Abstract
Background
The effect of combined oral contraceptives (COCs) and depot-medroxyprogesterone acetate (DMPA) on the area of cervical ectopy is not well understood.
Study Design
From 1996–1999, we recruited women not using hormonal contraception from two family planning centers in Baltimore, Maryland. Upon study entry and 3, 6 and 12 months after the initial visit, participants were interviewed and received visual cervical examinations with photography. Ectopy was measured from digitized photographs and was analyzed both continuously and categorically (small (≤0.48 cm2) vs. large (>0.48 cm2)).
Results
Of 1,003 enrolled women, 802 returned for at least one follow-up visit. At 12 months, the numbers of women using COCs, DMPA, or no hormonal method at least 50% of the time since the prior visit were 230, 76, and 229 respectively. After multivariable adjustment, COC use (vs. no hormonal use) was associated with large area of ectopy (odds ratio (OR): 1.8, 95% confidence interval (CI) 1.0 to 3.3). No significant relationship was observed between DMPA and large area of ectopy (OR: 0.5, 95% CI: 0.2–1.3). The incidence of large area of ectopy by contraceptive exposure (COC, DMPA, or no hormonal method) was 17.4 (CI: 11.8, 24.6), 10.9 (CI: 4.4, 22.4) and 4.6 (CI: 2.2, 8.4) per 100 woman-years, respectively.
Conclusions
Use of COCs, but not DMPA, was associated with large area of cervical ectopy. Area of ectopy at baseline was the strongest predictor of area of ectopy at follow-up.
Keywords: cervix, ectopy, cervical ectropion, cervical ectopia, hormonal contraceptives, COC, DMPA
1. Introduction
Cervical ectopy, the presence of columnar cells from the endocervix on the ectocervix, is common in young women. Ectopy is present in up to 80% of sexually active adolescents, and prevalence then declines as women enter their 30s and 40s [1]. However, factors associated with the evolution and devolution of cervical ectopy are not well understood. Because ectopy is seen frequently in adolescent girls and pregnant women, it may vary in response to hormonal fluctuations [1]. Increasing hormone levels may trigger intracervical edema, forcing the columnar epithelium from the endocervix downward and out onto the ectocervix [2].
Hormonal contraceptives (HC), including combined oral contraceptives (COCs) and depot-medroxyprogesterone acetate (DMPA), are also suspected of altering area and incidence of cervical ectopy [3–9]. These exogenous hormones may exert different effects on cervical ectopy since COCs contain both estrogen and progestin, whereas DMPA contains progestin alone. A number of studies, primarily cross-sectional, have investigated whether COC or DMPA use is associated with cervical ectopy [3–6, 8–12]. However, there is a paucity of well-measured, prospective data on change in cervical ectopy after the initiation of COCs or DMPA. Prior research also suggests that the presence of cervical ectopy may increase risk of some sexually transmitted infections (STIs), including chlamydial infection [9, 13–18], human papillomavirus infection [19, 20], human immunodeficiency infection [21, 22], and cytomegalovirus infection [23].
Since ectopy may alter susceptibility to infections, understanding the etiologic factors that contribute to ectopy is an important research priority. We designed a large prospective cohort study to examine the effect of initiation of HC on the development and degree of cervical ectopy over a one-year period.
2. Materials and methods
Detailed methods have been published elsewhere [24]. Briefly, from 1996 through 1999, women (n=1,003) seeking reproductive health care but not currently using HC were recruited from two Planned Parenthood centers in Baltimore, MD. One site, in the inner city, served a largely minority clientele; the other was a suburban clinic with a predominantly white, college-aged clientele. Exclusion criteria included age younger than 15 years or older than 45 years; current pregnancy or pregnancy planned for the following year; abortion in the last two weeks; use of HC since the start of the preceding menses; or DMPA injection in the previous 120 days. Women with a history of cervical cancer, hysterectomy, cone biopsy, or cryotherapy were also ineligible.
Institutional review boards at the University of North Carolina Chapel Hill and Family Health International approved the study. The National Medical Division of Planned Parenthood Federation of America also granted approval. All eligible women who wished to participate gave written informed consent.
Participants were interviewed, underwent a pelvic exam, and had follow-up visits at 3, 6, and 12 months after the initial interview. A trained interviewer administered a standardized questionnaire at each visit to capture data on sociodemographic factors and reproductive and sexual behavior, including contraceptive use.
At each visit, participants received a standardized pelvic exam with placement of a speculum and visualization of the cervix. Our ectopy measurement methods have been described in detail previously [25]. A clinician applied 4% acetic acid to the cervix. The clinician placed a vinyl dot (diameter 0.476 cm) on the face of the cervix and took three cervical photographs using a 35-mm Dental Eye II Camera with ring flash (Yashica Inc., Somerset, NJ, USA) and slide film (Ektachrome EPR 64). The photographs were taken at a standardized magnification (1:1.8). The vinyl dot was then removed from the participant’s cervix.
The photographic slides were scanned and digitized using Sigma Scan imaging analysis software (Sigma Scan, Jandel Scientific, San Rafael, CA). A trained independent rater (IY) used a cursor to trace the area of columnar epithelium on the digitized image, measuring the area of ectopy from the computer screen. The rater then traced the diameter of the vinyl dot. The absolute area of ectopy was calibrated by comparing the outlined area of ectopy against the diameter of the vinyl dot. The independent rater also calculated the proportion of the cervix covered by ectopy [25].
2.1 Statistical analyses
The primary outcome of these analyses is the area of cervical ectopy at each follow-up visit. The primary exposure is contraceptive use since the prior visit. We documented the exposure at each visit, on a month-by-month basis, with self-reports of contraceptive use validated against client records and clinic dispensation logs.
We used three different exposure definitions to evaluate the robustness of the contraception-ectopy association. First, we considered “exposed” those women who had used COC or DMPA (analyzed separately) at least 50% of the person-time since the last visit, and “unexposed” were women who had used no hormonal methods since the last visit. Participants who used a method less than 50% of the time or used both COCs and DMPA since the previous visit were excluded. Second, we considered a three-level exposure variable: exclusive COC use, exclusive DMPA use, and no hormonal method use during the 30 days prior to the visit. Women with mixed use in the 30 days before the visit were excluded. Finally, we assessed the effect of consistent COC use (defined as women who reported using only COCs during their entire study participation) vs. no hormonal method use. From this consistent-COC analysis we excluded women with mixed or any DMPA use. We were not able to assess consistent DMPA use because of the small number of women who used DMPA throughout their entire study participation.
In preliminary analyses, we evaluated the outcome – area of ectopy – both as a continuous and categorical measurement. Because a categorical outcome measure would allow for greater comparability to prior studies and because clinicians are more likely to estimate presence and degree of cervical ectopy in a categorical manner, we conducted our primary analyses using the categorical ectopy variable. To insure that our choice of a categorical outcome measure did not strongly influence our results, we also reran the final multivariable models substituting a transformed continuous outcome measure in place of the categorical measure (data not shown).
The absolute area and the proportion of the cervix with ectopy were highly correlated (Pearson’s r = 0.91) [26] with both the categorical and continuous ectopy measures. We chose to report the absolute area of ectopy (categorized) in our multivariable analyses because we believe it offers a more biologically meaningful measure of ectopy exposure compared to the proportion of the cervix affected by ectopy.
The cut-points for the ectopy categories were derived from quartiles of ectopy area for the 1,003 participants, measured at baseline (the smallest quartile, the middle two quartiles collapsed together, and the largest quartile): none/small (<0.09 cm2), medium (0.09–0.48 cm2), and large (> 0.48 cm2). In multivariable modeling, the none/small and medium categories were further collapsed and compared against the outcome of large ectopy for a dichotomous outcome.
We computed descriptive statistics on all participants and compared frequency distributions of selected covariates by inclusion/exclusion in the analysis population (Table 1). We evaluated the association between covariates and categorical and continuous measures of ectopy using chi-square tests and Student’s t-tests [26, 27]. Multivariable logistic regression analysis was conducted using generalized estimating equations (GEE) to accommodate repeated measures and differing intervals between visits [28, 29]. Separate GEE models were run for each of the three exposure variables.
Table 1.
Baseline characteristics of participants included and excluded from the analysis population.*
| Characteristics | Included n=802 (%) | Excluded* n=201 (%) |
|---|---|---|
| Sociodemographic factors | ||
| Age (years) | ||
| 15–17 | 14.6 | 11.9 |
| 18–24 | 50.6 | 52.7 |
| 25–34 | 26.1 | 29.8 |
| 35+ | 8.7 | 5.5 |
| Race | ||
| African-American/Black | 42.9 | 39.3 |
| White | 52.4 | 54.2 |
| Single†,‡ | 77.1 | 69.6 |
| Monthly income <$1000 | 49.1 | 45.7 |
| Site: Inner city †,§ | 48.1 | 59.2 |
| Reproductive health | ||
| Never pregnant | 52.4 | 46.3 |
| Ever sexually transmitted infection | 34.8 | 31.8 |
| 2–5 lifetime sexual partners | 7.9 | 39.7 |
| Coital debut age 15–17 years | 52. 4 | 53.5 |
| Never vaginal douching | 63.8 | 62.5 |
| Ever smoked | 45.8 | 44.0 |
| Area of ectopy†,‡ | ||
| None/Small (<0.09 cm2) | 34.0 | 21.6 |
| Medium (0.09–0.48 cm2) | 46.1 | 59.8 |
| Large (>0.48 cm2) | 17.2 | 18.6 |
| Sexual behavior | ||
| One current sexual partners | 74.3 | 77.5 |
| 5–12 coital acts per month | 30.9 | 37.7 |
| Past contraceptive use | ||
| Ever COCs | 63.3 | 65.8 |
| Ever DMPA | 14.2 | 18.4 |
Women with only one ectopy measurement or who were pregnant for entire study participation were excluded.
p-value for Mantel-Haenzel chi-square test for general association.
p<0.05.
p≤0.01.
We evaluated many potential confounders, including demographic variables (age, site, race, education, income, marital status); reproductive factors (live births, lifetime pregnancies, recency of last pregnancy, baseline ectopy level, prior HC use, douching, age of first menses, days since last menses); sexual behavior variables (age at first intercourse, lifetime sexual partners, coital frequency, recent sexual partners, condom use); and other factors potentially associated with the development of ectopy (e.g., current and previous smoking). By controlling for baseline ectopy level, we hoped to adjust for unmeasured and potentially confounding factors that exerted an effect on ectopy prior to enrollment (and prior to HC exposure).
Because of a priori concerns about confounding, we retained age, parity, and center in all models. Covariates modifying the HC-ectopy relationship were retained in the model (with product interaction terms between that covariate and HC) if the interaction term was found to be statistically significant at α=0.05. Covariates were retained in the final multivariate model as confounders if their removal changed the HC-ectopy relationship by 15% or more. Any variable identified as a confounder using any of the three HC exposure definitions was retained in all models to facilitate comparisons across models.
Data analyses were conducted using SUDAAN (Version 7.5.3) and SAS (Version (9.1)
3. Results
Of 1,003 enrolled participants, 802 (80.0%) are included in this analysis. The most common reason for exclusion from the analysis (n=113 women, 56% of those excluded) was failure to contribute non-pregnant follow-up visits. A comparison of measured characteristics between women included and excluded in the study showed that the two populations were largely similar (Table 1). Significant differences, however, were observed in marital status, center, prior pregnancy, and baseline ectopy level.
Of 802 included participants, 162 (20.2%) completed only one follow-up visit in addition to the baseline visit, 178 (22.2%) completed only two visits, and 462 (57.6%) completed all three follow-up visits. After 3 months, the numbers of women using COCs, DMPA, or no hormonal method at least 50% of the person-time since the baseline visit were 307, 98, and 253, respectively; after 6 months, the numbers of women with at least 50% use were 231, 87, and 218, respectively. After 12 months, the numbers were 230, 76, and 229, respectively.
Participant characteristics by initial contraceptive use have been described elsewhere (24). Briefly, participants using COCs were more likely than DMPA users or controls to be young, white, and nulliparous. Although the proportion of women with multiple sex partners was similar across exposure groups, COC and DMPA users had sex more frequently than control group participants. At baseline, more controls than COC or DMPA users had signs of STI, including abnormal vaginal discharge, a friable cervix, and cervical discharge. However, DMPA users had a somewhat higher baseline prevalence of cervical infections than COC users or controls (24).
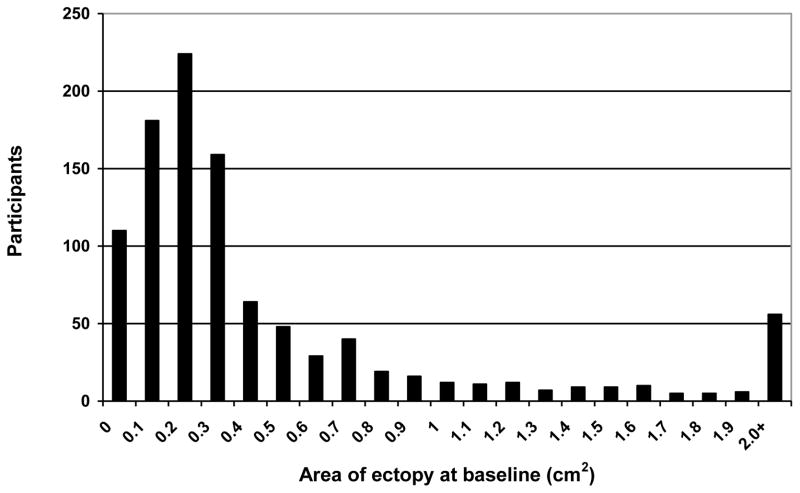
Baseline ectopy measurements were highly skewed with many women having no or very little ectopy but with a smaller number of women having very large area of ectopy (Fig. 1). Overall area of ectopy underwent the most change between the baseline and first follow-up visit (Table 2) with ectopy area diminishing from a mean of 0.49 cm2 at baseline to a mean of 0.36 cm2 after three months. Ectopy area remained relatively stable at subsequent follow-up visits. Among participants that did not have a large area of ectopy at baseline, the overall incidence rate for developing a large area of ectopy at follow-up was 10.4 per 100 women years (95% confidence interval (CI): 7.7, 13.8). When stratified by contraceptive exposure (COC, DMPA, or no hormonal method use at least 50% of the time since the last visit) the incidence rates were 17.4 (95% CI: 11.8, 24.6), 10.9 (CI: 4.4, 22.4) and 4.6 (95% CI: 2.2, 8.4) per 100 woman-years, respectively.
Fig. 1.
Area of cervical ectopy (cm2) at baseline for enrolled participants (n=1,003)
Table 2.
Continuous and categorical measures of cervical ectopy (cm2) and change in cervical ectopy by study visit
| Baseline | 3-month visit | 6-month visit | 12-month visit | |
|---|---|---|---|---|
| Area of cervix with ectopy | ||||
| N | 802 | 682 | 568 | 602 |
| Mean | 0.49 | 0.36 | 0.34 | 0.33 |
| Median | 0.21 | 0.12 | 0.12 | 0.14 |
| Categorized area of cervix | n (%) | n (%) | n (%) | n (%) |
| Small (<0.09 cm2) | 191 (23.8) | 266 (39.0) | 236 (41.5) | 201 (33.4) |
| Medium (0.09–0.48 cm2) | 403 (50.2) | 276 (40.5) | 242 (42.6) | 298 (49.5) |
| Large (>0.48 cm2) | 208 (25.9) | 140 (20.5) | 90 (15.8) | 103 (17.1) |
| Ectopy difference | Change between baseline and month 3 | Change between month 3 and month 6 | Change between month 6 and month 12 |
|---|---|---|---|
| Mean (95% CI)* | −0.13 (−0.17, −0.09) | −0.02 (−0.07, 0.02) | −0.02 (−0.06, 0.03) |
| Median (IQR)* | −0.05 (−0.19, 0.01) | 0.00 (−0.11, 0.07) | 0.00 (−0.08, 0.10) |
| p-value† | <0.01 | 0.06 | 0.27 |
CI: confidence interval; IQR: interquartile range
According to the Wilcoxon signed-rank test
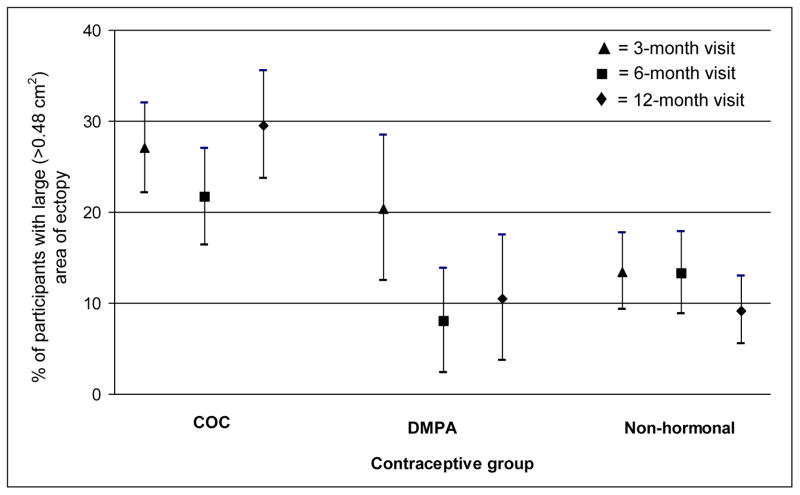
At each follow-up visit, the number of women who used COCs was similar to the number using a non-hormonal method, while the number of participants using DMPA was much lower. COC users were more likely to retain a large area of ectopy across visits compared to DMPA users or women not using hormonal methods, whose prevalence of large ectopy declined somewhat over time (Fig. 2). In addition, at each visit, the proportion of COC users with large area of ectopy was greater than the proportion with small ectopy (odds ratio (OR): 1.1, 95% CI: 1.0, 1.3), while this was not true for controls and DMPA users (data not shown).
Fig. 2.
Prevalence and 95% confidence intervals of large area of cervical ectopy, by follow-up visit and contraceptive group
* Contraceptive group defined as use for at least 50% of person-time since last visit
In bivariable analysis, COC use predicted large area of ectopy during follow-up when compared with women not using HC, regardless of the COC exposure definition used. Our assessment using the exposure of ≥50% COC use (vs. no hormonal use) since the previous visit yielded an OR of 2.9 (95% CI: 2.0, 4.3) for presence of large vs. small area of ectopy. Considering an exposure of exclusive COC use (vs. no hormonal use) in last 30 days, the OR was 2.8 (95% CI: 1.9, 4.0). Using a definition of continuous COC use since study enrollment, the OR for large vs. small area of ectopy was 3.3 (95% CI: 2.0, 5.5). In contrast, DMPA use (whether defined as ≥50% use or exclusive use in the last 30 days) did not predict large area of ectopy. Considering other covariates, large baseline area of ectopy and white race predicted large area of ectopy, while age ≥25 years, having >2 lifetime sexual partners, and previous COC use for more than 1 year were found to be inversely associated with large area of ectopy during follow-up.
In multivariable analysis, COC use again consistently predicted large ectopy, regardless of the exposure definition. However, adjusted ORs for the COC-ectopy association were attenuated compared to the unadjusted analyses. Compared to no hormonal use, multivariable models based on the exposure definitions of exclusive COC use in the 30 days prior to the visit and continuous COC use since study enrollment also found elevated ORs for large vs. small area of ectopy (OR: 1.8, 95% CI: 1.0, 3.1 and OR: 1.7, 95% CI: 1.1, 2.7, respectively) (Table 3). Compared to no hormonal use, no significant relationship between DMPA and large area of ectopy was observed for either DMPA use ≥ 50% of time or exclusive DMPA use in the 30 days prior to the visit. Both of these DMPA exposure definitions yielded OR of 0.5 (95% CI: 0.2, 1.3). Insufficient data were available to assess the effect of continuous DMPA use since study enrollment on large area of ectopy.
Table 3.
Adjusted measures of the effect of hormonal contraceptive (HC) method use vs. non-hormonal method use on large cervical ectopy at follow-up.
| HC use ≥50% since last visit *,‡ | Continual HC use† | |||
|---|---|---|---|---|
|
| ||||
| OR‡ | 95% CI‡ | OR | 95% CI | |
| COC *, †,‡ | 1.8 | 1.0, 3.3 | 1.7 | 1.1, 2.7 |
| DMPA *,‡ | 0.5 | 0.2, 1.3 | N/A | |
| Age ≥ 25 years | 0.4 | 0.2, 0.8 | 0.5 | 0.3, 0.9 |
| Inner city center | 2.1 | 1.2, 3.7 | 2.4 | 1.4, 4.3 |
| >1 live births | 1.2 | 0.6, 2.3 | 1.2 | 0.6, 2.2 |
| Large baseline area of ectopy | 55.1 | 30.9, 98.2 | 47.2 | 26.5, 84.0 |
| White race | 1.8 | 1.0, 3.2 | 2.5 | 1.4, 4.7 |
| Age of menarche >14 years | 0.8 | 0.4, 1.5 | 1.0 | 0.5, 1.7 |
| Always condom use | 0.7 | 0.4, 1.3 | 0.8 | 0.5, 1.4 |
| ≥6 lifetime sex partners | 0.4 | 0.3, 0.7 | 0.4 | 0.2, 0.6 |
| 5–15 days since LMP ‡ | 0.9 | 0.6, 1.5 | 1.0 | 0.6, 1.8 |
| 16–28 days since LMP | 0.8 | 0.5, 1.3 | 1.0 | 0.6, 1.7 |
| Income < $417/month | 1.4 | 0.8, 2.4 | 1.4 | 0.8, 2.3 |
| Age at coital debut <15 years | 1.1 | 0.6, 1.9 | 1.2 | 0.7, 2.1 |
| Previous COC use >12 months | 1.2 | 0.6, 2.1 | 1.3 | 0.7, 2.2 |
| Monthly coital frequency 1–4 acts | 1.0 | 0.6, 1.7 | 0.9 | 0.6, 1.5 |
Categorical hormonal contraception (HC) exposure variables: COC use for ≥50% of person-time since last visit and DMPA use ≥50% of person-time since last visit (referent group is no hormonal use since last visit).
Continuous HC exposure variable: continuous COC use since the study start (referent group is no hormonal use since study start).
HC: hormonal contraception; OR: odds ratio; CI: confidence interval; COC: combined oral contraceptive; DMPA: depot-medroxyprogesterone acetate; LMP: last menstrual period.
Participants using COCs tended to have a larger area of ectopy at baseline. In multivariable analyses, baseline area of ectopy was the strongest predictor of future ectopy (Table 3). White race and parity were also associated with large area of ectopy, while smoking and multiple sexual partners were inversely associated with large area of ectopy (Table 3). Relatively few women had cervical infections during follow-up, and this variable did not measurably confound the HC-ectopy association.
4. Discussion
We conducted this large prospective study to evaluate the effect of initiation of hormonal contraception on the subsequent area of cervical ectopy. We found that after multivariable adjustment, initiation of COC was associated with large area of ectopy. We observed no significant association between initiation of DMPA use and the area of cervical ectopy.
Our findings are consistent with the preponderance of previous literature. Several cross-sectional studies report that COC use was associated with cervical ectopy [3–5, 10]. Two longitudinal studies that examined changes in ectopy over time further support our findings [8, 30]; however, neither of these was designed to evaluate the effect of initiation of HC on area or incidence of ectopy. In contrast, a cross-sectional investigation of adolescents found no effect of COC use on ectopy [6]. Since adolescents are at increased risk of cervical ectopy due to their age, the age-associated risk may overwhelm any additional susceptibility conferred by COC use. Two previous studies had also indicated that DMPA use had no effect on ectopy [6, 31]. Estrogen is thought to stimulate maturation of squamous epithelium. Thus, the estrogen component of COCs may lead to a larger transformation zone (the area where columnar cells are replaced by squamous metaplasia and which contributes to the area of ectopy) [32].
Several important covariates in our analysis have also been previously associated with ectopy. For example, parity was associated with large area of ectopy in our data, similar to previous research [4, 6, 31]. Jacobson et al. [6] note that the effect of parity on ectopy may be due to “an increase in fibromusculature, stromal edema and hyperplasia of columnar epithelium probably resulting from hormonal changes especially during the third trimester [33,34].” The speculum may also open the cervix more widely in parous women [6]. Smoking was associated both in our data and previous research with reduced area of ectopy [3], as was sexual activity [35]. Prior research has suggested that sexual activity increases metaplasia, the process by which squamous epithelium replaces columnar epithelium [35,36].
In order to examine the effect of initiating HC, we controlled for many physiologic variables before enrollment, including baseline area of ectopy. The motivation for this strategy was to adjust for factors that may have influenced area of ectopy prior to study initiation (such as previous contraceptive exposure), and allowed us to better identify variables that predict changes in area of ectopy over time. However, the strongest predictor of future large area of ectopy by a large margin was baseline area of ectopy, suggesting that area of ectopy remains fairly stable over at least a 12-month period.
Our cut-points for small and large ectopy were empirical, based on collapsing the middle two quartiles for ectopy area. Jacobson et al. [6] used a similar method to categorize ectopy, but used tertiles rather than quartiles. Our cut-points resulted in a “large” area of ectopy categorization that was equal to 13% or more of the ectocervix covered by ectopy (when assessing proportion). This area was similar to several studies that used 10% as a cut-point [11,12, 21]. Others used 25% as a cut-point [5, 9], or simply “presence” or “absence” of ectopy [8]. If a threshold for area of ectopy is linked to morbidity, creating a cut-point for ectopy that reflects that threshold would be appropriate for future studies.
We did not randomize participants to different contraceptive methods for both ethical and practical reasons. Participants who self-select different contraceptive methods may differ in both measured and unmeasured factors, which could bias our observed estimates of effect. We attempted to control for such differences through careful measurement of both fixed and time-varying demographic, reproductive and sexual behavior variables; however, unmeasured variables may continue to confound our analysis.
Some participants were lost to follow-up. Of 1003 enrolled participants, 802 provided follow-up data. Fewer women from the inner city health center (compared to the suburban site) and with large area of ectopy are included in these analyses. However, it is unlikely that our results are highly influenced by attrition because the proportion of women using different types of contraception remained fairly consistent across follow-up visits. In addition, more excluded women than participants fell into the ‘medium’ ectopy category at baseline (59.8% v. 46.1%). As a result we may have underestimated the proportion of women with large area of ectopy at follow-up, because there were fewer included women with large ectopy and thus fewer who were poised to shift from ‘medium’ to ‘large’ ectopy classification. However, because the proportion of women with a history of COC or DMPA use was similar, we do not expect the exclusions to affect our observed measures of the effect of contraceptive use on area of ectopy.
Our study has a number of important strengths. We followed changes in ectopy prospectively. We enrolled women who were not currently using HC so were, to some extent, hormonally naïve. We adjusted for prior contraceptive use and baseline ectopy level to control for possible lingering effects from previous contraceptive exposure. We collected a large number of potentially confounding variables that are hypothesized to be associated with the development of ectopy and adjusted for them in multivariable models. We used standardized photographic images to characterize area of ectopy. The robustness of our observed HC-ectopy associations was confirmed by agreement across three different exposure definitions.
COC use was associated with increased incidence of large area of cervical ectopy over time, but no significant relationship was observed between DMPA use and large area of ectopy. However, incidence rates were not adjusted for confounding variables, such as age, which differed across exposure groups.
Nevertheless, although more cervical ectopy has been associated with increased risk of HIV and other STIs, in a well-controlled observational study in a general population of African women, DMPA use but not COC use was associated with increased risk of HIV acquisition [37]. Likewise, in the same US population in which the current analyses were performed, DMPA use, but not COC use, was associated with increased risk of cervical infection [24]. Among high-risk African women, both COC and DMPA use were associated with increased risk of HIV and chlamydial infection [38–41]. Among women in the present study, initial area of ectopy was the strongest predictor of subsequent large area of ectopy. Future studies that examine whether hormonal methods alter HIV/STI risk would benefit from particular focus on longitudinal changes in the area of ectopy as a mediator of infection risk.
Acknowledgments
Funding Support
This study was supported by the US Agency for International Development (USAID) through a Cooperative Agreement with Family Health International (CCP-A-00-95-00022-02) and by the National Institute for Child Health and Human Development (NICHD), through an Interagency Agreement (Y1-HD-0034-01) with USAID.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services or FHI, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling PF, Mårdh P, et al., editors. Sexually Transmitted Diseases. 3. New York: McGraw-Hill; 1999. pp. 407–22. [Google Scholar]
- 2.Anderson MC. A text and atlas of integrated colposcopy. St. Louis: Mosby-Year Book; 1991. [Google Scholar]
- 3.Critchlow CW, Wolner-Hanssen P, Eschenbach DA, et al. Determinants of cervical ectopia and of cervicitis: age, oral contraception, specific cervical infection, smoking, and douching. Am J Obstet Gynecol. 1995;173:534–43. doi: 10.1016/0002-9378(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 4.Goldacre MJ, Loudon N, Watt B, et al. Epidemiology and clinical significance of cervical erosion in women attending a family planning clinic. Br Med J. 1978;1:748–50. doi: 10.1136/bmj.1.6115.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison HR, Costin M, Meder JB, et al. Cervical Chlamydia trachomatis infection in university women: relationship to history, contraception, ectopy, and cervicitis. Am J Obstet Gynecol. 1985;153:244–51. doi: 10.1016/s0002-9378(85)80105-8. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson DL, Peralta L, Farmer M, Graham NM, Wright TC, Zenilman J. Cervical ectopy and the transformation zone measured by computerized planimetry in adolescents. Int J Gynaecol Obstet. 1999;66:7–17. doi: 10.1016/s0020-7292(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson DL, Peralta L, Farmer M, Graham NM, Gaydos C, Zenilman J. Relationship of hormonal contraception and cervical ectopy as measured by computerized planimetry to chlamydial infection in adolescents. Sex Transm Dis. 2000;27:313–9. doi: 10.1097/00007435-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Louv WC, Austin H, Perlman J, Alexander WJ. Oral contraceptive use and the risk of chlamydial and gonococcal infections. Am J Obstet Gynecol. 1989;160:396–402. doi: 10.1016/0002-9378(89)90456-0. [DOI] [PubMed] [Google Scholar]
- 9.Rahm VA, Odlind V, Pettersson R. Chlamydia trachomatis in sexually active teenage girls. Factors related to genital chlamydial infection: a prospective study. Genitourin Med. 1991;67:317–21. doi: 10.1136/sti.67.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mati JK, Hunter DJ, Maggwa BN, Tukei PM. Contraceptive use and the risk of HIV infection in Nairobi, Kenya. Int J Gynaecol Obstet. 1995;48:61–7. doi: 10.1016/0020-7292(94)02214-3. [DOI] [PubMed] [Google Scholar]
- 11.Mauck CK, Callahan MM, Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 12.Moscicki AB, Ma Y, Holland C, Vermund SH. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis. 2001;183:865–70. doi: 10.1086/319261. [DOI] [PubMed] [Google Scholar]
- 13.Richmond SJ, Milne JD, Hilton AL, Caul EO. Antibodies to Chlamydia trachomatis in cervicovaginal secretions: relation to serum antibodies and current chlamydial infection. Sex Transm Dis. 1980;7:11–5. doi: 10.1097/00007435-198001000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lee V, Tobin JM, Foley E. Relationship of cervical ectopy to chlamydia infection in young women. J Fam Plann Reprod Health Care. 2006;32:104–6. doi: 10.1783/147118906776276440. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs GT, Westcott M, Rusden J, et al. The prevalence of Chlamydia trachomatis in a young, sexually-active population. Med J Aust. 1987;147:550–2. [PubMed] [Google Scholar]
- 16.Porras C, Safaeian M, Gonzalez P, et al. Epidemiology of genital Chlamydia trachomatis infection among young women in Costa Rica. Sex Transm Dis. 2008;35:461–8. doi: 10.1097/OLQ.0b013e3181644b4c. [DOI] [PubMed] [Google Scholar]
- 17.Dowe G, Smikle M, King SD, Wynter H, Frederick J, Hylton-Kong T. High prevalence of genital Chlamydia trachomatis infection in women presenting in different clinical settings in Jamaica: implications for control strategies. Sex Transm Infect. 1999;75:412–6. doi: 10.1136/sti.75.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunham RC, Kimani J, Bwayo J, et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996;173:950–6. doi: 10.1093/infdis/173.4.950. [DOI] [PubMed] [Google Scholar]
- 19.Rocha-Zavaleta L, Yescas G, Cruz RM, Cruz-Talonia F. Human papillomavirus infection and cervical ectopy. Int J Gynaecol Obstet. 2004;85:259–66. doi: 10.1016/j.ijgo.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Castle PE, Jeronimo J, Schiffman M, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66:1218–24. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 21.Moss GB, Clemetson D, D’Costa L, et al. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J Infect Dis. 1991;164:588–91. doi: 10.1093/infdis/164.3.588. [DOI] [PubMed] [Google Scholar]
- 22.Plourde PJ, Pepin J, Agoki E, et al. Human immunodeficiency virus type 1 seroconversion in women with genital ulcers. J Infect Dis. 1994;170:313–7. doi: 10.1093/infdis/170.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Collier AC, Handsfield HH, Ashley R, et al. Cervical but not urinary excretion of cytomegalovirus is related to sexual activity and contraceptive practices in sexually active women. J Infect Dis. 1995;171:33–8. doi: 10.1093/infdis/171.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Morrison CS, Bright P, Wong EL, et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis. 2004;31:561–7. doi: 10.1097/01.olq.0000137904.56037.70. [DOI] [PubMed] [Google Scholar]
- 25.Morrison CS, Bright P, Blumenthal PD, et al. Computerized planimetry versus clinical assessment for the measurement of cervical ectopia. Am J Obstet Gynecol. 2001;184:1170–6. doi: 10.1067/mob.2001.113125. [DOI] [PubMed] [Google Scholar]
- 26.Selvin S. Statistical analysis of epidemological data. 3. New York: Oxford University Press; 2004. [Google Scholar]
- 27.Gosset WS. The probable error of a mean. Biometrika. 1908;6:1–25. [Google Scholar]
- 28.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 30.Rahm VA, Gnarpe H, Odlind V. Chlamydia trachomatis among sexually active teenage girls. Lack of correlation between chlamydial infection, history of the patient and clinical signs of infection. Br J Obstet Gynaecol. 1988;95:916–9. doi: 10.1111/j.1471-0528.1988.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn L, Denny L, Pollack AE, Wright TC. Prevalence of visible disruption of cervical epithelium and cervical ectopy in African women using Depo-Provera. Contraception. 1999;59:363–7. doi: 10.1016/s0010-7824(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson DL, Peralta L, Graham NM, Zenilman J. Histologic development of cervical ectopy: relationship to reproductive hormones. Sex Transm Dis. 2000;2:252–8. doi: 10.1097/00007435-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Coppleson M, Reid BL. A colposcopic study of the cervix during pregnancy and the puerperium. J Obstet Gynaecol Br Commonw. 1966;73:575–85. doi: 10.1111/j.1471-0528.1966.tb15536.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferenczy A, Wright TC. Anatomy and histology of the cervix. In: Kurman RJ, Blaustein A, editors. Blaustein’s pathology of the female genital tract. 4. New York: Springer-Verlag; 1994. pp. 185–201. [Google Scholar]
- 35.Gottardi G, Gritti P, Marzi MM, Sideri M. Colposcopic findings in virgin and sexually active teenagers. Obstet Gynecol. 1984;63:613–5. [PubMed] [Google Scholar]
- 36.Moscicki AB, Winkler B, Irwin CE, Jr, Schachter J. Differences in biologic maturation, sexual behavior, and sexually transmitted disease between adolescents with and without cervical intraepithelial neoplasia. J Pediatr. 1989;115:487–93. doi: 10.1016/s0022-3476(89)80863-7. [DOI] [PubMed] [Google Scholar]
- 37.Morrison CS, Chen P, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–81. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–7. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 39.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 40.Lavreys L, Baeten JM, Martin HL, Jr, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS. 2004;18:695–7. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 41.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–9. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]