Abstract
Purpose:
The double row cuff repair with suture bridging is commonly used for arthroscopic rotator cuff repair (RCR). Despite its biomechanical qualities, the rate of iterative tears with this technique is important. The aim of our study was to evaluate the effect of autologous conditioned plasma (ACP) on functional results and on the rate of iterative tears after RCR by suture bridging.
Materials and Methods:
A consecutive series of 65 patients who underwent arthroscopic double-row suture bridge (Speed-Bridge, Arthrex) primary cuff repair of symptomatic full-thickness supraspinatus tear (retraction <3 in the Patte classification) were evaluated. Mean patient age was 60 (+/-8). The supraspinatus was repaired by knot-less bridging (SwiveLock, Arthrex) with suture tape material. 2 homogenous groups were created (A: 33 patients, B: 32 patients). In group A, all patients received, besides the cuff repair, an intra-tendinous ACP injection. Constant scores and Simple Shoulder Tests (SST) were measured pre-operatively and after a minimum follow-up period of 12 months post-operatively. Structural integrity of the repairs was evaluated by MRI according to the Sugaya classification. Sugaya >4 were considered as iterative tears.
Results:
Mean follow-up was 19 months (+/−42) in the 2 groups. The mean quantity of ACP injected was 6ml. (+/−1.5) and no specific complication of the injection was found. Mean preoperative Constant-Murley scores were 41,2 (±7,7) and 38 (±11)in group B. Mean normalized Constant-Murley score increased from 41 points (±7) pre-operatively to 70 points (±8) post-operatively in group A and from 38 points (±11) to 73 points (±11) in group B. There were no significative differences between the two groups (P > 0.05). In group A, 31 repairs were Sugaya 1-3 (94%), vs. 30 in group B (93%), and 1 was type 4 in group A (5%) vs. 2 in group B (8%).
Conclusion:
In both groups, RCR with suture bridging gave successful functional outcomes, with a low rate of iterative tear. In this preliminary study, the adjuvant effect of ACP injections could not be showed on both functional and structural results. Longer follow-up is needed to evaluate potential differences.
Keywords: Double row, platelet-rich plasma, rotator cuff tear, suture-bridging
INTRODUCTION
Rotator cuff injuries are very frequent, especially in patients above 60 years old, reaching more than 30% on cadaveric studies.[1] Arthroscopic rotator cuff repair is a common procedure known to restore function and provide satisfactory pain relief when nonoperative treatment has failed.[2,3] Nevertheless, these arthroscopic repairs have an important rate of postoperative re-tear that varies between 11% and 94% in the literature.[1,4] Numerous techniques have been proposed and controversies still surround ideal arthroscopic rotator cuff repair.
The arthroscopic double row cuff repair with suture-bridging is now a widely used technique in rotator cuff tears. The suture-bridging system allows better load-sharing between the four anchors, especially in rotation movements and creates a larger tendon-bone interface for better healing.[5,6] Moreover, the use of suture tapes of 2-mm width maximizes the compression of the tendon-bone interface and improves tissue cut-through resistance.
In order to improve tendon healing, many leads have been followed.[7,8] Several studies have reported the healing potential of autologous platelet-rich plasma (PRP) on tendons and especially on those of the rotator cuff.[9,10,11,12,13] They have been shown to decrease postoperative pain and to improve early clinical results.[14] These autologous conditioned plasma (ACP) are rich in growth factors, contained in the a-granules of the platelets. These growth factors improve tissue healing and regeneration, promote new capillary growth, and accelerate epithelialization in chronic wounds. Animal and in-vivo studies have proven the effects of ACP on collagen repair and on tissue vascularization.[15,16] Many clinical studies have also shown promising results of ACP application for a variety of indications, and a range of platelet preparations have been approved by the US Food and Drug Administration and are now commercially available.
The aim of the study was to evaluate if APC injection after tape-bridging improved functional and structural results in rotator cuff repair.
Our hypothesis was that the functional and structural results should be improved by the injection of ACP. Based on this rationale we injected APC after arthroscopic double row rotator cuff repair with tape-bridging to prevent postoperative re-tear and to increase healing potential.
MATERIALS AND METHODS
A consecutive series of patients who underwent arthroscopic primary rotator cuff repair by suture-bridging between March 2010 and September 2012 were enrolled in the present comparative study.
The inclusion criteria were symptomatic full-thickness supraspinatus tear, retraction I or II (according to the Patte classification) confirmed during surgery, fatty infiltration <3 (according to Goutallier and Bernageau scales), and minimum follow-up of 12 months with control imaging. Patients were excluded if they had shoulder stiffness, previous shoulder surgery, shoulder osteoarthritis, partial-thickness rotator cuff tear or rotator cuff tear involving the sub-scapularis tendon. Sixty-five patients met the inclusion criteria.
The patients were firstly separated in two groups depending on surgeon habit regarding ACP injection combined with arthroscopic repair: A Group A of 33 patients operated by one surgeon who used ACP and a Group B of 32 patients were operated by another surgeon who did not. In Group A, all patients were injected in the tendon with ACP in addition to the rotator cuff repair. Both surgeons were experienced in rotator cuff repair and shoulder arthroscopy. The two groups were similar in terms of preoperative functional scores.
Patient assessment
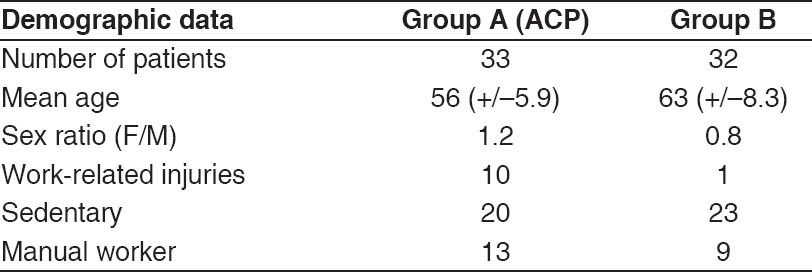
The senior surgeons clinically followed their patients at regular intervals, from the preoperative period until the last follow-up. All patients were evaluated by Constant-Murley score (absolute, normalized), simple shoulder test (SST) and visual analogue scale (VAS). Antero-posterior extension of the tears was evaluated by Bateman's classification[17]. in Group A, 13 tears were grade 1 (tear <1 cm), 15 were grade 2 (tear 1-3 cm), and 5 grade 3 (tear <5 cm). In Group B, 14 were grade 1 (tear <1 cm), 17 grade 2 (tear 1-3 cm), and 1 grade 3 (tear <5 cm). In both groups, no cuff tear was classified as grade 4 (no cuff left). Postoperative control imaging by magnetic resonance imaging (MRI) was performed systematically in both groups by one of the authors (JDW) and by an experienced radiologist. Postoperative cuff integrity was classified into five categories according to Sugaya et al.[18,19] with coronal, axial, and sagittal views of T2-weigthed images: Type 1, sufficient thickness; type 2, sufficient thickness with partial high intensity; type 3, insufficient thickness without discontinuity; type 4, minor discontinuity; type 5, major discontinuity. Types 4 and 5 were considered as failure of tendon repair. Whatever the technique, intact repair was defined as complete anatomical footprint reconstruction. The demographic data of each group are reported in Table 1.
Table 1.
Demographic data
All complications were recorded, and no patients were lost to follow-up.
Surgical procedure
All the patients underwent the same surgical procedure with the same technique by two senior shoulder surgeons experienced in this repair technique.
All procedures were performed on patients in the beach-chair position under general anesthesia. A posterior portal was first established for initial assessment of the gleno-humeral joint, with completion during the procedure via 3 or 4 additional portals. During the examination, tear location and size, delamination and associated biceps tendon lesions were inspected carefully. Tenotomy or tenodesis was undertaken in case of lesion. Every patient was treated by the same knotless suture-bridge fiber tape technique [Figure 1].
Figure 1.
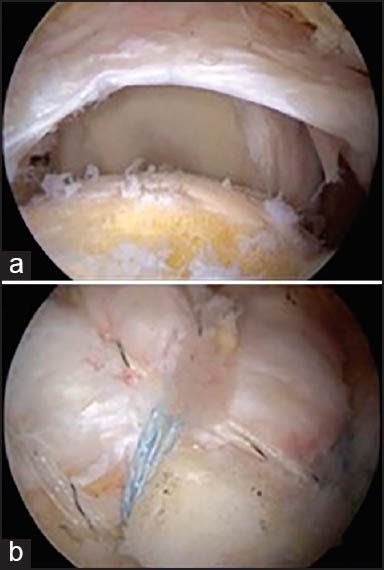
Rotator cuff repair using a knotless suture-bridge fiber tape technique (a) before, (b) after
In most cases an acromioplasty with bursectomy was performed, with an abrasion of the footprint in all cases.
In Group A, at the end of the procedure, the patients were injected with ACP. The autologous PRP was prepared with the ACP© Arthrex system with no adjuvant. Two syringes of 10 ml. of peripheral venous blood were taken (usually at the ankle) at the end of the intervention as soon as the suture of the cuff had been completed. The blood sample was centrifuged for 5 min at 1500 revolutions/min and the supernatant was collected for the surgical injection.. The injection was performed immediately after centrifugation for maximal efficiency.
A 10 cc syringe with a 21-gauge, 4 mm. Needle were used for the injection. The needle was introduced under arthroscopic guidance in the thickness of the repaired tendon between the two rows of anchors. Once the needle was well positioned, all the portals were sutured except the one for the arthroscope and the irrigation of the arthroscopic pump was stopped to prevent any leaks and out-flow of the plasma. Several injections in different areas of the repaired tendon were performed under arthroscopic control [Figure 2].
Figure 2.
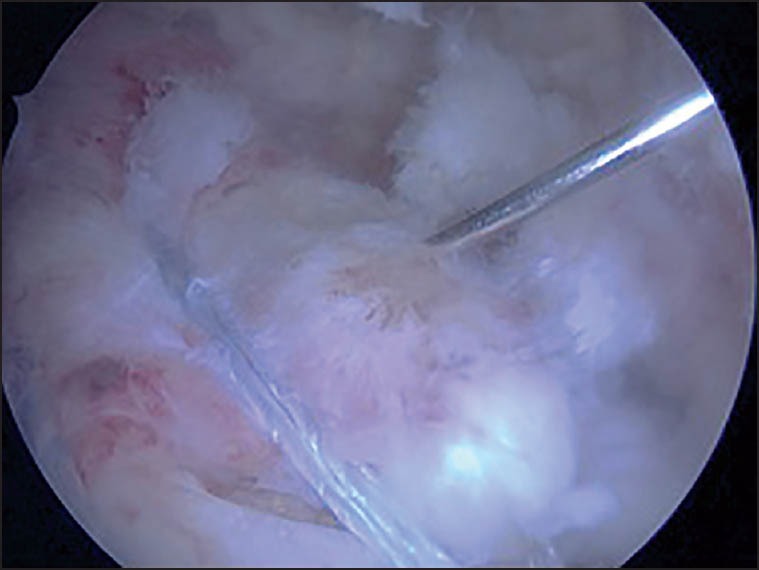
Arthroscopic intratendinous injection of autologous conditioned plasma
Postoperative protocol
The postoperative protocol was identical in both groups. The shoulders were immobilized for 6 weeks with a sling. Shoulder rehabilitation consisted of free passive range of motion exercises starting from postoperative day 1. Active motion was initiated at 6 weeks. Rotator cuff strengthening exercises began 10 weeks after surgery. Full return to sports and heavy labor were allowed after 6 months.
Statistical analysis
The data were analyzed by SAS 9.1 software (SAS Institute, Cary, IN, USA). Continuous data were assessed for a normal distribution by the Mann-Whitney U-test and a Kruskal-Wallis test for noncontinuous data. Significance was set at P < 0.05.
RESULTS
Functional results
Mean follow-up at the time of postoperative functional evaluation was 19 months (±4.2) in the two groups. There was no statistical difference of follow-up between the two groups. The mean quantity of ACP injected was 6 (4.5-7.5) ml. Mean preoperative Constant-Murley scores were 41.2 (±7.7) and 38 (±11) in Group B. Mean SST scores were 4.5 (±1.9) in Group A and 4.1 (±1.9) in Group B.
Mean normalized Constant-Murley score increased from 41 points (±7) preoperatively to 70 points (±8) postoperatively in Group A and from 38 points (±11) to 73 points (±11) in Group B. However, there was no statistical difference in mean postoperative Constant-Murley score between groups (no significant).
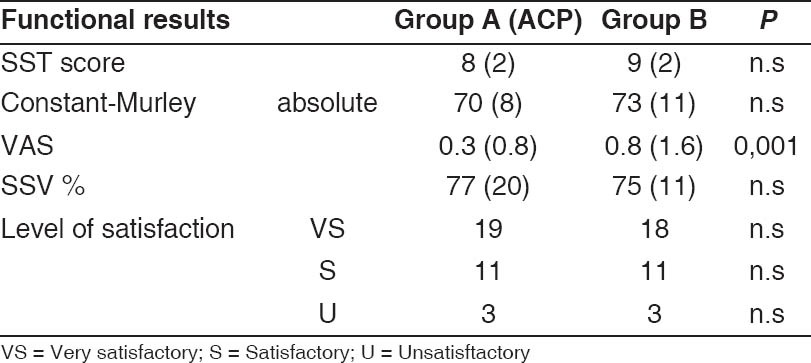
There were no significant differences between the two groups per- or post-operatively (no significant), except for the pain. Indeed, the VAS at last follow-up was 0.3 (±0.8) in Group A and 0.8 (±1.6) in Group B (P = 0.001). The functional results in each group are reported in Table 2.
Table 2.
Functional results
There were no major intra- or post-operative complications especially infection. One shoulder in each group developed a frozen shoulder after repair that evolved favorably after medical treatment.
Structural integrity
Healing of rotator cuff repair was assessed systematically by control MRI. In Group A, at last follow-up, 31 cuff repairs (94%) were classified as Sugaya type 1-3 and 2 was type 4 (minor discontinuity). In Group B, 30 (93%) repairs were classified as Sugaya type 1-3 and 2 were type 4. There were 1 re-tear in Group A (5%) and 2 in Group B (8%). Statistical analysis did not reveal any significant difference in the healing rate between Groups A and B.
DISCUSSION
The aim of the study was to evaluate if APC injection improved functional and structural results in rotator cuff repair. The present study did not establish any significant difference between the two groups functionally and structurally except for the VAS score at last follow-up (P < 0.01). This result is similar with the results of other studies[14] and this could be explained by the analgesic effect of the APC via the protease-activated receptors 4 peptides.[20]
The present study also shows that rotator cuff repair by knotless tape-bridging whatever the group improves significantly the Constant-Murley score and relieves pain efficiently. These results are in accordance with those of previous studies where knotless, self-reinforcing, double row system achieved a lower re-tear rate compared to other repair techniques.[5] Several biomechanical reasons may explain such findings. The construct with suture tape material could provide better-high footprint compression between the tendon and bone to help promote healing. In addition, the theoretical risk of cut-through resistance on medial rows may have been lower because tension was better shared. The use of screw anchors rather than impacted anchors could also be an explanation for the good results observed in both groups.[5]
Despite its biomechanical qualities, suture-bridging is often criticized particularly because it may have a deleterious effect on the tendon vascularization. In a recent study, blood flow at the repair site showed a significant decline (44.6%) after placement of the second row of implants.[21] Although the implications of vasculature reduction in tendon healing are still unknown, excessive tensioning of the lateral row is not recommended. In addition, marginal dog-ear deformities of the repaired rotator cuff are not uncommon with these suture-bridging techniques. Diminished blood flow because of these deformities and excessive cuff compression are responsible for insufficient healing. Therefore, it has been thought that APC injections in the rotator cuff could improve tendon healing rates after double row suture-bridging.
Indeed, the beneficial effect of APC injections in tendon surgical repair has been shown in several studies, and could have potential applications in many fields in orthopedic surgery (Reconstruction of anterior cruciate ligament,[22] tendon surgery, treatment of joint injuries,[23,24] tendinopathy[25] or muscle tears). Mishra et al. report the positive effect of ACP injection in patients with chronic rotator cuff tendinopathy.[26] However, despite these promising results, our findings are in accordance with those of previous studies as shown in Table 3.
Table 3.
Results of previous studies comparing rotator cuff repairs with and without autologous conditioned plasma
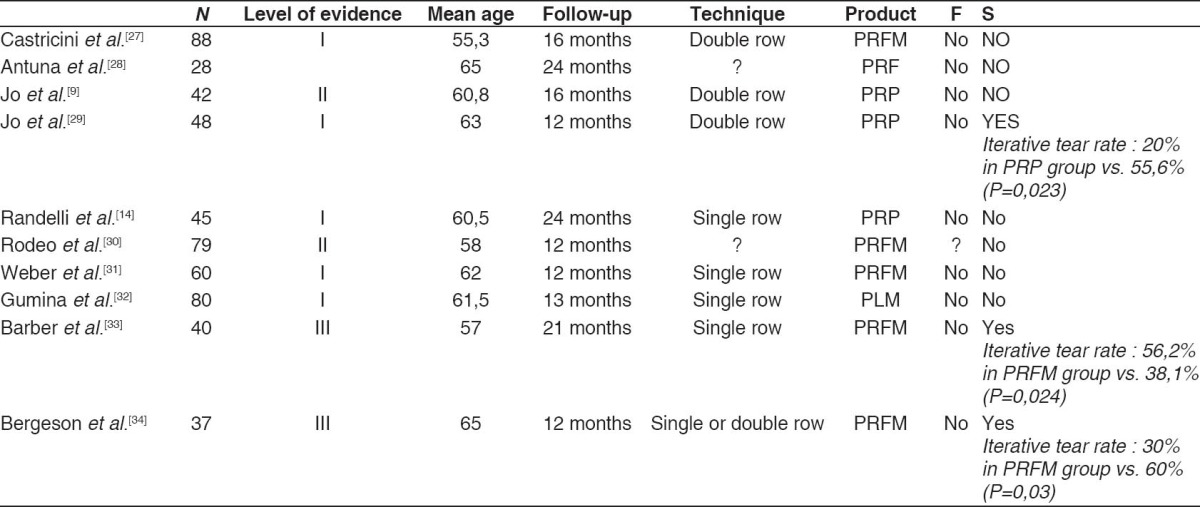
In all those studies, as in our, there were no statistical differences on the functional and structural outcome whether PRP had been injected or not. These results are confirmed by the findings of Zhang et al.[27]. In their meta-analysis comparing prospective studies, they showed no benefits of the PRP on the overall clinical outcomes and re-tear rate for the arthroscopic repair of full-thickness rotator cuff tears. However, they noticed a decrease of the re-tear among the patients treated by PRP for small- and medium-sized rotator cuff tears, but not for large- and massive-sized tears. There is a discrepancy between these results and those of Jo et al.[9,28] Indeed, in their two studies, they only found a statistical difference in the two groups (PRP vs. no PRP) for the structural outcome in the large to massive rotator cuff repairs with no consequence on the clinical outcome.
This study had several limitations. First of all, only a small number of patients were available for evaluation, follow-up was short. It is possible that a longer delay may negatively impact the results reported here. In addition, the population in Group A had more work-related injuries. Consequently, the functional outcome in Group A could have been underestimated.[29] The patients in each group were operated by a different surgeon. A bias could thus have been created, even though, both surgeons were senior surgeons experienced in arthroscopic rotator cuff repair. The ACP injection was not randomized, resulting in potential selection bias. Lastly, there was a significant age difference in both groups (P = 0.003) which could possibly explain the significant difference in pain between the two groups at the last follow-up.
The ideal dose of ACP to inject is as yet unknown, and no study makes it clear whether an activator is needed or not. Many different injection systems of ACP are available [listed in Table 4] with different platelet concentrations.
Table 4.
Different injection systems of ACP
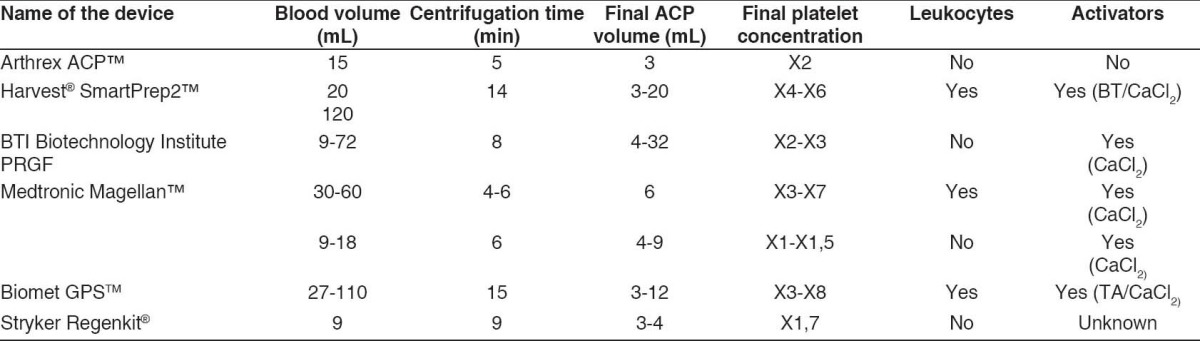
The platelet concentration in the PRP varies greatly from person to person and is, therefore, expressed as a multiplication factor of the platelet concentration in native blood.[30] The ACP is not the most concentrated PRP, but it seems that there is an ideal platelet concentration above which there may be a deleterious effect of the PRP on tendon healing.[31] It appears also important to control the leukocyte concentration. Indeed, the leukocytes are involved in the catabolic signaling, and an excessive leukocyte concentration in the PRP could have a negative effect on tendon healing.[31,32] The ACP we used for this study has no leucocytes and does not require the adjunction of an activator. The platelet activators (bovine thrombin or calcium chloride) are used to release the growth factors from the platelets, but they release them very quickly (in an hour for the bovine thrombin[33] and in 7 days for the calcium chloride.[34] On the other hand, the in-vivo activation using no activators enables to have a sustained release of the growth factors during the healing process.
CONCLUSION
First of all in this preliminary study, no adjuvant effect of ACP on healing and functional results was demonstrated except for a possible analgesic effect. Secondly, this study demonstrates successful functional and structural outcome as well as reproducibility of arthroscopic, double row cuff repair with knotless tape-bridging. Follow-up is being continued, to assess results at long-term and determine whether this analgesic effect can be attributed to PRP.
Footnotes
Source of Support: Nil
Conflict of Interest: There are no conflicts of interest.
REFERENCES
- 1.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: A review. Clin Orthop Relat Res. 2010;468:1476–84. doi: 10.1007/s11999-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19:234–8. doi: 10.1053/jars.2003.50036. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart SS, Danaceau SM, Pearce CE., Jr Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17:905–12. doi: 10.1053/jars.2001.26821. [DOI] [PubMed] [Google Scholar]
- 4.Randelli P, Spennacchio P, Ragone V, Arrigoni P, Casella A, Cabitza P. Complications associated with arthroscopic rotator cuff repair: A literature review. Musculoskelet Surg. 2012;96:9–16. doi: 10.1007/s12306-011-0175-y. [DOI] [PubMed] [Google Scholar]
- 5.Boyer P, Bouthors C, Delcourt T, Stewart O, Hamida F, Mylle G, et al. Arthroscopic double-row cuff repair with suture-bridging: A structural and functional comparison of two techniques. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2401-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Elattrache NS, Tibone JE, Jun BJ, DeLaMora SN, Kvitne RS, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34:407–14. doi: 10.1177/0363546505281238. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery SR, Petrigliano FA, Gamradt SC. Biologic augmentation of rotator cuff repair. Curr Rev Musculoskelet Med. 2011;4:221–30. doi: 10.1007/s12178-011-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21:181–90. doi: 10.1016/j.jse.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Jo CH, Kim JE, Yoon KS, Lee JH, Kang SB, Lee JH, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39:2082–90. doi: 10.1177/0363546511413454. [DOI] [PubMed] [Google Scholar]
- 10.Mei-Dan O, Carmont MR. Autologous blood products in rotator cuff repair. Med Sport Sci. 2012;57:65–75. doi: 10.1159/000328904. [DOI] [PubMed] [Google Scholar]
- 11.Nixon AJ, Watts AE, Schnabel LV. Cell- and gene-based approaches to tendon regeneration. J Shoulder Elbow Surg. 2012;21:278–94. doi: 10.1016/j.jse.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30:1584–9. doi: 10.1080/09638280801906081. [DOI] [PubMed] [Google Scholar]
- 13.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:S191–7. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: A prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–28. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 16.Visser LC, Arnoczky SP, Caballero O, Egerbacher M. Platelet-rich fibrin constructs elute higher concentrations of transforming growth factor-ß1 and increase tendon cell proliferation over time when compared to blood clots: A comparative in vitro analysis. Vet Surg. 2010;39:811–7. doi: 10.1111/j.1532-950X.2010.00739.x. [DOI] [PubMed] [Google Scholar]
- 17.Bateman JE. The diagnosis and treatment of ruptures of the rotator cuff. Surg Clin North Am. 1963;43:1523–30. doi: 10.1016/s0039-6109(16)37139-0. [DOI] [PubMed] [Google Scholar]
- 18.Sugaya H, Maeda K, Matsuki K, Moriishi J. Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: Single-row versus dual-row fixation. Arthroscopy. 2005;21:1307–16. doi: 10.1016/j.arthro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–60. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 20.Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, et al. Protease-activated receptor-4: A novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150:176–85. doi: 10.1038/sj.bjp.0706975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christoforetti JJ, Krupp RJ, Singleton SB, Kissenberth MJ, Cook C, Hawkins RJ. Arthroscopic suture bridge transosseus equivalent fixation of rotator cuff tendon preserves intratendinous blood flow at the time of initial fixation. J Shoulder Elbow Surg. 2012;21:523–30. doi: 10.1016/j.jse.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Seijas R, Ares O, Catala J, Alvarez-Diaz P, Cusco X, Cugat R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet-rich plasma. J Orthop Surg (Hong Kong) 2013;21:10–4. doi: 10.1177/230949901302100105. [DOI] [PubMed] [Google Scholar]
- 23.Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411–7. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 24.Andia I, Maffulli N. Platelet-rich plasma for muscle injury and tendinopathy. Sports Med Arthrosc. 2013;21:191–8. doi: 10.1097/JSA.0b013e318299972b. [DOI] [PubMed] [Google Scholar]
- 25.Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg. 2013;79:10–5. [PubMed] [Google Scholar]
- 26.Mishra A, Randelli P, Barr C, Talamonti T, Ragone V, Cabitza P. Platelet-rich plasma and the upper extremity. Hand Clin. 2012;28:481–91. doi: 10.1016/j.hcl.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Ge H, Zhou J, Cheng B. Are platelet-rich products necessary during the arthroscopic repair of full-thickness rotator cuff tears: A meta-analysis. PLoS One. 2013;8:e69731. doi: 10.1371/journal.pone.0069731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo CH, Shin JS, Lee YG, Shin WH, Kim H, Lee SY, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: A randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41:2240–8. doi: 10.1177/0363546513497925. [DOI] [PubMed] [Google Scholar]
- 29.Nové-Josserand L, Liotard JP, Godeneche A, Neyton L, Borel F, Rey B, et al. Occupational outcome after surgery in patients with a rotator cuff tear due to a work-related injury or occupational disease. A series of 262 cases. Orthop Traumatol Surg Res. 2011;97:361–6. doi: 10.1016/j.otsr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–16. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 31.Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42:42–9. doi: 10.1177/0363546513507566. [DOI] [PubMed] [Google Scholar]
- 32.McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. (1-8).J Bone Joint Surg Am. 2012;94:e143. doi: 10.2106/JBJS.L.00019. [DOI] [PubMed] [Google Scholar]
- 33.Bennett NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–37. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad Z, Howard D, Brooks RA, Wardale J, Henson FM, Getgood A, et al. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep. 2012;3:40. doi: 10.1258/shorts.2011.011148. [DOI] [PMC free article] [PubMed] [Google Scholar]


