Abstract
Study Objectives:
To characterize and compare insomnia symptoms within two common phenotypes of Shift Work Disorder.
Design:
Observational laboratory and field study.
Setting:
Hospital sleep center.
Participants:
34 permanent night workers. Subjects were classified by Epworth Sleepiness Scale and Insomnia Severity Index into 3 subgroups: asymptomatic controls, alert insomniacs (AI), and sleepy insomniacs (SI).
Measurements:
Sleep parameters were assessed by sleep diary. Circadian phase was evaluated by dim-light salivary melatonin onset (DLMO). Objective sleepiness was measured using the multiple sleep latency test (MSLT). Brain activity was measured using the N1 event-related potential (ERP). A tandem repeat in PER3 was genotyped from saliva DNA.
Results:
(1) AI group showed normal MSLT scores but elevated N1 amplitudes indicating cortical hyperarousal. (2) SI group showed pathologically low MSLT scores but normal N1 amplitudes. (3) AI and SI groups were not significantly different from one another in circadian phase, while controls were significantly phase-delayed relative to both SWD groups. (4) AI showed significantly longer sleep latencies and lower sleep efficiency than controls during both nocturnal and diurnal sleep. SI significantly differed from controls in nocturnal sleep parameters, but differences during diurnal sleep periods were smaller and not statistically significant. (5) Genotype × phenotype χ2 analysis showed significant differences in the PER3 VNTR: 9 of 10 shift workers reporting sleepiness in a post hoc genetic substudy were found to carry the long tandem repeat on PER3, while 4 of 14 shift workers without excessive sleepiness carried the long allele.
Conclusions:
Our results suggest that the sleepy insomnia phenotype is comprehensively explained by circadian misalignment, while the alert insomnia phenotype resembles an insomnia disorder precipitated by shift work.
Citation:
Gumenyuk V, Belcher R, Drake CL, Roth T. Differential sleep, sleepiness, and neurophysiology in the insomnia phenotypes of shift work disorder. SLEEP 2015;38(1):119–126.
Keywords: insomnia, shift work, event-related brain potentials (ERPs), Insomnia Severity Index (ISI), multiple sleep latency test (MSLT), circadian phase
INTRODUCTION
Shift Work Disorder (SWD) is a circadian rhythm disorder characterized by a chronic mismatch between a shift worker's sleep-wake schedule and his or her circadian clock.1–5 Clinically, this mismatch manifests in insomnia and/or excessive sleepiness (ES). Diagnostic criteria for SWD require the presence of one or both of these symptoms, temporally related to a shift work schedule for at least three months and not better explained by another medical, psychiatric, or sleep disorder.1
Many patients meeting diagnostic criteria for SWD report sleep difficulties consistent with those reported by patients with an insomnia disorder, while others report excessive sleepiness (either alone or in combination with insomnia symptoms).6 Survey studies comparing night shift workers with day workers estimate that 44.8% of night workers score > 10 on the Ep-worth Sleepiness Scale (compared to 32.7% of day workers), and 24.7% score > 13 (compared to 15.5% of day workers); 18.5% of night workers meet DSM-IV criteria for insomnia, while only 8.6% of day workers did.6 In conducting previous studies on SWD,4,5 we have observed that the population of shift workers reporting insomnia can be classified into two subgroups: those reporting insomnia only, and those reporting insomnia alongside excessive sleepiness. We call the corresponding phenotypes “sleepy insomniacs” (SI) and “alert insomniacs” (AI). The phenotype presenting excessive sleepiness without insomnia (“sleepy non-insomniacs” SN) appears to be less common and is not considered in this study (see Methods).
The near-ubiquity of insomnia in shift workers—with or without excessive sleepiness—raises an interesting and important question, since 20% to 25% of the general population also report insomnia.7–9 What, if anything, differentiates the insomnia-only phenotype (AI) of SWD from insomnia in the day-working population? We hypothesize that a subgroup of SWD patients experiences sleep problems primarily caused by circadian misalignment (SI), while another subgroup has a vulnerability to insomnia per se, which is precipitated by the circadian challenge associated with shift work (AI). Accordingly, we hypothesize that AI patients and SI patients will show similar difficulty sleeping during diurnal sleep (work days), but will experience different qualities of nocturnal sleep (days off). Those showing symptoms similar to an insomnia disorder (the AI phenotype) will experience some degree of sleep disruption even while sleeping at night on days off, while those with insomnia and comorbid sleepiness (SI) will sleep well when they have the opportunity to sleep at night. Accounting for their different clinical presentations, we hypothesize that these two insomnia phenotypes seen among patients with SWD may stem from different etiologies: the SI phenotype may be comprehensively traced to circadian misalignment, while the AI phenotype may be more similar to an insomnia disorder per se with the presence of 24-h hyperarousal.10
Further phenotypic differences may exist between SWD patient types. Studies using the multiple sleep latency test (MSLT) for evaluating differences in sleepiness between insomniacs and normal sleepers during a daytime waking period showed that insomnia patients may have sleep latencies similar to (or even higher than) normal sleepers, despite having chronic sleep loss related to insomnia.11–13
Studies using event-related brain potentials (ERP) indicate that insomniacs present an enhanced amplitude of N1 response following auditory stimuli compared to normally sleeping individuals.14–16,17 This is consistent with previous research showing characteristics of cortical hyperarousal in insomnia patients during wake.18,19 Extending this research into the realm of shift work, we test whether the N1 elevation may be present in night workers with one or both insomnia phenotypes of SWD.
This study used electrophysiological measures of sleepiness (MSLT), evoked brain potentials (ERPs), self-reports of sleep parameters, and a circadian biomarker assay (DLMO) to better characterize the two most prominent phenotypes of SWD: alert insomniacs (AI) and sleepy insomniacs (SI). Finally, a post hoc substudy also examined Period 3 tandem repeat distribution20–27 in the phenotypes of SWD and in asymptomatic night workers to test for differential genetic vulnerability to SWD-related insomnia.
METHODS
Subjects
Night workers were recruited from healthcare and industrial settings to participate in an overnight laboratory sleep deprivation study. Subjects were required to have worked exclusively on a night shift for ≥ 6 months (≥ 3 night shifts per week, with each shift lasting 8–12 h and occurring between 19:00 and 08:00) and must not have worked a rotating shift or “picked up” daytime shifts during the 6 months preceding the laboratory study. They were required to have low probability for obstructive sleep apnea, restless leg syndrome, narcolepsy, and psychiatric disorders by providing responses in the normal range on the Berlin Questionnaire and Hamilton Depression Scale, as well as in a clinical interview with a sleep medicine physician. Subjects who had been diagnosed with insomnia or excessive sleepiness prior to beginning their nighttime work shift were excluded. They were required to be free from head injury, hearing problems, and alcohol or substance abuse; must have been nonsmokers; and must not have consumed > 300 mg of caffeine, on average, per day. Finally, they were required to be free from all CNS-acting medication as well as β-adrenergic blocking agents and other drugs known to affect the circadian rhythm, sleep-wake function, or melatonin production. Drugs encountered and disqualified included sertraline, tamoxifen, paroxetine, zolpidem, metoprolol, atenolol, bupropion, alprazolam, venlafaxine, methylphenidate, gabapentin, tramadol, diazepam, triazolam, levocetirizine, buspirone, citalopram, lisdexamfetamine, and trazodone. An exception was made for shift workers with insomnia symptoms who had taken melatonin or sleep-promoting agents while working nighttime shifts but had subsequently discontinued medication and had been free of the drug and all potential metabolites for ≥ 2 weeks prior to the laboratory study.
Ninety-five night workers initially responded to flyers and online newsletter advertisements. Initial advertisements did not specify symptoms of SWD, while subsequent advertisements recruited night workers experiencing “sleepiness or trouble sleeping during the day,” based on recruitment needs. Of the 95 initial respondents, 35 (36.8%) were disqualified based on an online survey; 13 (13.7%) did not comply with pre-screening requests to keep a sleep diary or failed to keep a screening appointment; 5 (5.3%) were disqualified at the screening appointment; 3 (3.2%) were eligible but not enrolled; and 1 (1.1%) dropped out following enrollment. Thirty-eight subjects (40.0%) qualified based on inclusion criteria. Excluding one subject released for noncompliance, 37 subjects completed the study (35.41 ± 9.35 years, 62.2% female).
At the screening appointment, 26 of 37 qualifying subjects (70.2%) were diagnosed with SWD upon a clinical interview with a sleep medicine physician. The remaining 11 (29.7%) were asymptomatic (controls).
Sleep Parameters
All subjects completed a sleep diary for 2 weeks prior to the overnight laboratory assessment. This assessment queried bed time, rise time, sleep latency, number of awakenings, and total time spent awake after sleep onset. From these reported variables, subjective time in bed (TIB) and total sleep time (TST) were calculated, as well as sleep efficiency (TST divided by TIB).
Laboratory Procedures
The laboratory study was scheduled to begin on an evening after the subject had worked ≥ 3 consecutive night shifts. They were instructed to sleep during the day before coming to the laboratory, as if sleeping before a subsequent night shift. On the study day, they arrived at 16:00 and were housed in a dimly lit (< 15 lux), sound-attenuated private bedroom and bathroom that they would occupy for 25 hours. Subjects were aware of clock time. They were not permitted to sleep at any point during their entire visit, and were required to remain out of bed except for nap trials during the multiple sleep latency test (see below). Internet access, television, and cellular phone usage were permitted during unscheduled time, and three meals were provided at the subject's request, in addition to snacks and water. Meals did not contain foods or drinks known to affect sleep or circadian rhythms such as caffeine, ethanol, milk, bananas, or tomatoes. Subjects were also prohibited from consuming crunchy foods that may injure the gums and permit blood contamination of the saliva samples (see below). Registered polysomnographic technologists and research associates continuously monitored subjects for wakefulness throughout the study.
Questionnaires
At 18:00, each subject completed an Epworth Sleepiness Scale (ESS) and 2 separate Insomnia Severity Index (ISI) questionnaires. Items on the separate ISIs were identical, but both verbal and written instructions prompted subjects to separately evaluate their diurnal sleep on one questionnaire (ISI-D) and nocturnal sleep on the other (ISI-N).
Phenotype Classification
Twenty-six subjects with SWD were classified into 3 phenotypes using scores from the ESS and ISI as well as a diagnostic interview with a sleep medicine physician. Twelve subjects presented pathologic scores (≥ 10) on the ISI-D, ISI-N, or both, while scoring in the normal range (< 10) on the ESS. These subjects were classified “alert insomniacs” (AI). Within this subgroup, 11 subjects (91.7%) were flagged for pathologic scores on the ISI-D, and 8 (66.7%) were flagged for pathologic scores on the ISI-N.
Eleven subjects with elevated scores on one or both ISIs as well as the ESS were classified into the SI group (42.3%). Within this subgroup, 10 subjects (90.9%) were flagged for pathologic scores on the ISI-D and 4 (36.4%) were flagged for pathologic scores on the ISI-N. Eleven controls showed normal scores on the ESS and ISI, corroborating the diagnostic interview at the screening appointment. Finally, 3 subjects showed pathologic sleepiness (ESS ≥ 10) but normal scores on both insomnia indices (ISI-D and ISI-N < 10). These subjects were classified “sleepy non-insomniacs” (SN) and are excluded from these analyses, as they do not represent an insomnia phenotype.
Circadian Phase Assessment
Beginning at 17:00, saliva samples were collected at 30-min intervals using a Salivette tube with a cotton insert (Sarstedt Group, Numbrecht, Germany). Prior to 17:00, subjects were instructed on the procedure of saliva collection and the amount of saliva required for each sample. Each sample was weighted to ensure that ≥ 1 mL of saliva was provided. Saliva was then extracted from the cotton insert in a frozen centrifuge, and samples were frozen at -20°C until being shipped over dry ice to SolidPhase, Inc. (Portland, Maine, USA), where they were radio-immunoassayed. The intra-assay precision was 2.6% to 20.1% with functional sensitivity of 0.9 pg/mL and analytical sensitivity of 0.2 pg/mL.
Dim light melatonin onset (DLMO) is an individual's characteristic point in clock time marking the onset of elevated melatonin levels that normally coincide with the timing of the sleep period.28–30 DLMO was calculated as the time that the amplitude of the fitted LOWESS curve for melatonin concentration rose and remained above a subject's melatonin threshold for ≥ 1 hour. The threshold used was the average of the 5 lowest concentrations of melatonin during the 24-h phase assessment, plus 15% of the average of the 5 highest concentrations. DLMOoff was calculated as the time at which the LOWESS curve amplitude fell and remained below the individual's threshold for ≥ 2 hours.4,5,31
Objective and Subjective Sleepiness Assessment
Objective sleepiness was assessed with nocturnal/diurnal MSLT. Eight naps were conducted at 2-h intervals from 22:30 to 12:30, following standard research protocol. Electrode integrity was checked by physical inspection and electrical bio-calibration before each nap. The first 5 naps (22:30 to 06:30) coincided with night shift hours, while the last 3 (08:30 to 12:30) evaluated the effects of acute sleep deprivation. In this report we are presenting the results of a 5-nap MSLT collected between 22:30 and 06:30. Subjective sleepiness was assessed by the Epworth Sleepiness Scale at 18:00.
N1 Event-Related Brain Potential
The typical oddball paradigm consisting of 3 types of sounds was used for passive auditory processing while subjects were watching a self-chosen muted film with subtitles. Seventy percent of the sounds were simple tones (duration = 100 ms, frequency 800 Hz) that served as standards. Ten percent of the sounds were simple tones with deviant frequencies (duration = 100 ms, frequency of 1000 Hz), and another 10% of the sounds were simple tones with deviant durations (duration = 150 ms, frequency of 800 Hz). The final 10% of sounds were novel tones, with timbre and tone distinct from the simple tones; these novel sounds were not considered in our analysis. All sounds were presented through earplugs binaurally at a 75 dB SPL (sound pressure level) with 5 ms rise/fall time. All stimuli were presented at a constant interstimulus interval of 800 ms. Each session lasted 7.3 min. A total of 2 sessions with a short inter-session break (2 min) were presented to each subject. During this task, subjects were instructed to ignore the sounds and focus on the movie. The clock time of the task was between 18:00 and 19:00 for each subject, a time in the 24-h cycle that occurs at neither the nadir for well-adjusted night workers nor the nadir for unadjusted night workers.5 It is, thus, a “neutral zone” in the circadian cycles of our subjects, and timing the ERP in this window permitted the examination of cognitive differences shortly after waking without the confounding effects of peak sleepiness or peak alertness in the circadian melatonin profile.
EEG Recording and ERP Data Analysis
EEG data were recorded via a 32-channel EEG cap (10-20 system, Easy Cap, Gilching, Germany) and an ASA system (ANT, the Netherlands). Electrooculogram (EOG) was recorded by 2 electrodes at the left and right canthus and 2 electrodes above and below the left eye of the subject. Impedances were kept < 10 kΩ, and a band-pass filter was set from 0.1 to 100 Hz. The sampling rate was 1024 Hz.
Data were analyzed off-line using Brain Vision Analyzer software (Brain Products GmbH, Gilching, Germany). ERP data were separately analyzed for standard and deviant tones. Each segment began 100 ms prior to the stimulus onset and continued for 400 ms after the stimulus onset. A band-pass filter from 0.1 to 30 Hz was applied to segmented data. Segments in which the EEG or EOG exceeded ± 75 microvolts were excluded from the average. ERPs in response to standard, deviant-frequency and deviant-duration stimuli were averaged separately. Baseline correction (100 ms pre-stimulus interval) was applied to the averaged data. On average, ≥ 300 trials for the standard tone and ≥ 100 trials for the deviant tones were included for each subject.
The obligatory auditory N1 waveform was identified as the largest negative wave at latency 80 to 130 ms from sound onset in each individual grand average corresponding to each type of stimulus (standard and deviant). The peak amplitude and latency of the N1 were measured within an 80–100 ms time window from sound onset at the FCz and Cz electrodes, as these are typically shown in studies to present the largest N1 amplitude.
Genotyping
Twenty-four randomly selected subjects (63%) from our sample were asked to provide a saliva DNA sample for a post hoc substudy of the tandem repeat on PER3, a gene on the first human chromosome that is expressed in the suprachiasmatic nucleus on a circadian rhythm and known to be associated with characteristic sleepiness and morningness/eveningness preference.20–27 Participating subjects deposited 2 mL of saliva into an Oragene OG-500 collection kit. Genomic DNA was extracted and genotyped for a variable number tandem repeat on PER3. This VNTR has 2 alleles: a 4-repeat (short) allele, and a 5-repeat (long) allele. Following a dominant genetic analysis model, individuals heterozygous (PER34/5) and homozygous (PER35/5) for the long allele are considered together for comparison with homozygotes of the more common short allele (PER34/4). Preparation and analysis of the samples was performed by the Applied Genomics Technology Center at Wayne State University (Detroit, MI).
All elements of this protocol were approved by the Institutional Review Board of Henry Ford Health System. The principal investigator or a research associate fully explained the procedures and tests to subjects, all of whom provided informed consent. Subjects were compensated for their participation.
Statistical Analyses
The primary aim in this study was to evaluate the ERP, sleep, and circadian phase differences between the two most common phenotypes of Shift Work Disorder (AI and SI) relative to night-working asymptomatic controls. To this end, we compared objective and subjective measures between the AI and SI phenotypes and compared attributes of each phenotype to the asymptomatic night-workers (controls). Comparisons were made in assessments related to the diagnostic classification of the disorder (MSLT for objective sleepiness and separated day/night Insomnia Severity Indices); the hypothesized circadian etiology of the disorder (circadian biomarker—dim light melatonin onset [DLMO]); neurophysiology (N1 standard tone response and deviant tone response); and measures of habitual sleep (sleep diary). Phenotypic differences were analyzed using one-way ANOVA and two-tailed Student's t-tests where appropriate. Linear modeling was used to examine the differential contributions of SWD symptoms (insomnia and sleepiness) to neurophysiological findings.
The evaluation of a variable number tandem repeat in the sleepiness-related PER3 gene was performed in a subsample of 24 subjects (35.88 ± 9.16 years, 18 female). Frequency differences by SWD phenotype were compared using likelihood ratio χ2.
RESULTS
Sleep
Self-reported measures of sleep latency, total sleep time, and number of awakenings, as well as sleep efficiency, were compared among the three groups (see Table 1). The AI group had significantly longer sleep latency and lower sleep efficiency than controls during both nocturnal and diurnal sleep. The SI group differed from controls in sleep efficiency, total sleep time, and number of awakenings during daytime sleep, while differences were smaller during nocturnal sleep periods and only statistically significant in the case of wake time. ISI-D scores were substantially and significantly different from controls in both the AI and SI groups, in both cases representing insomnia in the pathological range. On the ISI-N, however, only the AI group showed scores statistically different from controls and in the pathological range. The mean score of the SI group was < 10 and did not reach statistical significance when compared with controls. Despite differences between the two phenotypes, both groups showed suboptimal quality and duration of sleep during both daytime and nighttime (total sleep times < 7 h and sleep efficiencies below 90%).
Table 1.
Self-reported sleep parameters by SWD phenotype (mean ± SD).
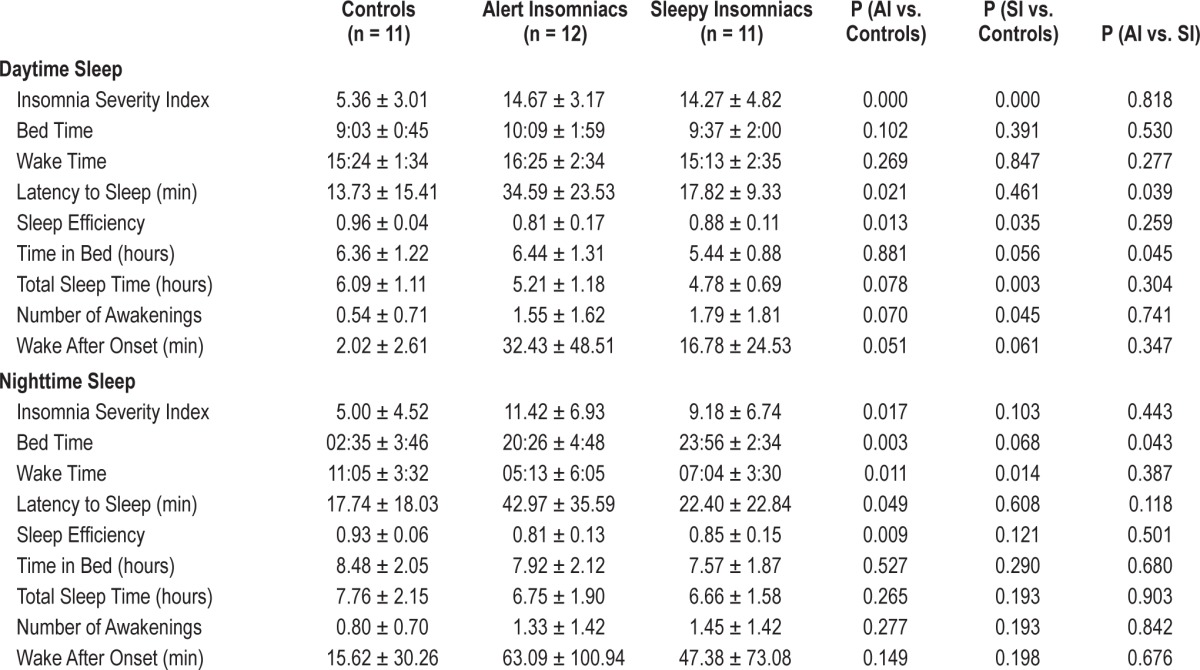
To assess whether perceptions of insomnia severity were related to reported sleep disturbances, we examined correlations between the Insomnia Severity Indices and sleep diary parameters (latency to sleep, sleep efficiency, total sleep time, and wake after sleep onset). The ISI-D significantly correlated with daytime wake after sleep onset in the SI group (r = 0.726, P = 0.011) but not in the AI group (r = −0.337, P = 0.284) or in controls (r = 0.269, P = 0.424). No other daytime sleep parameters were significantly correlated with the ISI,-D nor were any of the nighttime sleep parameters significantly correlated with the ISI-N.
Circadian Phase
One-way analysis of variance showed a significantly different DLMO time as a function of group (F2,31 = 10.90, P < 0.001). Controls presented the most delayed DLMO of the three groups (05:00 ± 3:31), while AI and SI largely remained in a daytime phase (22:25 ± 5:04 and 20:33 ± 4:38, respectively), suggesting that asymptomatic night workers made a greater circadian adjustment to shift work than their symptomatic counterparts. Post hoc two-sample t-tests show DLMO in both SWD phenotypes was significantly different from controls (P < 0.01), and that the two SWD groups were not significantly different from one another. DLMOoff was also significantly different in analysis of variance among the three groups (P = 0.01): controls had the latest DLMOoff (16:05 ± 3:23), followed by the AI (10:17 ± 5:13) and SI (08:05 ± 4:57) groups. Figure 1 presents individual DLMO times separated by phenotype.
Figure 1.
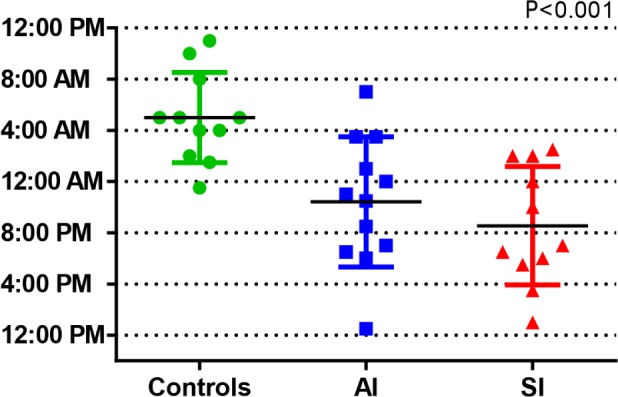
DLMO times by SWD phenotype, individual and mean ± SD.
Sleepiness
A comparison of mean nocturnal MSLT latencies showed a significant main effect of group (F2,31 = 5.39, P = 0.01). Post hoc analyses showed that mean (± SD) MSLT latencies were highest in controls (8.14 ± 3.58 min). AI patients were not significantly different from controls (7.85 ± 5.13 min), while SI patients had significantly lower mean latencies than either of the other groups (3.12 ± 3.01 min, P < 0.01). All five individual nap times in the SI group were significantly lower than controls (P < 0.05). This difference was largest at the 00:30 nap (mean difference = 8.41 min) and smallest at the 06:30 nap (mean difference = 2.00 min). Sleepy insomniacs also had a significantly lower MSLT latency than alert insomniacs at the 22:30, 00:30, and 04:30 naps (mean difference = 6, 22; 5, 14, and 5.81 min respectively, P < 0.05). AI did not significantly differ from controls overall or on any of the naps (see Figure 2).
Figure 2.
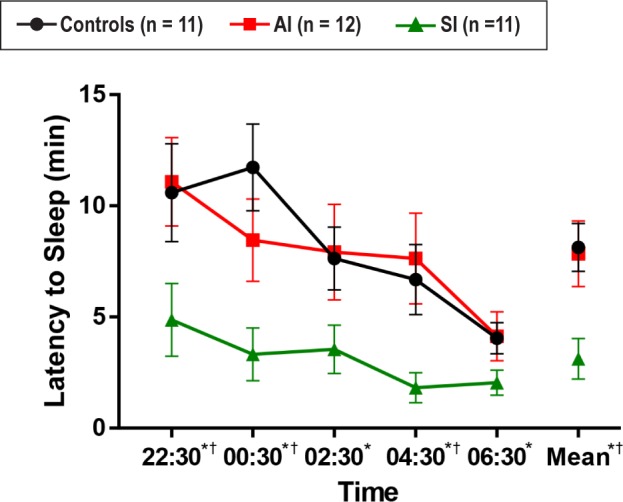
MSLT scores by SWD phenotype (mean latency ± SD). * SI vs. Controls, P < 0.05. † SI vs. AI, P < 0.05. AI vs. Controls, all n/s.
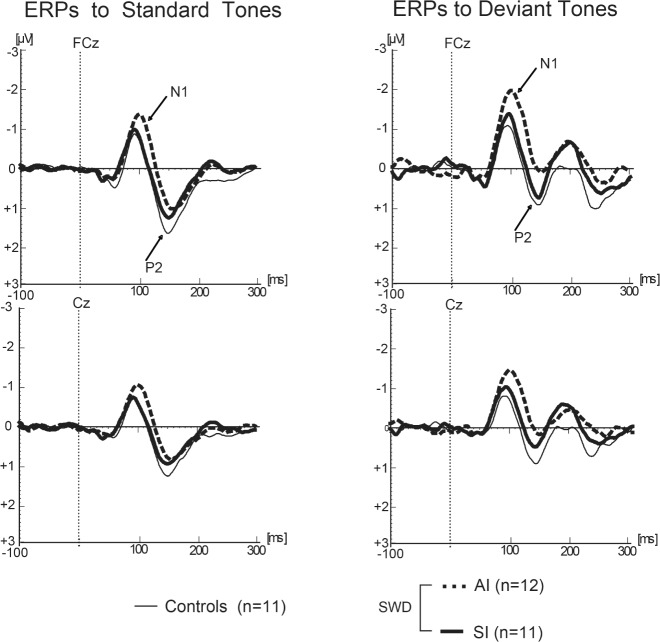
N1 Evoked Response Potential
Figure 3 represents the results of each group's averaged ERPs to standard and deviant tones. Comparison of peak N1 amplitudes showed a significant main effect of group when elicited by standard tones (F2,31 = 5.843, P < 0.01) as well as by deviant tones (F2,31 = 6.139; P < 0.01). Post hoc independent samples t-tests revealed that the SI group was not significantly different from controls (Mean ± SD: −0.93 μV ± 0.64 μV vs. −0.89 μV ± 0.49 μV for the standard tone, and −1.58 μV ± 0.81 μV vs. −1.30 μV ± 0.76 μV for the deviant tone, respectively). However, N1 responses in the AI group were significantly enhanced with respect to both the control and sleepy groups (−1.53 μV ± 0.36 μV for the standard tone, and −2.38 μV ± 0.79 μV for the deviant tone, both P < 0.01 with respect to controls, and P < 0.05 with respect to sleepy insomniacs). These data suggest that alert insomniacs, but not sleepy insomniacs, show cortical hyperarousal.
Figure 3.
N1 waveforms in response to auditory stimuli.
Isolated Consideration of Sleepy Insomniacs
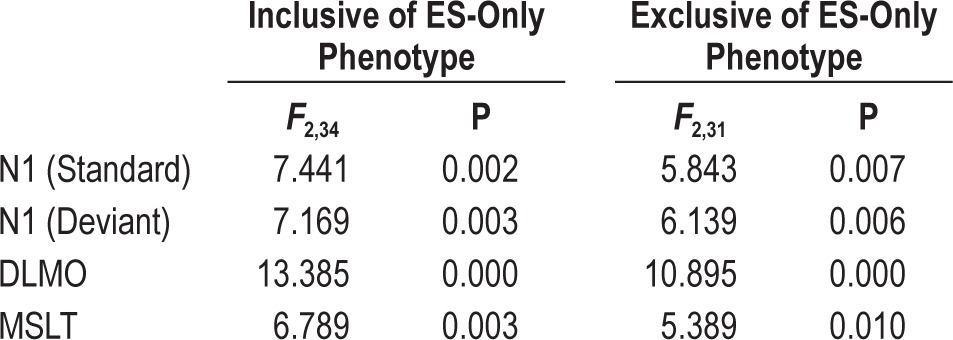
As described in the Methods section, our primary endpoint analyses considered sleepy insomniacs (n = 11) and excluded 3 subjects who showed excessive sleepiness without insomnia (ESS ≥ 10, ISI-D < 10 and ISI-N < 10). We considered them not directly relevant to the aim of this study, which focused on characterizing the differential insomnia phenotypes. To ensure that the exclusion of the three ES-only subjects without insomnia did not affect our results, we ran secondary analyses for all primary endpoints (DLMO; MSLT; N1 standard tone; and N1 deviant tone) with an inclusive regrouping which added the ES-only subjects to the sleepy insomniac group. Accordingly, these recategorized groups can be thought of as controls (n = 11), alert SWD patients (n = 12), and sleepy SWD patients (n = 14). As presented in Table 2, the exclusion of these 3 subjects did not alter any of our results.
Table 2.
Comparison of ANOVA results including and excluding subjects with ES-only phenotype.
Genetics
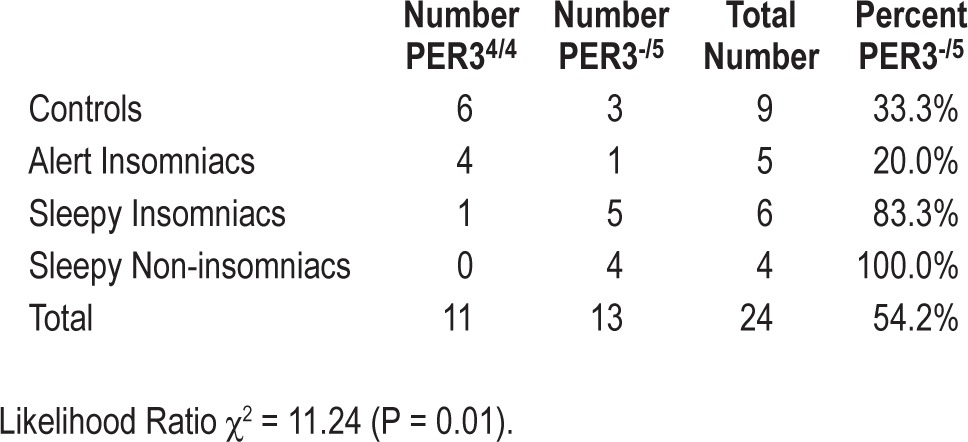
A post hoc genetic substudy was performed to test the allelic frequencies of the PER3 gene in 3 study groups. Although this analysis was only performed on a random subsample of 24 subjects, our preliminary evidence identified significantly different polymorphism distributions in the 3 groups (likelihood ratio χ2 = 11.24, P = 0.01). Three of 9 controls (33%) and 1 of 5 AI (20%) were found to carry the long (5-repeat) variant of the PER3 VNTR. However, 5 of 6 SI (83.3%) presented the long allele, as did 4 of 4 subjects with ES alone (100%). These frequencies are presented in Table 3. Note that one ES-only subject included in the genetic substudy was not included in the larger laboratory protocol due to noncompliance.
Table 3.
Allelic frequencies of period 3 polymorphism by SWD phenotype.
DISCUSSION
Our data suggest that the two insomnia phenotypes of Shift Work Disorder are different from one another in many clinically relevant and etiologically informative respects. These differences are most pronounced on the MSLT and the N1 ERP. Taken together, our results suggest that the AI phenotype is likely an insomnia disorder per se precipitated by shift work, while the SI phenotype is more consistent with the circadian misalignment typically attributed to SWD.
The N1 brain response represents a relatively early sensory processing function, and can be affected by exogenous (parameters of the stimulus) and endogenous (attention focusing) factors.17 Electrophysiological data from our ERP analysis showed cortical hyperarousal in the AI group very similar to that observed in day-working insomniacs in other studies.15,16 The enhanced amplitude of N1 in the AI group with respect to SI and controls suggests two inferences: either individuals in the AI group were physiologically hyperaroused to sounds during the task, or they were less able than the other groups to ignore distracting sounds despite instructions to do so. In this and other studies, insomnia patients demonstrated robustly enhanced N1 amplitudes compared to normal sleepers as well as to sleepy patients. Interestingly, insomnia comorbid with other conditions may also be associated with an increased N1 response. Ahveninen and colleagues found that the first week after alcohol withdrawal in chronic alcoholics was associated with augmentation of the N1 response to unattended tones.32 It was also correlated with impaired memory performance in these patients with respect to controls. Alcohol withdrawal results in neural hyperexcitability caused by reduced GABAergic inhibitory transmission and increased glutamatergic excitatory transmission.33 Therefore, alcohol withdrawal is also associated with sleep disturbance and insomnia, as reflected by an augmented N1 amplitude.
Since our study excluded subjects with insomnia prior to shift work, it appears that the night shift may be a powerful precipitant of insomnia in vulnerable individuals. On the other hand, the SI phenotype showed symptoms which are temporally associated with shift work and may reasonably be attributed to circadian misalignment. The sleep disturbances experienced by these sleepy shift workers occur predominantly when trying to sleep during the day, as the SI group showed comparatively normal nocturnal sleep which was not statistically distinguishable from controls in our sample. Based on their MSLT results, the SI group also demonstrated high sleep pressure (in contrast to hyperarousal in the AI group). The SI group's normal N1, normal nocturnal sleep, normal relationship between perceived insomnia on the ISI and reported sleep disturbances on a sleep diary, and high sleep propensity on the MSLT suggest that this phenotype has little in common with an insomnia disorder. The AI phenotype, on the other hand, showed hyperarousal (enhanced N1), pathological scores on both the ISI-D and ISI-N, and no significant correlation between perceived insomnia and reported sleep disturbance—all characteristics resembling a primary insomnia disorder.
Previous literature has suggested that circadian misalignment is etiologically responsible for the symptoms of Shift Work Disorder. In this study, we did observe that controls tend to be more phase-delayed than subjects with SWD. In addition, the circadian phases of the two SWD phenotypes were not significantly different from one another. More research is clearly needed to identify common impediments to physiological adjustment so we may better parse out the reasons that some shift workers remain asymptomatic while others suffer on the night shift. However, our data add nuance to the proposed role of circadian misalignment in SWD, since we characterized two clinically distinct phenotypes, both of which are misaligned. We encourage future research on the clinical treatment of insomnia within SWD, since the AI phenotype may benefit from insomnia management beyond the circadian alignment typically advised for symptomatic shift workers. As one example, CBT-I has been shown to have some benefit for insomnia symptoms within SWD.34
Our genetic substudy offers preliminary evidence of a genomic predisposition to sleepiness within SWD. Alert insomniacs and controls were much less likely to carry the long polymorphism of PER3 (28.6%), while sleepy insomniacs and sleepy non-insomniacs were much more likely to carry it (90.0%). Consistent with other research linking the long polymorphism to a faster rate of sleep propensity accumulation and greater sleepiness at the circadian nadir,23 this study suggests that the phenotypes of SWD presenting excessive sleepiness may share a genetic predisposition, while asymptomatic controls and SWD patients presenting the phenotype without sleepiness are more likely to be immune from this genetic vulnerability.
This study has a few notable limitations. First, we excluded shift workers who consumed large amounts of caffeine, smoked, had high pretest probability for sleep apnea or psychiatric conditions, or used hypnotics or other medications likely to affect the sleep-wake cycle. While this allowed a more precise understanding of the insomnia phenotypes of Shift Work Disorder, it did not permit us to take into consideration the sleep, sleepiness, or neurophysiological effects of these highly prevalent environmental or physiological influences. Thus, we offer the caveat that our study pertains specifically to SWD and not to a population of shift workers in a more general sense.
Second, the genetic substudy was not performed on the entire sample. Although results indicate a high level of statistical significance, we feel that further research is warranted to confirm and better characterize our findings of discrepant geno-types among different phenotypes of SWD. Additionally, this study does not consider an objective measure of sleep (e.g., PSG). Further studies are needed to objectively evaluate sleep across SWD phenotype and determine with higher resolution the specific sleep characteristics of the AI and SI phenotypes. It would be interesting and important to understand whether AI or SI patients show overestimations of sleep latency and underestimations of total sleep time, which can be objectively determined by PSG.35–37
Another limitation of our study is the lack of evaluation of performance differences in night workers with the AI vs. SI types of SWD. One study showed that among day-working subjects, insomnia lowered performance across 24 hours regardless of the type and parameters of the task with respect to healthy controls, despite similar circadian phase between groups,38 although other studies have failed to demonstrate performance differences (for review see Fortier-Brochu et al.39). In our study, SWD patients have circadian phases similar to day-active individuals and evaluating the impact of any interaction between circadian mismatch and insomnia/sleepiness on work or occupational performance would be an important priority.
A final limitation is that our laboratory study was only carried out once for each subject. It is, therefore, not possible to determine whether circadian phase, MSLT, or ERP differences between phenotypes are stable or variable across multiple nights (e.g., depending on whether testing occurred following subsequent work shifts, as in this study, or after subsequent days off).
In summary, our findings clearly demonstrate that patients with SWD who suffer from apparent insomnia should be more carefully diagnosed and treated, since the insomnia symptoms in SWD are not a homogeneous problem and may be related to distinct physiological phenotypes.
CONCLUSION
We interpret these findings as evidence for heterogeneity in the insomnia associated with Shift Work Disorder. Differences in sleep, neurophysiology, circadian phase, and sleepiness suggest a differential etiology between the two insomnia phenotypes: SI patients show symptoms likely related to circadian misalignment, while AI patients appear to have symptoms consistent with an insomnia disorder, perhaps precipitated by the circadian insult of shift work. We recommend that further research on SWD take into account the different symptoms, and perhaps different treatments, of these distinct phenotypes.
DISCLOSURE STATEMENT
This was not an industry supported study. This study is supported by grant K01OH009996-03 from CDC/NIOSH. Dr. Drake has served as a consultant or received financial support from Merck, Teva, Purdue, and Jazz. Dr. Roth has served as a consultant for Abbott, Arcadia, AstraZeneca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, GlaxoSmithKline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon, and Transcept. He has received research support from Cephalon, Merck, Transcept, Speakers Bureau, Purdue, Proctor and Gamble. The other authors have indicated no financial conflicts of interest. This study does not involve off-label or investigational use of a drug.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the hard work of Ryan Howard, Laura Spear, Mike Gable, Jennifer Philport, Elaine Douglas, Brian King, and Drs. Niraj Parikh, Rami Abboud, Chirag Popat, Victor Gordon, Venkatesh Basappa-Krishnamurthy, and Narayan Neupane.
REFERENCES
- 1.American Academy of Sleep Medicine. 3rd edition. Darien, IL: American Academy of Sleep Medicine; 2014. International Classification of Sleep Disorders. [Google Scholar]
- 2.Wright KP, Jr., Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 4.Gumenyuk V, Roth T, Drake CL. Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int. 2012;29:928–36. doi: 10.3109/07420528.2012.699356. [DOI] [PubMed] [Google Scholar]
- 5.Gumenyuk V, Howard R, Roth T, Korzyukov O, Drake CL. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep. 2014;37:545–56. doi: 10.5665/sleep.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 7.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5(Suppl 1):S23–30. doi: 10.1016/s1389-9457(04)90004-4. [DOI] [PubMed] [Google Scholar]
- 9.Eldevik MF, Flo E, Moen BE, Pallesen S, Bjorvatn B. Insomnia, excessive sleepiness, excessive fatigue, anxiety, depression and shift work disorder in nurses having less than 11 hours in between shifts. PloS One. 2013;8:e70882. doi: 10.1371/journal.pone.0070882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. 24—Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 11.Stepanski E, Lamphere J, Badia P, Zorick F, Roth T. Sleep fragmentation and daytime sleepiness. Sleep. 1984;7:18–26. doi: 10.1093/sleep/7.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Activity, arousal, and the MSLT in patients with insomnia. Sleep. 2000;23:205–12. [PubMed] [Google Scholar]
- 13.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turcotte I, St-Jean G, Bastien CH. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int J Psychophysiol. 2011;81:177–90. doi: 10.1016/j.ijpsycho.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Bastien CH. Insomnia: neurophysiological and neuropsychological approaches. Neuropsychol Rev. 2011;21:22–40. doi: 10.1007/s11065-011-9160-3. [DOI] [PubMed] [Google Scholar]
- 17.Kertesz RS, Cote KA. Event-related potentials during the transition to sleep for individuals with sleep-onset insomnia. Behav Sleep Med. 2011;9:68–85. doi: 10.1080/15402002.2011.557989. [DOI] [PubMed] [Google Scholar]
- 18.Wu YM, Pietrone R, Cashmere JD, et al. EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9:1031–7. doi: 10.5664/jcsm.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 20.Viola AU, Chellappa SL, Archer SN, et al. Interindividual differences in circadian rhythmicity and sleep homeostasis in older people: effect of a PER3 polymorphism. Neurobiol Aging. 2012;33:1010, e17–27. doi: 10.1016/j.neurobiolaging.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 22.Maire M, Reichert CF, Gabel V, et al. Sleep ability mediates individual differences in the vulnerability to sleep loss: evidence from a PER3 polymorphism. Cortex. 2014;52C:47–59. doi: 10.1016/j.cortex.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PloS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chellappa SL, Viola AU, Schmidt C, et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–7. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- 25.Barclay NL, Eley TC, Mill J, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:681–90. doi: 10.1002/ajmg.b.31210. [DOI] [PubMed] [Google Scholar]
- 26.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31:608–17. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 28.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 29.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 30.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–65. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 32.Ahveninen J, Jaaskelainen IP, Pekkonen E, et al. Global field power of auditory N1 correlates with impaired verbal-memory performance in human alcoholics. Neurosci Lett. 2000;285:131–4. doi: 10.1016/s0304-3940(00)01041-7. [DOI] [PubMed] [Google Scholar]
- 33.Becker HC. Alcohol withdrawal: neuroadaptation and sensitization. CNS Spect. 1999;4:38–65. [Google Scholar]
- 34.Jarnefelt H, Lagerstedt R, Kajaste S, Sallinen M, Savolainen A, Hublin C. Cognitive behavioral therapy for shift workers with chronic insomnia. Sleep Med. 2012;13:1238–46. doi: 10.1016/j.sleep.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 36.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–9. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 37.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 38.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 39.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]