Scholar]
- 33.Gucuyener K, Erdemoglu AK, Senol S, Serdaroglu A, Soysal S, Kockar AI. Use of methylphenidate for attention-deficit hyperactivity disorder in patients with epilepsy or electroencephalographic abnormalities. J Child Neurol. 2003;18:109–12. doi: 10.1177/08830738030180020601. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette's syndrome: Effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36:589–96. doi: 10.1097/00004583-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Kurlan R. Methylphenidate to treat ADHD is not contraindicated in children with tics. Mov Disord. 2002;17:5–6. doi: 10.1002/mds.10094. [DOI] [PubMed] [Google Scholar]
- 36.Roessner V, Robatzek M, Knapp G, Banaschewski T, Rothenberger A. First-onset tics in patients with attention-deficit-hyperactivity disorder: Impact of stimulants. Dev Med Child Neurol. 2006;48:616–21. doi: 10.1017/S0012162206001290. [DOI] [PubMed] [Google Scholar]
- 37.Tallian KB, Finley PR, Perry P, Kuperman S. Attention deficit hyperactivity disorder in children, adolescents, and adults. In: Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, et al., editors. Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Philadelphia, USA: Lippincott Williams and Wilkins; 2012. p. 2006. [Google Scholar]
- 38.Vetter VL, Elia J, Erickson C, Berger S, Blum N, Uzark K, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407–23. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- 39.Vitiello B. Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function. Child Adolesc Psychiatr Clin N Am. 2008;17:459–74. doi: 10.1016/j.chc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheumatol. 2008;14:30–3. doi: 10.1097/RHU.0b013e3181639aaa. [DOI] [PubMed] [Google Scholar]
- 41.Cohen HA, Ashkenazi A, Nussinovitch M, Gross S, Frydman M. Fixed drug eruption of the scrotum due to methylphenidate. Ann Pharmacother. 1992;26:1378–9. doi: 10.1177/106002809202601107. [DOI] [PubMed] [Google Scholar]
- 42.Confino-Cohen R, Goldberg A. Successful desensitization of methylphenidate-induced rash. J Child Adolesc Psychopharmacol. 2005;15:703–5. doi: 10.1089/cap.2005.15.703. [DOI] [PubMed] [Google Scholar]
- 43.Solanto MV, Arnsten AF, Castellanos F ×. Oxford: Oxford University Press; 2001. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. [Google Scholar]
- 44.Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: Incidence, prevention and management. Drug Saf. 2008;31:109–26. doi: 10.2165/00002018-200831020-00002. [DOI] [PubMed] [Google Scholar]
- 45.Merkel RL, Jr, Kuchibhatla A. Safety of stimulant treatment in attention deficit hyperactivity disorder: Part I. Expert Opin Drug Saf. 2009;8:655–68. doi: 10.1517/14740330903279956. [DOI] [PubMed] [Google Scholar]


