Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by a selective loss of dopamine in the striatum. Problems remain in the accurate diagnosis of PD. The diagnosis of idiopathic PD is based on the interpretation of clinical signs and symptoms could be incorrect at the time of initial presentation. In vivo imaging of the dopaminergic system has the potential to improve the diagnosis of PD in its early stages. The imaging of dopamine transporter (DAT) with 99mTc-labeled tropane derivative (TRODAT-1) single photon emission computer tomography/computer tomography (SPECT/CT) has been proposed to be a valuable and feasible means of assessment of the integrity of dopamine neurons. The purpose of this study was to investigate the potential usefulness of 99mTc-TRODAT-1 imaging in the evaluation of patients with PD and classify into different stages of the disease. SPECT imaging with 99mTc-TRODAT-1 was conducted in 16 consecutive PD patients (9 men; 7 women) and in 6 age matched healthy volunteers (4 men; 2 women). The images were obtained 3 h after the intra-venous injection of the tracer. Specific uptake in the striatum and its sub-regions, including the putamen and caudate nucleus was calculated and the ratios of specific striatal binding to nonspecific occipital binding were calculated. ANOVA with Dunnett C post-hoc analysis was conducted using SPSS 20. A stepwise reduction in specific striatal uptake of 99mTc-TRODAT-1 with increasing disease severity between healthy control versus Stage I versus Stage II versus Stage III was found in PD patients (i.e., 3.77 vs. 2.56 vs. 1.57 vs. 0.63, P < 0.05). The changes were magnified by measurement of specific putaminal uptake (1.43 vs. 0.79 vs. 0.54 vs. 0.19, P < 0.05) and specific caudate uptake (1.90 vs. 1.47 vs. 0.73 vs. 0.27, P < 0.05). No remarkable adverse reactions were found in either healthy volunteers or PD patients during or after imaging. 99mTc-TRODAT-1 is accurate and widely available for the assessment of DAT activity, which might shed light on the integrity of the presynaptic nigrostriatal function. Our preliminary study results confirm the potential of using 99mTc-TRODAT-1 for DAT measurement, which is clinically important for the staging of PD.
Keywords: Dopamine receptor, labeled tropane derivative, Parkinson's disease, single photon emission computer tomography-computer tomography
Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by the motor features of rigidity, tremors, akinesia, and changes in speech and gait which are associated with the loss of dopaminergic neurons in the substantia nigra and the subsequent deficiency in striatal dopaminergic system. The current diagnosis of idiopathic PD is substantially based on the clinical symptoms as well as a favorable response to levodopa therapy. An easily applicable marker for the diagnosis is still unavailable. Several proposed clinical criteria have been established[1] and the most recent one has classified PD as possible PD, probable PD, and definite PD.[2] For definite PD, the presence of Lewy bodies in the pathologic findings is required. However, Lewy bodies are also present in many other diseases[3] and are probably absent in autosomal recessive juvenile Parkinsonism.[4] The clinicopathologic correlation is not totally satisfactory.[5] For clinical diagnosis and further management, practical laboratory assistance is required. The severity of PD was assessed using the Hoehn and Yahr scale (HYS).[6]
Neuroimaging approaches using 18F-dopa positron emission tomography (PET) have been the gold standard for the functional evaluation of nigral dopaminergic neurons in PD[7] and play a major role in research. Dopamine transporter (DAT) is a protein located on the membrane of the dopamine terminal in the striatum. The reduction of DAT correlates with the loss of dopaminergic neurons in the striatum.[8] Since 99mTc has an optimal energy for imaging, is much cheaper than the other available radionuclides, and is readily available in every nuclear medicine department, 99mTc-labeled ligands would be more suitable for routine imaging. 99mTc labeled tropane derivative (TRODAT-1) is a potential agent for PD patients. More studies have found that it binds with high selectivity to the basal ganglia, especially to the caudate and putamen. Mozley et al. and Huang et al.[9,10] have reported studies of 99mTc-TRODAT-1 single photon emission computer tomography (SPECT) in the diagnosis of PD. They showed the correlation between the clinical manifestations and the uptake of 99mTc-TRODAT-1 in the sub-regions of the basal ganglia. The current study is performed as a preliminary study to test the feasibility of using 99mTc-TRODAT-1 as a tool for evaluating patients with PD and stage the disease.
Materials and Methods
The clinical protocol was approved by the Institutional Ethics Committee in Kovai Medical Center and Hospital. All subjects, including the PD patients and healthy volunteers, gave written informed permission before the SPECT scanning.
Subjects
Sixteen patients with PD were enrolled in this study (9 men, 7 women; Mean (SD) age - 64 (10.4) years). PD was diagnosed according to generally accepted criteria.[11] The severity of PD was assessed using the HYS. In addition, the patients we selected showed response to a single-dose levodopa challenge. Four patients were in HYS Stage I (3 men, 1 woman; mean (standard deviation [SD]) age - 57 (11.9) years), five were in HYS Stage II (1 man, 4 women; mean (SD) age - 65 (10.3) years) and seven were HYS Stage III (5 men, 2 women; mean (SD) age - 67 (9.4) years). Inclusion criteria mandate the presence of at least two of the following signs: resting tremor, rigidity, bradykinesia, or postural reflex impairment, at least one of which had to be either resting tremor or bradykinesia. Specific uptake in the striatum, putamen, and caudate nucleus was measured bilaterally.
Six age-matched healthy volunteers (4 men, 2 women; Mean (SD) age - 62 (12.7) years) served as controls. On the basis of a screening interview, none of the healthy volunteers had a history of neuropsychiatric disorders or a family history of movement disorders. For at least 3 month, none had taken medication known to affect brain function.
Radiopharmaceutical preparation
99mTc-labeled tropane derivative-1 was prepared from a preformulated lyophilized kit. Freeze dried single vial cold kit suitable for preparation of 99mTc-TRODAT at hospital radiopharmacy was developed at Radiopharmaceutical Division, Bhabha Atomic Research Centre BARC, Mumbai, India. Each kit vial contains 100 μg TRODAT-1, 500 μg ethylenediaminetetraacetic acid, 5 mg glucoheptonate and 35 μg SnCl2 in freeze dried form. The kits were tested for sterility and bacterial endotoxin as per pharmacopeia procedures to assure quality and safety of the product. In a standardized radio-labeling procedure, the kit was reconstituted with 2220 MBq (60 mCi) freshly eluted sodium pertechnetate (99mTc-O4−) in 1 mL normal saline solution and was heated at 100°C in a boiling water bath for 40 min. After cooling to room temperature, the radiochemical quality control was carried out using polyamide 6 coated PET strips (Fluka) was used for thin layer chromatography (TLC). Acetonitrile and 5 M 3,3-dimethylglutaric acid buffer at a ratio of 80:20 was used as the mobile phase. The distribution of radioactivity was determined by TLC scanner or NaI (Tl) radioactive detector.
Imaging and data acquisition
After clinical evaluation, patients were brought to the Nuclear Medicine Department. A dose of 740-814 MBq (20-22 mCi) 99mTc-TRODAT-1 in normal saline solution was injected intravenously into each patient soon after the preparation. Patients were examined in the supine position with a head holder to avoid motion. SPECT/computer tomography (CT) images were acquired 3 h later by using a Siemens dual-head-camera, with low energy high-resolution collimators and 64 equally spaced projections over 360°, 30 s/step and using a 128 × 128 matrix size.
Data processing
Attenuation correction was done by a low energy CT. AC data were reconstructed by using Filtered back projection with Butterworth filter, cut-off frequency 0.3, and an order of 10. The slice thickness was 3 mm. For the analysis of striatal 99mTc-TRODAT-1 binding, we selected the slice with the highest activity. Regions were drawn over the whole striatum, putamen, and caudate nucleus of each hemisphere on the slice with the highest basal ganglia activity. The values for the occipital cortex (OC) were also drawn in the same way and served as background areas. The ratios of specific to nonspecific striatal 99mTc-TRODAT-1 binding were then calculated as: 99mTc-TRODAT-1 binding ratio = (ROI counts - OC counts)/OC counts. We also calculated the (putamen - OC)/OC and (caudate - OC)/OC ratio to determine the specific pattern and the selective loss of DATs in the PD group. All images underwent a blind review by a senior nuclear physician.
Statistical methods
Descriptive statistics like mean and standard deviations were calculated for the demographic variable age, both for patients and the healthy volunteers. Mean standardized uptake values were calculated for striatum, caudate nucleus, and putamen. The ratios were calculated by applying the following formula (region counts - OC)/OC. Values for patients and controls were compared. Values are not corrected for multiple comparisons. Data were analyzed using IBM SPSS version 20 (International Business Machines Statistical Package for Social Sciences). Multiple group comparison was carried out using ANOVA and mean difference between the individual variables were compared using Dunnett C post-hoc analysis. Mean difference between the groups thus identified were further identified as significant based on P values, that is, P < 0.05. Mean plots comparing the different groups for each of the location were also provided. Error bars showing the comparison in the mean values between the different groups were also plotted. Median value with 95% confidence limits is depicted in the graphs for each of the stages.
Result
The formulated kit was found to be pharmaceutically pure when tested for sterility and endotoxin. The radio-chemical purity of prepared 99mTc-TRODAT-1 were >90% and remained stable up to 2 h.
The images were interpreted both visually and by semiquantitative analysis. In the dynamic studies, we found that radioactivity accumulated in the basal ganglia area of each subject. On SPECT images, a better contrast of radioactivity between the striatum and adjacent brain tissue was observed in healthy volunteers than in patients.
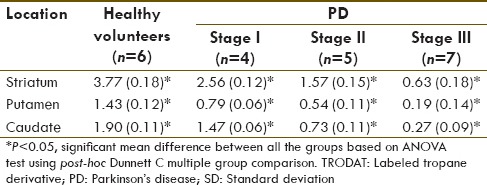
A continuous reduction in specific striatal uptake of 99mTc-TRODAT-1 with increasing disease severity was found in PD patients (healthy normal vs. Stage I vs. Stage II vs. Stage III, 3.77 vs. 2.56 vs. 1.57 vs. 0.63, respectively). Greater loss of uptake occurred in the putamen than in the caudate nucleus, although striatal uptake was bilaterally reduced. The changes were magnified by measurement of specific putaminal uptake (control vs. Stage I vs. Stage II, vs. Stage III, 1.43 vs. 0.79 vs. 0.54 vs. 0.19 respectively, P < 0.05) and specific caudate uptake (control vs. Stage I vs. Stage II, vs. Stage III, 1.90 vs. 1.47 vs. 0.73 vs. 0.27, respectively, P < 0.05) [Table 1].
Table 1.
Specific uptake of 99mTc-TRODAT-1 at basal ganglia comparing the mean (SD) between healthy volunteers and patients with various stages of PD
One could usually distinguish PD patients from healthy subjects by viewing the images. In the control group, the 99mTc-TRODAT-1 binding ratios were symmetric. The 99mTc-TRODAT-1 binding ratios of the PD group were significantly asymmetric. No significant change in mean uptake by the caudate nucleus - either in its entirety or for each side considered separately - was observed between Stage I patients and healthy volunteers, but a significant decrease was seen in putamen between Stage I patients and healthy volunteers. Significant decrease was seen in putamen and caudate between Stage II patients and Stage III patients. No remarkable adverse reactions were found in either healthy volunteers or PD patients during or after imaging. There was no sexual difference in the 99mTc-TRODAT-1 binding ratios in the PD and control groups. Representative images are shown in [Figures 1 and 2].
Figure 1.
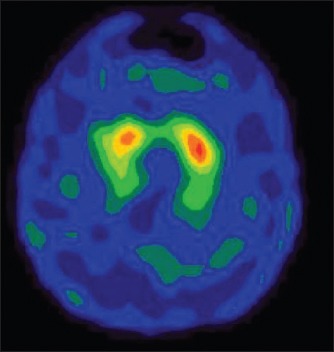
Normal 99mTc-labeled tropane derivative image
Figure 2.
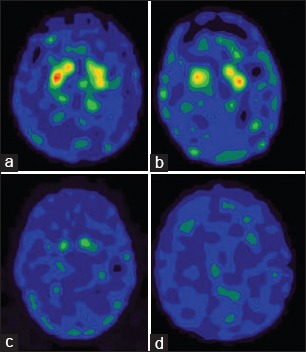
The 99mTc-labeled tropane derivative -1 single photon emission computer tomography images of Stage I (a), Stage II (b), Stage III (c) and Stage IV (d)
The images above clearly state that the specific uptake gradually and sequentially decreased as the disease progresses through Stage I to Stage III and also in comparison to healthy controls. The Confidence limits seen in the error plots do not overlap and gives us an idea that the differences are statistically significant. This statement holds good for all the three locations in the basal ganglia where measurements were taken.
Discussion
We have demonstrated that there was a significant decrease of striatal 99mTc-TRODAT-1 binding in PD patients compared with age-matched healthy controls. In the majority of patients, this distinction is obvious on simple observation of the images. In patients with early-stage PD, a significant loss of striatal uptake of 99mTc-TRODAT-1 at 170-200 min after injection was found using Siemens double head Gamma Camera. Our results support previous observations using SPECT[12,13] and suggest that 99mTc-TRODAT-1 may be an useful agent for DAT imaging to assess early dopaminergic neuron loss. In addition, 99mTc-TRODAT-1 DAT imaging appears to be safe for humans.[14]
The most commonly used SPECT ligands bind to DAT, reduction of which correlates with the loss of presynaptic dopamine. Ligands for DAT include: 123I FP-CIT (N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-[123I] iodophenyl) nortropane, ioflupane, DaTscan™); 99mTc-TRODAT-1[2-[2-[3-(4-chlorophenyl)-8-methyl-8-azabicyclo] 3.2.1]oct-2-yl] methyl](2-mercaptoethyl) amino] ethyl] amino] ethanethiolato (3-)-N2, N20, S2, S20]oxo-[1R-(exo-exo)]; and123I-CIT (2β-carbomethoxy-3β-(4-123I iodophenyl) tropane, iometopane). The first two of these ligands have more rapid binding kinetics, enabling imaging 3-6 h after administration rather than 20-30 h for 123I β-CIT.[15,16]
A comparative study between 123I-FP-beta-CIT and 99mTc-TRODAT-1, assessing their differential diagnostic power in early Parkinsonism and sensitivity for detection of disease progression demonstrated maximal classification accuracy for 123I-FP-beta-CIT of 93% (sensitivity 95% and specificity 86%). For 99mTc-TRODAT-1, the corresponding values were 87% accuracy, 92% sensitivity, and 70% specificity. ant correlation was found (all P > 0.5). In conclusion, both123I-FP-beta-CIT and 99mTc-TRODAT-1 showed high sensitivity in a clinically relevant setting, but FP had superior accuracy for early differential diagnosis of idiopathic Parkinsonism and nondegenerative extrapyramidal disorders, as well as better sensitivity for disease follow-up.[17]
A cross-over study comparing 99mTc-TRODAT-1 SPECT and (18) F-FDOPA PET suggested that 99mTc-TRODAT-1 SPECT may provide a reliable alternative to (18) F-FDOPA PET in the evaluation of clinical PD patients and an acceptable correlation was found between SPECT and PET in both the striatum and the putamen.[18]
High sensitivity and specificity of 99mTc-TRODAT-1 uptake values in discriminating PD patients from healthy subjects has been reported by Mozley et al.[9] These researchers believed that most of the inaccuracy in their study could be accounted for by the hemi-PD patients and the elderly volunteers. Similar to123I β-CIT, the binding capability of 99mTc-TRODAT-1 to the DAT is highly specific. However, 99mTc as a label is far superior to123Ifor routine nuclear medicine use. The striatum-background ratios at 240-280 min showed greater differences between the healthy subjects and PD patients.[19]
It is important that only a 3-h waiting period was required to perform 99mTc-TRODAT-1 SPECT, instead of the 20 h needed for 123I β-CIT SPECT.[19] The shorter time requirement allowed for a 1-d scan session and gave the 99mTc-TRODAT-1 SPECT improved applicability. Both the ease in preparation and the widespread availability of 99mTc-TRODAT-1 are certainly other advantages. Our results support previous findings that have suggested that 99mTc-TRODAT-1 SPECT is useful in detecting the decrease of striatal DAT concentration. We have further affirmed that 99mTc-TRODAT-1 SPECT is a good tool for clinical applications. We used the OC as a reference region because of its low density of DAT. However, it should be noted that 99mTc-TRODAT-1 is not universally available and it has been approved for clinical use only recently in India.
Progress in neuroscience has made evident that nigral dopaminergic projections to the striatum, especially those to the putamen, are targeted in PD, whereas those to the caudate nucleus are relatively spared.[8] Our results agree with previous SPECT and PET studies that showed a correlation between DAT density and disease severity.[20] On the other hand, pathologic evidence has disclosed a more severe depletion of dopamine in the putamen than in the caudate nucleus in PD patients,[12] in contrast to many PD - like disorders, which usually show a more uniform and symmetric striatal loss of dopaminergic activity.[21]
These histopathologic differences provide an assessable approach for the early diagnosis of PD and perhaps can be used to discriminate etiologies of Parkinsonism by measuring specific putaminal uptake, especially in the posterior portion.[22] In this study, the specific putaminal uptake contralateral to symptoms in the Stage I group was also significantly reduced compared with the ipsilateral side and the healthy volunteers. These results agree with recent studies showing that binding of 99mTc-TRODAT-1 to DAT is sensitive to minimal changes in the availability of DAT.[23] The greater decrease of 99mTc-TRODAT-1 uptake in putamen than in the caudate nucleus may enhance the sensitivity of 99mTc-TRODAT-1 imaging for early detection of PD.[14,20]
We chose the OC as a reference region because of the low density of monoamine transporters there and the ability to easily compare data between the striatum and the OC from the same slice. However, controversy revolves around the use of OC or cerebellum as reference regions, because these small regions sometimes have low counting rates.[11] In addition, because 99mTc-TRODAT-1 provides images that are of good quality, have high target-to-nontarget ratios, and show uneven patterns of dopamine loss in PD patients,[24] visual interpretation of the images may be a useful supplement for assessing patient status.
Conclusion
This study showed that 99mTc-TRODAT-1 SPECT is a safe, convenient and reliable tool for measuring DATs and for evaluating and monitoring nigrostriatal degeneration. Although the clinical rating scales and DAT SPECT imaging have some limitations, the complementary measurements of mean ratio of specific striatal-to-nonspecific uptake of 99mTc-TRODAT-1 by SPECT should provide adequate monitoring of disease progression and evaluation of potential neuroprotective effects. Our results confirm the potential of using 99mTc-TRODAT-1 for DAT measurement, and staging the patients, which is clinically important for the early diagnosis of PD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1932;32(Suppl):S125–7.1. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 2.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Gibb WR, Scaravilli F, Michund J. Lewy bodies and subacute sclerosing panencephalitis. J Neurol Neurosurg Psychiatry. 1990;53:710–1. doi: 10.1136/jnnp.53.8.710-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa A, Takahashi H. Clinical and neuropathological aspects of autosomal recessive juvenile parkinsonism. J Neurol. 1998;245:P4–9. doi: 10.1007/pl00007745. [DOI] [PubMed] [Google Scholar]
- 5.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–8. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 6.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 7.Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–55. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson's disease. Neurology. 1996;47:718–26. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- 9.Mozley PD, Acton PD, Barraclough ED, Plössl K, Gur RC, Alavi A, et al. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999;40:1812–7. [PubMed] [Google Scholar]
- 10.Huang WS, Lin SZ, Lin JC, Wey SP, Ting G, Liu RS. Evaluation of early-stage Parkinson's disease with 99mTc-TRODAT-1 imaging. J Nucl Med. 2001;42:1303–8. [PubMed] [Google Scholar]
- 11.Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson's disease. Neurology. 1996;46:231–7. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- 12.Tatsch K, Schwarz J, Mozley PD, Linke R, Pogarell O, Oertel WH, et al. Relationship between clinical features of Parkinson's disease and presynaptic dopamine transporter binding assessed with [123I] IPT and single-photon emission tomography. Eur J Nucl Med. 1997;24:415–21. doi: 10.1007/BF00881814. [DOI] [PubMed] [Google Scholar]
- 13.Brucke T, Asenbaum S, Pozzera A. Dopaminergic nerve cell loss in Parkinson's disease quantified with [123I] b-CIT and SPECT correlates with clinical findings [abstract] Mov Disord. 1994;9(Suppl 1):120P. [Google Scholar]
- 14.Mozley PD, Stubbs JB, Plössl K, Dresel SH, Barraclough ED, Alavi A, et al. Biodistribution and dosimetry of TRODAT-1: A technetium-99m tropane for imaging dopamine transporters. J Nucl Med. 1998;39:2069–76. [PubMed] [Google Scholar]
- 15.Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I] FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med. 2001;28:266–72. doi: 10.1007/s002590000460. [DOI] [PubMed] [Google Scholar]
- 16.Hwang WJ, Yao WJ, Wey SP, Ting G. Reproducibility of 99mTc-TRODAT-1 SPECT measurement of dopamine transporters in Parkinson's disease. J Nucl Med. 2004;45:207–13. [PubMed] [Google Scholar]
- 17.Van Laere K, De Ceuninck L, Dom R, Van den Eynden J, Vanbilloen H, Cleynhens J, et al. Dopamine transporter SPECT using fast kinetic ligands: 123I-FP-beta-CIT versus 99mTc-TRODAT-1. Eur J Nucl Med Mol Imaging. 2004;31:1119–27. doi: 10.1007/s00259-004-1480-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang WS, Chiang YH, Lin JC, Chou YH, Cheng CY, Liu RS. Crossover study of (99m) Tc-TRODAT-1 SPECT and (18) F-FDOPA PET in Parkinson's disease patients. J Nucl Med. 2003;44:999–1005. [PubMed] [Google Scholar]
- 19.Kao PF, Tzen KY, Yen TC, Lu CS, Weng YH, Wey SP, et al. The optimal imaging time for [99Tcm] TRODAT-1/SPET in normal subjects and patients with Parkinson's disease. Nucl Med Commun. 2001;22:151–4. doi: 10.1097/00006231-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Bao SY, Wu JC, Luo WF, Fang P, Liu ZL, Tang J. Imaging of dopamine transporters with technetium-99m TRODAT-1 and single photon emission computed tomography. J Neuroimaging. 2000;10:200–3. doi: 10.1111/jon2000104200. [DOI] [PubMed] [Google Scholar]
- 21.Brooks DJ. Functional imaging in relation to parkinsonian syndromes. J Neurol Sci. 1993;115:1–17. doi: 10.1016/0022-510x(93)90061-3. [DOI] [PubMed] [Google Scholar]
- 22.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 23.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–47. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tedroff J, Ekesbo A, Rydin E, Långström B, Hagberg G. Regulation of dopaminergic activity in early Parkinson's disease. Ann Neurol. 1999;46:359–65. [PubMed] [Google Scholar]


