Abstract
Study Objectives:
Hypoglossal motoneurons were recorded intracellularly to determine whether postsynaptic inhibition or disfacilitation was responsible for atonia of the lingual muscles during rapid eye movement (REM) sleep.
Design:
Intracellular records were obtained of the action potentials and subthreshold membrane potential activity of antidromically identified hypoglossal motoneurons in cats during wakefulness, nonrapid eye movement (NREM) sleep, and REM sleep. A cuff electrode was placed around the hypoglossal nerve to antidromically activate hypoglossal motoneurons. The state-dependent changes in membrane potential, spontaneous discharge, postsynaptic potentials, and rheobase of hypoglossal motoneurons were determined.
Analyses and Results:
During quiet wakefulness and NREM sleep, hypoglossal motoneurons exhibited spontaneous repetitive discharge. In the transition from NREM sleep to REM sleep, repetitive discharge ceased and the membrane potential began to hyperpolarize; maximal hyperpolarization (10.5 mV) persisted throughout REM sleep. During REM sleep there was a significant increase in rheobase, which was accompanied by barrages of large-amplitude inhibitory postsynaptic potentials (IPSPs), which were reversed following the intracellular injection of chloride ions. The latter result indicates that they were mediated by glycine; IPSPs were not present during wakefulness or NREM sleep.
Conclusions:
We conclude that hypoglossal motoneurons are postsynaptically inhibited during naturally occurring REM sleep; no evidence of disfacilitation was observed. The data also indicate that glycine receptor-mediated postsynaptic inhibition of hypoglossal motoneurons is crucial in promoting atonia of the lingual muscles during REM sleep.
Citation:
Fung SJ, Chase MH. Postsynaptic inhibition of hypoglossal motoneurons produces atonia of the genioglossal muscle during rapid eye movement sleep. SLEEP 2015;38(1):139–146.
Keywords: atonia, hypoglossal, IPSP, motoneuron, OSA, postsynaptic inhibition, REM sleep
INTRODUCTION
Patency of the upper airway, which is essential to maintain ventilatory processes during wakefulness as well as nonrapid eye movement (NREM) and rapid eye movement (REM) sleep, is dependent on the degree of contraction, i.e., tone, of the genioglossus muscle. In turn, the genioglossal muscle is activated by hypoglossal motoneurons.1–4 In pathological conditions such as obstructive sleep apnea (OSA), episodic periods of apnea/hypopnea occur during sleep, most frequently during REM sleep. Patients with OSA are therefore subjected to recurrent episodes of hypoxia that can occur hundreds of time each night.4 In an attempt to elucidate the synaptic mechanisms that control the activity of hypoglossal motoneurons during naturally occurring REM sleep, we obtained intracellular records from hypoglossal motoneurons to directly measure their changes in membrane excitability. Knowledge of the state-dependent changes in excitability of hypoglossal motoneurons will not only to advance our understanding of the normal state-dependent control of hypoglossal motor processes but also serve as a foundation for determining and manipulating those processes that result in pathological conditions such as OSA.
Previously, human5 and animal studies6–8 of the state-dependent activity of hypoglossal motoneurons have used extracellular techniques to record the activity of the hypoglossal nerve or genioglossal muscle in conjunction with the ejection of neurotransmitter agonists and antagonists into the brainstem in the region of the hypoglossal nucleus. These studies were not designed to examine the underlying subthreshold and suprathreshold processes that determine the intrinsic excitability of hypoglossal (and other) motoneurons. Therefore, to obtain direct measures of the factors that are responsible for changes in hypoglossal motoneuron excitability during naturally occurring states of sleep and wakefulness, and specifically REM sleep, we used intracellular recording techniques in intact, un-anesthetized cats. We monitored directly the crucial membrane potential events (both suprathreshold and subthreshold) that are responsible for regulating the state-dependent discharge of hypoglossal motoneurons and the tone of the genioglossal muscles.
METHODS
Five adult male cats (3.5–4.5 kg) were used in a previous study of field potential activity,9 in addition to being used for intracellular recordings that were the subject of the current report. All animals were in good health and approved for research by veterinarians of the UCLA Department of Laboratory Animal Medicine. Experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011). Appropriate procedures were conducted to minimize pain, discomfort, or stress of the animals and only the minimum number of animals was used that was necessary to yield reliable scientific data.
Surgical Procedures
Details of the surgical procedures that were used to obtain electrophysiological data from hypoglossal motoneurons in the intact, unanesthetized cat and for monitoring electroencephalographic (EEG), electro-oculographic (EOG), pontogeniculo-occipital (PGO) and electromyographic (EMG) activity have been previously reported.9–11 Briefly, during isoflurane anesthesia, screw electrodes were placed in the calvarium to monitor EEG activity. A small hole (1 mm in diameter) was drilled in the caudal parietal bone to provide access for a microelectrode to the hypoglossal motor nucleus. A pair of screw electrodes was placed in the infraorbital region of the frontal bone to record EOG activity; flexible wire electrodes were inserted into the neck muscles to monitor EMG activity. A silicone-based stimulating cuff electrode9,12 was implanted (via a submandibular approach) around the trunk of the hypoglossal nerve, proximal to the bifurcation into its medial and lateral nerve branches to antidromically identify hypoglossal motoneurons. The leads from the cuff electrode were tunneled subcutaneously to the dorsal occipital bone, where they were externalized and bonded with acrylic resin to a head implant. All electrode leads were connected to a female Winchester plug, which was also bonded to the calvarium with acrylic resin. A head-restraining device was cemented to the acrylic mound for maintaining, without pain or discomfort, stereotaxic alignment of the head of the animal during recording sessions. Following a recovery period of 10 to 14 days, the animals were habituated daily (4 to 9 days; 2 to 4 h per day) to a stereotaxic apparatus (Kopf 880 head-holder, David Kopf Instruments, Tujunga, CA). Experiments (recording) were conducted when the animals exhibited normal amounts of and transitions between wakefulness and NREM and REM sleep states.13
Stimulation and Recording Procedures
Field potential recordings were obtained from each animal in order to localize the optimal site to record antidromically activated motoneurons.9 Subsequently, microelectrode penetrations were aligned subtentorially at 10–12° (posterior-to-anterior) to pass through the cerebellum to access the hypoglossal nucleus (stereotaxic coordinates: P12 to 14, L0.5 to 1.0, H-6.5).14 Intracellular recordings from hypoglossal motoneurons were obtained using glass microelectrodes filled with a solution of either 3 M KCl (tip resistance = 10–20 MΩ) or 2 M K-citrate (tip resistance = 20–35 MΩ). A reference (ground) silver wire electrode was placed, acutely, in contact with tissue surrounding the access hole during recording sessions. Intracellular current injections were carried out via recording microelectrodes using an Axoclamp 2A preamplifier (Axon Instruments, Inc., Union City, CA) operating in current clamp mode. Polysomnographic and intracellular signals (10x low-gain and 100x high-gain) were simultaneously monitored on a chart recorder (Grass Instruments, Middleton, WI) and oscilloscope, respectively, and were stored on a video cassette recorder using a pulse code modulation (PCM) multiplex adapter.
Data Analysis
Intracellular recordings were digitized off-line at 10 μs and 50 μs bin widths to examine action potentials and other moto-neuron membrane properties, respectively. Axograph software was used for measuring the magnitude of the digitized intra-cellular data (action potentials, membrane potentials, inhibitory postsynaptic potentials [IPSPs]) as described previously.9,12,15 Hypoglossal motoneurons were identified by antidromic activation by the ipsilateral hypoglossal nerve via the cuff electrode (single rectangular pulses, 0.05 ms pulse-width, 0.25 Hz frequency, 2–7 V intensity). The antidromic spike amplitude was measured from the baseline membrane potential to the peak of the spike.16 The membrane potential was obtained by subtracting the steady DC voltage drop of the cell from the extracellular voltage registered upon withdrawal of the electrode from the cell.17 For the analysis of the waveform and frequency of IPSPs, only cells recorded with citrate-filled microelectrodes were used because of the chloride-dependent nature of these potentials.18 To examine the ion dependency of the IPSP, chloridefilled microelectrodes were used. Accordingly, chloride ions were injected into the impaled cell by passive diffusion from the recording electrode or by passing steady hyperpolarizing currents (up to 5 nA). The rheobase of the cell was assessed by determining the threshold current necessary to produce an action potential in at least 90% of the trials with long (20–50 msec) depolarizing current pulses at a repetition rate of 1/sec.16
A paired two-tailed Student t-test was used for statistical analysis of changes in the membrane potential during NREM compared with REM sleep. Because rheobase data were collected from individual motoneurons during either NREM or REM sleep, the difference in these values was analyzed using the unpaired two-tailed Student t-test. Statistical significance was set at P < 0.05; data were expressed as means ± standard error of the mean.
RESULTS
Intracellular data were obtained from motoneurons (n = 28) that were antidromically identified following stimulation of the ipsilateral trunk of the hypoglossal nerve. Only data from cells that were healthy and undamaged from electrode placement were included in this study. All cells had antidromic spike amplitudes ranging from 60–91 mV (mean = 72.4 ± 1.4 mV) and stable potentials free from signs of membrane deterioration and/ or injury discharge. The mean duration for which recordings were obtained was: 4.0 ± 0.6 min (range = 0.5–10.2 min; n = 23 cells) and 2.1 ± 0.4 min (range = 0.6–6.3 min; n = 15 cells) for NREM and REM sleep, respectively. Cells with membrane potentials of -60.8 mV and -55.4 mV were recorded only during wakefulness for approximately 3 min. For the entire pool of motoneurons that were recorded during NREM or REM sleep states (n = 26), the mean membrane potential during NREM sleep was -58 ± 0.7 mV, compared to -68.6 ± 1.1 mV during REM sleep.
Figure 1 is a recording that was obtained during wakefulness from a hypoglossal motoneuron that was positively identified by antidromic activation. Following antidromic invasion, a representative full-sized (somadendritic) action potential with an overshoot spike potential was observed (spike size = 91 mV, antidromic latency = 1.1 msec, membrane potential = -60.8 mV; Figure 1A and 1B). In addition, this and other antidromic spikes were typically followed by a prolonged after-hyperpolarizing potential (AHP; duration = 63.6 msec, amplitude = 6.4 mV; Figure 1B).
Figure 1.
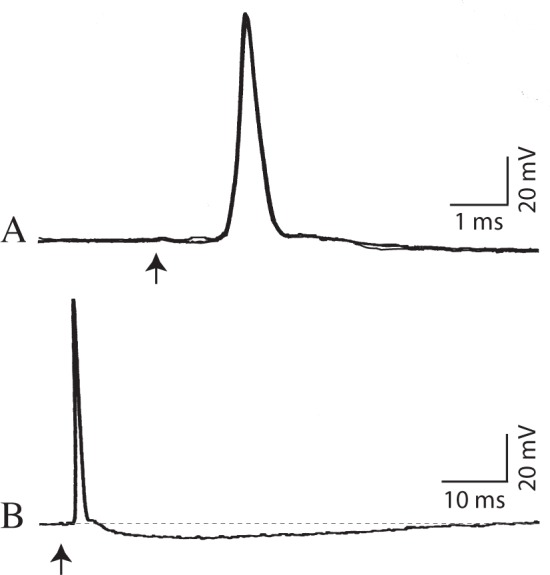
Positive identification of an intracellularly recorded hypoglossal motoneuron. (A) Superimposed traces of three consecutive trials of antidromic activation of the motoneruron. This representative hypoglossal motoneuron exhibited antidromically evoked action potentials with a constant latency following stimulation of the ipsilateral hypoglossal nerve (arrows) during naturally occurring wakefulness. (B) An example of a postspike after-hyperpolarizing potential is presented with an expanded time base. Hypoglossal nerve stimulus: 3.4 V (arrowheads) (A,B).
All intracellular data were collected during multiple recording sessions over a period of 2–4 w. Of the 28 motoneurons that were recorded, a subgroup of 12 hypoglossal motoneurons (spike size = 70.8 ± 2.3 mV, range = 60–82 mV) were selected for analysis of the membrane potential during naturally occurring NREM sleep, the transition to REM sleep, and during the subsequent period of REM sleep. The remaining cells were excluded from the analysis of hyperpolarization because they were only recorded during a single state (wakefulness, n = 2; NREM sleep, n = 11; REM sleep, n = 3).
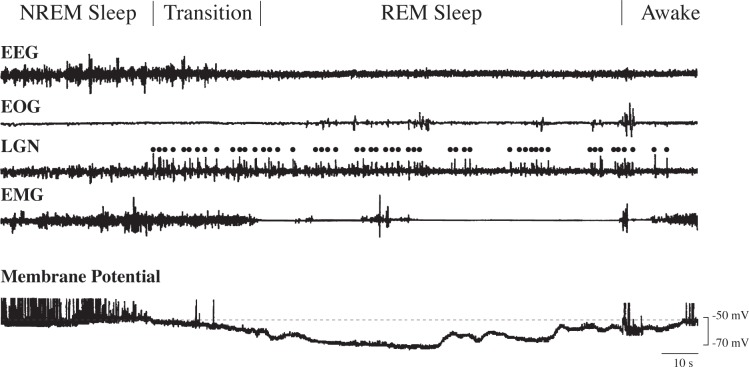
For those cells exhibiting state changes (n = 12), recordings within and across animals revealed that the membrane potential of hypoglossal motoneurons exhibited a consistent pattern of state-dependent activity, as shown in Figure 2. The transition from NREM to REM sleep began with the presence of PGO waves, which were correlated with a decrease in the amplitude of EMG activity, the cessation of slow EEG waves, and the occurrence of low voltage/fast EEG activity. The spontaneous discharge of hypoglossal motoneurons terminated at the onset of this transition period. The end of the transition phase was determined by the cessation of EMG activity, as shown in Figure 2. The membrane potential of the representative motoneuron (-52 mV during NREM sleep) as shown in Figure 2 began to hyperpolarize at the beginning of the transition phase (that lasted for approximately 30 sec). Hyperpolarization continued until the membrane potential reached a maximum of -71 mV, which occurred 50 sec after the onset of REM sleep (Figure 2). This cell exhibited a maximal hyperpolarization of 19 mV during REM sleep.
Figure 2.
Hypoglossal motoneuron hyperpolarization during the transition from nonrapid eye movement (NREM) to rapid eye movement (REM) sleep and throughout REM sleep. During the transition phase, only sporadic firing occurred. In contrast, during REM sleep, discharge ceased. The membrane potential (dashed line) returned to the same level as during NREM sleep when the animal awoke. Action potentials are truncated. PGO waves are indicated by dots above the LGN trace. EEG, electroencephalogram; EMG, electromyogram; EOG, electro-oculogram; LGN, lateral geniculate nucleus; PGO, pontogeniculo-occipital.
For the pool of 12 motoneurons with stable records during NREM and REM sleep, similar to that shown in Figure 2, the mean value of the membrane potential was -57.5 ± 0.9 mV during NREM sleep (range = -52 to -64 mV), compared to -68 ± 1.3 mV (range = -60 to -74 mV) during REM sleep. Quantitatively, there was maximal hyperpolarization of 10.5 ± 1.4 mV (range = 4–19 mV) during REM sleep. The REM sleep related membrane hyperpolarization, compared with the level of polarization during NREM sleep, was statistically significant (P < 0.0001, degrees of freedom = 11, t-value = 7.383, n = 12).
Spontaneous action potentials were observed in seven of the 12 hypoglossal motoneurons (58%) that were recorded during NREM sleep: these cells either ceased firing completely (n = 4) or exhibited sporadic discharge during the succeeding period of REM sleep (n = 3; Figure 3). Phasic discharge consisted of one or several spikes that occurred in conjunction with episodes of threshold depolarization that were superimposed on the tonic hyperpolarization that was present throughout REM sleep. Other phasic events (such as myoclonic twitches and eye movements) occurred in conjunction with the phasic periods of hypoglossal motoneuron depolarization. Of the seven cells that exhibited spontaneous discharges during NREM sleep, the mean firing frequency was 10.9 Hz (range = 7.5 to 15.6 Hz); three of these cells discharged intermittently during REM sleep at a mean frequency of 0.17 Hz (range = 0.02 to 0.26 Hz). The remaining five motoneurons (42%) did not exhibit spontaneous activity (spiking) throughout the entire period of NREM and REM sleep.
Figure 3.
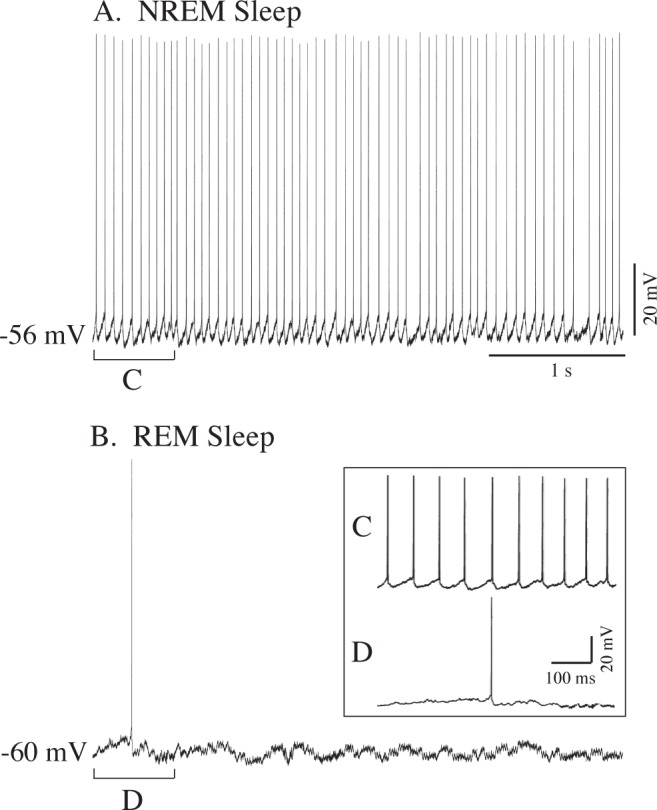
Example of the spontaneous discharge of a hypoglossal motoneuron during NREM sleep (A). At the onset of REM sleep, hyperpolarization occurred and discharge ceased, except momentarily in conjunction with REM sleep related phasic events (B) 60 sec intervened between panels A and B. Selected segments of traces in A and B as marked by brackets C and D are illustrated in the inset at an expanded time sweep.
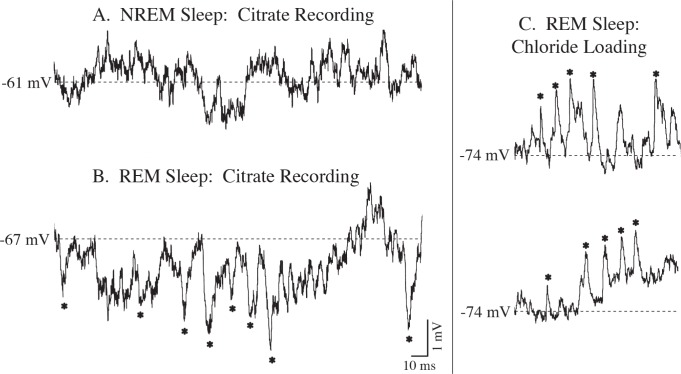
Prominent subthreshold membrane activity arose at the onset of the transition from NREM to REM sleep. Specifically, large-amplitude IPSPs (up to 2.5 mV), which began to appear during the transition phase, persisted throughout REM sleep (Figure 4B). The frequency of these IPSPs was 38.9 Hz and 46.5 Hz for the representative motoneurons shown in Figure 4B and 4C, respectively. REM sleep-specific IPSPs were present in eight motoneurons with membrane potentials that ranged from -60 to -69 mV (Figure 4B). The four additional motoneurons (whose membrane potentials ranged from -73 to -74 mV) that were recorded during REM sleep were subjected to chloride ion loading; in these cells polarity of the REM sleep-specific IPSPs was reversed (Figure 4C). The three motoneurons that did not exhibit IPSPs had membrane potentials that ranged from -71 to -72 mV, which is near or at the reversal potential of chlo-ride ions. In contrast to REM sleep, IPSPs did not occur during NREM (Figure 4A) or wakefulness (not shown) in spite of the relatively depolarized level of the membrane potential during these states (see Discussion).
Figure 4.
Spontaneous, large-amplitude inhibitory postsynaptic potentials (IPSPs, recorded using a potassium citrate-filled microelectrode) were present only during the nonrapid eye movement (NREM)-rapid eye movement (REM) sleep transition and throughout REM sleep (asterisks) (B). They were absent during NREM sleep (A). When chloride ions were injected by steady current (-3.9 nA), the REM sleep-specific IPSPs were reversed in polarity (asterisks) (C). A and B are records from a representative motoneuron during NREM and REM sleep, respectively; both top and bottom traces in C are records from another motoneuron that was recorded with a chloride-filled electrode.
The rheobase current of six motoneurons during NREM sleep and another three during REM sleep was quantified to determine this direct measure of the intrinsic membrane excitability of hypoglossal motoneuron during these states. Figure 5 presents examples of the marked increase in rheobase during REM sleep (2 nA, Figure 5B) compared to that recorded during NREM sleep (0.3 nA, Figure 5A). The mean rheobase was 0.65 ± 0.20 nA (range = 0.3–1.5 nA, n = 6) during NREM sleep, compared to 2.40 ± 0.89 nA (range = 1.1–4.1 nA, n = 3) recorded during REM sleep. This difference in rheobase between NREM and REM sleep was statistically significant (P = 0.03, degree of freedom = 7, t-value = 2.68, n = 9).
Figure 5.
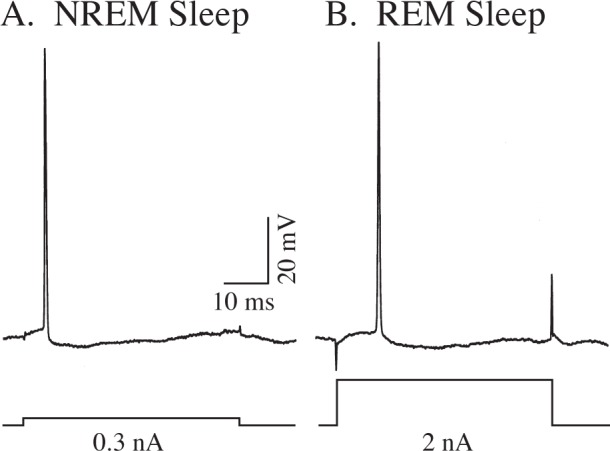
Examples of the increase in rheobase for motoneurons recorded during nonrapid eye movement (NREM) sleep (rheobase = 0.3 nA) (A) compared to that during rapid eye movement (REM) sleep (rheobase = 2 nA) (B). Top trace, intracellular potentials; bottom trace, current monitor.
DISCUSSION
This is the first description of the spontaneous changes in the membrane potential activity of hypoglossal motoneurons, which were documented by recording intracellularly during naturally occurring states of sleep and wakefulness. In addition, a coherent set of intracellularly documented state-dependent changes in subthreshold and suprathreshold patterns of hypoglossal motor control is presented. The data reveal that the normal state-dependent mechanisms that control hypoglossal motor activity during NREM and REM sleep are comparable to those that modulate the membrane potential of other brainstem as well as spinal cord motoneurons.10,19,20 Specifically, we postulate that during REM sleep, hypoglossal and other motoneurons are subjected to glycinergic postsynaptic inhibition, which constitutes the crucial mechanism that is responsible for atonia of the muscles that are innervated by these motoneurons (discussed in the next paragraphs). We are not aware of any previous intracellular studies (in any species) that have examined the synaptic control of hypoglossal motoneurons during sleep and wakefulness. All other intracellular experiments dealing with hypoglossal motoneurons have used current-clamp or whole-cell voltage-clamp techniques under acute conditions, i.e., either in anesthetized or decerebrate animals12,21–28 or have been conducted in slice preparations.2,29,30
In the current experiment, we examined the following intracellularly recorded properties of the membrane potential of hypoglossal neurons: membrane polarization, frequency of discharge, rheobase, and postsynaptic potentials. Determinations were made of the changes in these membrane potential parameters that were temporally and functionally correlated with variations in the animals' state of sleep and wakefulness. We determined that hypoglossal motoneurons exhibit a significant increase in hyperpolarization during REM sleep (10.5 mV) based on their membrane potential level during NREM. The degree of hyperpolarization during REM sleep compared to that present during NREM sleep was similar to that reported for digastric (11 mV),19 trigeminal (approximately 10 mV),10,31 and spinal motoneurons (8–9 mV)20,32,33 in comparable experimental paradigms during naturally occurring NREM and REM sleep.
The cessation of spontaneous discharge during the transition from NREM to REM sleep was anticipated because hyperpolarization shifted the membrane potential further from its “threshold” voltage and, in addition, there was a potent current shunt generated by IPSPs that were present during this state.12,27,34 Our determination of state-related changes in hypoglossal motoneuron discharge mirrors the pattern of activity that has been reported for EMG activity of the genioglossus muscle in humans,5 except for the fact that there was no evidence of respiratory-related activity of hypoglossal motoneurons in this and another study.35 Hypoglossal motoneurons are the final common pathway for execution of the volitional and reflexive motor function that are task dependent according to specific conditions of behavioral states.36 In addition, factors such as posture,37 vestibular stimuli,38 vagotomy,8 and metabolic stress (hypoxia/hypercapnia)21 also influence the degree to which individual motoneurons participate in respiratory and/ or nonrespiratory functions.36 The spontaneous discharge recorded in seven of 12 hypoglossal motoneurons (58%) during NREM sleep is consistent with another intracellular study that reported that 53% of the hypoglossal motoneurons that were recorded were nonrespiratory modulated.25 Our data are also in agreement with human findings that a majority (38 of 51, or 75%) of motor units of the genioglossus muscle exhibit tonic discharges that are nonrespiratory related during NREM sleep.39 Furthermore, spontaneous discharge, as illustrated in Figure 3, was different from the patterns of respiratory-modulated discharge described in animals28 and humans.39
In conjunction with membrane hyperpolarization, a significant increase in the rheobase of hypoglossal motoneurons during REM sleep was observed in the current report as well as in studies of spinal motoneurons.32,33 It is evident that both hyperpolarization and the current shunt produced by the REM sleep-specific IPSPs contributed to the suppression in excitability and discharge of hypoglossal motoneurons during REM sleep, as demonstrated by our determination of the cells' rheo-base. Because rheobase is a direct measure of membrane excitability,16 the cessation of tonic motoneuron discharge during REM sleep establishes the mechanistic basis for atonia of the lingual musculature and hence collapsibility of the upper airway that occurs during REM sleep, which is a critical factor in the production of airway obstruction in humans that results in OSA.4
The presence of IPSPs that we observed during REM sleep is additional direct evidence that an active inhibitory process is responsible for the suppression of motoneuron discharge during this state.18 The chloride channel-dependent nature of the REM sleep-specific IPSPs in hypoglossal motoneurons corresponds with findings that similar REM sleep-specific IPSPs bombard trigeminal, digastric, and spinal motoneurons during REM sleep.19,40,41 These chloride-dependent IPSPs have been demonstrated to be mediated by strychnine-sensitive glycine receptors.20,27,40 In humans with OSA, strychnine treatment is relatively efficacious in eliminating apnea, which supports the critical role of glycine receptor-mediated active inhibition during REM sleep.42 Functional anatomical (c-fos) studies have also demonstrated that glycinergic premotor interneurons that innervate hypoglossal motoneurons are activated during REM sleep, which provides an anatomical basis (i.e., source) for the inhibitory glycinergic inputs (IPSPs) to hypoglossal motorneurons.43 Note that REM sleep-specific IPSPs were not present during NREM sleep or wakefulness. If they had been present during these states, they would have been readily detected because the membrane potential during wakefulness and NREM sleep was more depolarized (-52 to -64 mV) than during REM sleep and the equilibrium potential for chloride is approximately -70 mV in motoneurons as calculated by the Nernst equation.44
Because chloride-dependent IPSPs could be activated by both glycine and gamma-aminobutyric acid A (GABAA) receptors,45 it is possible both these receptors are involved in mediating the REM sleep-specific IPSPs. In vitro voltage clamp studies have shown that both glycine- and GABAA-receptor mediated miniature inhibitory postsynaptic currents (mIPSCs) are present in hypoglossal motoneurons,30 although the likelihood of their being coreleased is challenged by another study that demonstrates differences between these two mIPSPs on the basis of their rise time, decay time, and their sensitivity to specific blockers.29 This study demonstrated that the chloride-mediated mIPSCs, as recorded in hypoglossal motoneurons in vitro, are mainly dependent on the activation of glycine rather than GABAA receptors. In addition, REM sleep-specific IPSPs (that appear to be identical to those we observed in this study) that are recorded from spinal motoneurons have been shown to be completely blocked by the glycine antagonist, strychnine, not by GABAA antagonists (picrotoxin or bicuculline).20 A similar complete blockade of strychnine-sensitive REM sleep-specific IPSPs that produce the carbachol-induced REM sleep atonia has also been documented in hypoglossal and masseter motoneurons.27,40
The findings that hypoglossal motoneuron hyperpolarization, an increase in rheobase, cessation of discharge, and the presence of IPSPs are phase-locked to REM sleep, and are not present during NREM sleep or wakefulness, confirm that postsynaptic inhibitory mechanisms are responsible for the suppression of genioglossal muscle activity during REM sleep. Therefore, postsynaptic (active) inhibition is the essential mechanism that underlies genioglossal muscle atonia during REM sleep. The current findings are in accordance with recent reports of a REM sleep related decrease in the antidromic field potential of hypoglossal nucleus in intact, unanesthetized animals.9,35 Furthermore, previous findings that glycinergic postsynaptic processes and a reduction in the excitability of hypoglossal motoneurons occur during pharmacologically (carbachol) induced REM sleep-like state in anesthetized cats.12,27
With regard to the circuitry of motor inhibition during REM sleep, it has been shown that there are REM-generator neurons in the pons that are responsible for producing REM sleep. These neurons, which selectively discharge during REM sleep, have also been shown to activate glycinergic premotor cells in the nucleus reticularis gigantocellularis and nucleus magnocellularis of the ventromedial reticular formation.1 These glycinergic premotor neurons project monosynaptically to motoneurons in the brainstem and spinal cord,1 wherein they produce potent postsynaptic inhibitory processes, as observed in this and other studies.10,19,31–34,41 Functionally, the inhibition of motoneurons at all levels of the neuraxis during REM sleep has been suggested to be a beneficial survival adaption that prevents animals from performing motor acts when they are essentially blind and are unaware of potentially harmful stimuli or environmental situations.1
It has been proposed that a multitude of synaptic mechanisms may contribute to REM sleep atonia of the genioglossus and other motoneurons.2,46–49 These include (1) postsynaptic inhibition as observed in this and previous studies,1,10,12,20,27,49 (2) disfacilitation caused by the withdrawal of premotor, aminergic inputs,7,50 and (3) presynaptic and postsynaptic inhibition that is mediated by muscarinic receptors.6,51 Microinjections of antagonists of norepinephrine (prazosin) and serotonin (methysergide) alone or in combination into the hypoglossal nucleus in anesthetized rats have shown that the withdrawal of both aminergic excitatory drives participate in the carbachol-induced depression of extracellularly recorded hypoglossal nerve activity.50 It is of note that the animals used in the latter studies were bilaterally vagotomized and anesthetized, and REM sleep was induced by carbachol. These procedures increase aminergic excitatory drives, which resulted in an increase in hypoglossal motoneuron activity.8 We believe that aminergic disfacilitation is important when there is an elevated level of excitability of hypoglossal motoneurons, which occurs in response to metabolic stress stimuli such as hypoxia.21 Therefore, aminergic disfacilitation is likely to be involved in mediating REM atonia, especially under pathophysiological conditions such as OSA.1 However, there is electrophysiological evidence that is incompatible with a disfacilitation hypothesis of aminergic inputs during naturally occurring REM sleep. For instance, both norepinephrine and serotonin are known to reduce the amplitude of post-spike AHP of brainstem and spinal motoneurons52–54 via a closure of potassium channels.55 However, the expected effect of a withdrawal of serotonin and/or norepinephrine on the AHP of action potentials is opposite to the observed decrease in AHP of motoneurons during this state.12,20,27 In addition, there is no effect of serotonin blockade on the genioglossal EMG activity across the sleep-waking states under normoxic conditions; serotonin's excitatory action on genioglossal EMG becomes apparent only under hypercapnic condition.8 Finally, clinical studies have demonstrated that serotonergic enhancement strategy is ineffective in reducing the apnea-hypopnea index of patients with OSA during REM sleep.56
Limitations
Generally, good-quality intracellular records can be obtained from a limited number of positively identified motoneurons per animal. However, we have shown that a sample size similar to that used in the current study is sufficient to produce meaningful data: we have also demonstrated, statistically, the presence of significant differences between study parameters. In addition, the number of neurons recorded was comparable to the number studied in similar intracellular experiments dealing with other brainstem and spinal cord motoneurons.20,31,32 We also believe that from a functional perspective there is no sampling problem. For example, despite the fact that the hypoglossal nucleus is composed of motoneurons that innervate the intrinsic and extrinsic lingual muscles,57 bilateral stimulation of the hypoglossal nerve produces protrusion of the tongue and hence increases upper airway patency.58 Similarly, coactivation of tongue protrudor and retractor muscles or independent activation of protrudor muscles improves upper airway flow mechanics.59 In humans with OSA, hypoglossal nerve stimulation has been shown to increase the inspiratory airflow during sleep.60
CONCLUSION
Based on electrophysiological and functional neuroanatomical studies from our laboratory and other reports, active postsynaptic inhibitory glycinergic drives are confirmed to play a direct role in producing the suppression of hypoglossal motoneuron discharge and atonia of genioglossal muscle that occurs during REM sleep. Disfacilitatory drives may arise under pathological conditions such as OSA.1
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by grants 1|01BX00819, TGS-109219 and 1R01HL116845. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Francisco Morales and Jack Yamuy for their constructive advice in developing the chronically-instrumented cat preparation for the intracellular investigation of hypoglossal motoneurons during naturally-occurring states of sleep and wakefulness. Pablo Torterolo and MingChu Xi contributed invaluable support in the preparation of the head and nerve cuff implants, respectively. We are also indebted to Peter Soja and Ron Harper for sharing their experience with respect to the fabrication of the cuff electrode for the hypoglossal nerve implant. We also thank Ida Shakhverdyan, Pandi Perumal, Naira Manukian, Andrui Nazarian, and Reza Khorsan for their excellent technical assistance.
ABBREVIATIONS
- AHP
after-hyperpolarizing potential
- c-fos
a proto-oncogene
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electro-oculogram
- IPSP
inhibitory postsynaptic potential
- K-citrate
potassium citrate
- KCl
potassium chloride
- LGN
lateral geniculate nucleus
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PGO
ponto-geniculo-occipital
- REM
rapid eye movement
REFERENCES
- 1.Chase MH. Motor control during sleep and wakefulness: clarifying controversies and resolving paradoxes. Sleep Med Rev. 2013;17:299–312. doi: 10.1016/j.smrv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Funk GD, Zwicker JD, Selvaratnam R, Robinson DM. Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms. Arch Ital Biol. 2011;149:426–53. doi: 10.4449/aib.v149i4.1271. [DOI] [PubMed] [Google Scholar]
- 3.Horner RL. Neural control of the upper airway: integrative physiological mechanisms and relevance for sleep disordered breathing. Compr Physiol. 2012;2:479–535. doi: 10.1002/cphy.c110023. [DOI] [PubMed] [Google Scholar]
- 4.White DP, Younes MK. Obstructive sleep apnea. Compr Physiol. 2012;2:2541–94. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 5.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–70. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 6.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–19. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 7.Kubin L, Kimura H, Tojima H, Davies R, Pack A. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–12. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 8.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–47. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 9.Fung SJ, Chase MH. Control of hypoglossal motoneurones during naturally occurring sleep and wakefulness in the intact, unanaesthetized cat: a field potential study. J Sleep Res. 2014;23:469–74. doi: 10.1111/jsr.12137. [DOI] [PubMed] [Google Scholar]
- 10.Chase MH, Chandler SH, Nakamura Y. Intracellular determination of membrane potential of trigeminal motoneurons during sleep and wakefulness. J Neurophysiol. 1980;44:349–58. doi: 10.1152/jn.1980.44.2.349. [DOI] [PubMed] [Google Scholar]
- 11.Fung SJ, Boxer PA, Morales FR, Chase MH. Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res. 1982;248:267–73. doi: 10.1016/0006-8993(82)90584-4. [DOI] [PubMed] [Google Scholar]
- 12.Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res. 2000;885:262–72. doi: 10.1016/s0006-8993(00)02955-3. [DOI] [PubMed] [Google Scholar]
- 13.Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–9. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- 14.Berman AL. Madison, WI: University of Wisconsin Press; 1968. The brainstem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. [Google Scholar]
- 15.Fung SJ, Xi M, Zhang J, Sampogna S, Chase MH. Apnea produces excitotoxic hippocampal synapses and neuronal apoptosis. Exp Neurol. 2012;238:107–13. doi: 10.1016/j.expneurol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Frank K, Fuortes MG. Stimulation of spinal motoneurons with intracellular electrodes. J Physiol. 1956;134:451–70. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brock LG, Coombs JS, Eccles JC. The recording of potentials from motoneurons with an intracellular electrode. J Physiol. 1952;117:431–60. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombs JS, Eccles JC, Fatt P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955;130:326–73. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedroarena C, Castillo P, Chase MH, Morales FR. The control of jaw-opener motoneurons during active sleep. Brain Res. 1994;653:31–8. doi: 10.1016/0006-8993(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 20.Soja PJ, López-Rodríguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–11. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierrefiche O, Bischoff AM, Richter DW, Spyer KM. Hypoxic response of hypoglossal motoneurons in the in vivo cat. J Physiol. 1997;505:785–95. doi: 10.1111/j.1469-7793.1997.785ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter R. Antidromic responses of hypoglossal motoneurons. Exp Neurol. 1968;20:624–34. doi: 10.1016/0014-4886(68)90113-1. [DOI] [PubMed] [Google Scholar]
- 23.Sun QJ, Pilowsky P, Llewellyn-Smith IJ. Thyrotropin-releasing hormone inputs are preferentially directed towards respiratory motoneurons in rat nucleus ambiguus. J Comp Neurol. 1995;362:320–30. doi: 10.1002/cne.903620303. [DOI] [PubMed] [Google Scholar]
- 24.Takata M, Ogata K. Two components of inhibitory postsynaptic potentials evoked in hypoglossal motoneurons by lingual nerve stimulation. Exp Neurol. 1980;69:299–310. doi: 10.1016/0014-4886(80)90213-7. [DOI] [PubMed] [Google Scholar]
- 25.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–51. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 26.Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport. 1995;6:2085–8. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- 27.Yamuy J, Fung SJ, Xi MC, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–5. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Barillot JC, Bianchi AL. Patterns of membrane potentials and distributions of the medullary respiratory neurons in the decerebrate rat. Brain Res. 1991;546:261–70. doi: 10.1016/0006-8993(91)91490-r. [DOI] [PubMed] [Google Scholar]
- 29.Donato R, Nistri A. Relative contribution by GABA or glycine to Cl(-)-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol. 2000;84:2715–24. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–41. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Goldberg LJ, Chandler SH, Chase MH. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–7. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- 32.Glenn LL, Dement WC. Membrane potential and input resistance of cat spinal motoneurons in wakefulness and sleep. Behav Brain Res. 1981;2:231–6. doi: 10.1016/0166-4328(81)90060-7. [DOI] [PubMed] [Google Scholar]
- 33.Morales F, Chase MH. Postsynaptic control of lumbar motoneuron excitability during active sleep in the chronic cat. Brain Res. 1981;225:279–95. doi: 10.1016/0006-8993(81)90836-2. [DOI] [PubMed] [Google Scholar]
- 34.Engelhardt JK, Fung SJ, Yamuy J, Xi MC, Morales FR, Chase MH. The unique inhibitory potentials in motoneurons that occur during active sleep are comprised of minimal unitary potentials. Brain Res. 2004;1018:26–31. doi: 10.1016/j.brainres.2004.05.094. [DOI] [PubMed] [Google Scholar]
- 35.Fenik VB, Fung SJ, Lim V, Chase MH. Quantitative analysis of the excitability of hypoglossal motoneurons during natural sleep in the rat. J Neurosci Methods. 2013;212:56–63. doi: 10.1016/j.jneumeth.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lunteren E, Dick TE. Intrinsic properties of pharyngeal and diaphragmatic respiratory motoneurons and muscles. J Appl Physiol. 1992;73:787–800. doi: 10.1152/jappl.1992.73.3.787. [DOI] [PubMed] [Google Scholar]
- 37.Megirian D, Hinrichsen CF, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–28. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- 38.Rossiter CD, Yates BJ. Vestibular influences on hypoglossal nerve activity in the cat. Neurosci Lett. 1996;211:25–8. doi: 10.1016/0304-3940(96)12710-5. [DOI] [PubMed] [Google Scholar]
- 39.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 40.Kohlmeier KA, López-Rodríguez F, Liu RH, Morales FR, Chase MH. State-dependent phenomena in cat masseter motoneurons. Brain Res. 1996;722:30–8. doi: 10.1016/0006-8993(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 41.Morales FR, Chase MH. Repetitive synaptic potentials responsible for inhibition of spinal cord motoneurons during active sleep. Exp Neurol. 1982;78:471–6. doi: 10.1016/0014-4886(82)90065-6. [DOI] [PubMed] [Google Scholar]
- 42.Remmers JE, Anch AM, deGroot WJ, Baker JP, Jr, Sauerland EK. Oropharyngeal muscle tone in obstructive sleep apnea before and after strychnine. Sleep. 1980;3:447–53. doi: 10.1093/sleep/3.3-4.447. [DOI] [PubMed] [Google Scholar]
- 43.Morales FR, Sampogna S, Rampon C, Luppi PH, Chase MH. Brainstem glycinergic neurons and their activation during active (rapid eye movement) sleep in the cat. Neuroscience. 2006;142:37–47. doi: 10.1016/j.neuroscience.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 44.Ichinose T, Miyata Y. Recurrent excitation of motoneurons in the isolated spinal cord of newborn rats detected by whole-cell recording. Neurosci Res. 1998;31:179–87. doi: 10.1016/s0168-0102(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien JA, Sebe JY, Berger AJ. GABAB modulation of GABAA and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol. 2004;141:35–45. doi: 10.1016/j.resp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Lydic R. The motor atonia of REM sleep: a critical topics forum. Sleep. 2008;31:1471–2. doi: 10.1093/sleep/31.11.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger AJ. What causes muscle atonia in REM? Sleep. 2008;31:1477–8. doi: 10.1093/sleep/31.11.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horner RL. The tongue and its control by sleep state-dependent modulators. Arch Ital Biol. 2011;149:406–25. doi: 10.4449/aib.v149i4.1256. [DOI] [PubMed] [Google Scholar]
- 49.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32:9785–95. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–30. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–70. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- 52.White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989;502:205–13. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- 53.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–8. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 54.Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–9. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- 55.Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamineand noradrenaline-evoked depolarization of adult rat facial motoneurons. J Physiol. 1992;456:473–90. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraiczi H, Hedner J, Dahlöf P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22:61–7. [PubMed] [Google Scholar]
- 57.Uemura M, Matsuda K, Kume M, Takeuchi Y, Matsushima R, Mizuno N. Topographical arrangement of hypoglossal motoneurons: an HRP study in the cat. Neurosci Lett. 1979;13:99–104. doi: 10.1016/0304-3940(79)90024-7. [DOI] [PubMed] [Google Scholar]
- 58.Bennett GA, Hutchinson RC. Experimental studies on the movements of the mammalian tongue; the protrusion mechanism of the tongue (dog) Anat Rec. 1946;94:57–72. doi: 10.1002/ar.1090940107. [DOI] [PubMed] [Google Scholar]
- 59.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–13. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am. 2003;36:501–10. doi: 10.1016/s0030-6665(02)00178-0. [DOI] [PubMed] [Google Scholar]