Abstract
Study Objectives:
We examined how sleep deprivation alters physiological responses to psychosocial stress by evaluating changes in skin conductance.
Design:
Between-subjects design with one group allocated to 24 h of total sleep deprivation and the other to rested wakefulness.
Setting:
The study took place in a research laboratory.
Participants:
Participants were 40 healthy young adults recruited from a university.
Interventions:
Sleep deprivation and feedback.
Measurements and Results:
Electrodermal activity was monitored while participants completed a difficult perceptual task with false feedback. All participants showed increased skin conductance levels following stress. However, compared to well-rested participants, sleep deprived participants showed higher skin conductance reactivity with increasing stress levels.
Conclusions:
Our results suggest that sleep deprivation augments allostatic responses to increasing psychosocial stress. Consequentially, we propose sleep loss as a risk factor that can influence the pathogenic effects of stress.
Citation:
Liu JC, Verhulst S, Massar SA, Chee MW. Sleep deprived and sweating it out: the effects of total sleep deprivation on skin conductance reactivity to psychosocial stress. SLEEP 2015;38(1):155–159.
Keywords: affect, allostasis, skin conductance, sleep loss, stress, sympathetic nervous system
INTRODUCTION
Psychosocial stress, characterized by loss of control and social threat,1 is a risk factor for disease.2,3 Stressors ranging from isolation to low socioeconomic status have been implicated in the initiation or progression of viral infections, cardiovascular disease, cancer, asthma, and overall mortality.2 These findings are robust, and have been observed across animal models (e.g., monkeys,4 rabbits5) and experimental designs.
Nonetheless, the pathogenic effects of stress show interindividual variation3 and may be influenced by situational variables such as sleep loss. Even in the absence of external stressors, sleep deprivation has been shown to increase basal sympathetic activity, activate the hypothalamic-pituitary-adrenal axis, and can elevate inflammation markers (see Meerlo et al. and Mullington et al.6,7 for reviews). In addition to these changes, sleep deprivation may also modulate reactivity to episodic psychosocial stress.6
To date, only two human studies have explored the effects of sleep deprivation on stress reactivity. These studies suggest that sleep deprivation magnifies responses to stress, as shown in heightened subjective stress during low-stress conditions,8 and greater stress-related increases in systolic blood pressure.9 Here, we probed how the sympathetic nervous system might contribute to altered reactivity in sleep deprived persons. This is a key mediator of the acute response to stress, with activity leading to downstream changes in effectors such as the cardiovascular system.10
Changes in skin conductance occur with eccrine sweating and constitute a relatively pure assay of sympathetic activity.11 Alterations in sweating are mediated by cholinergic nerves and are not affected by beta-blockers, allowing evaluation of the sympathetic system even when a person is being treated for hypertension.12 Along these lines, we hypothesized that sleep deprivation would add to skin conductance responses to psychosocial stress, reflecting greater activation of the sympathetic nervous system.
METHODS
Participants
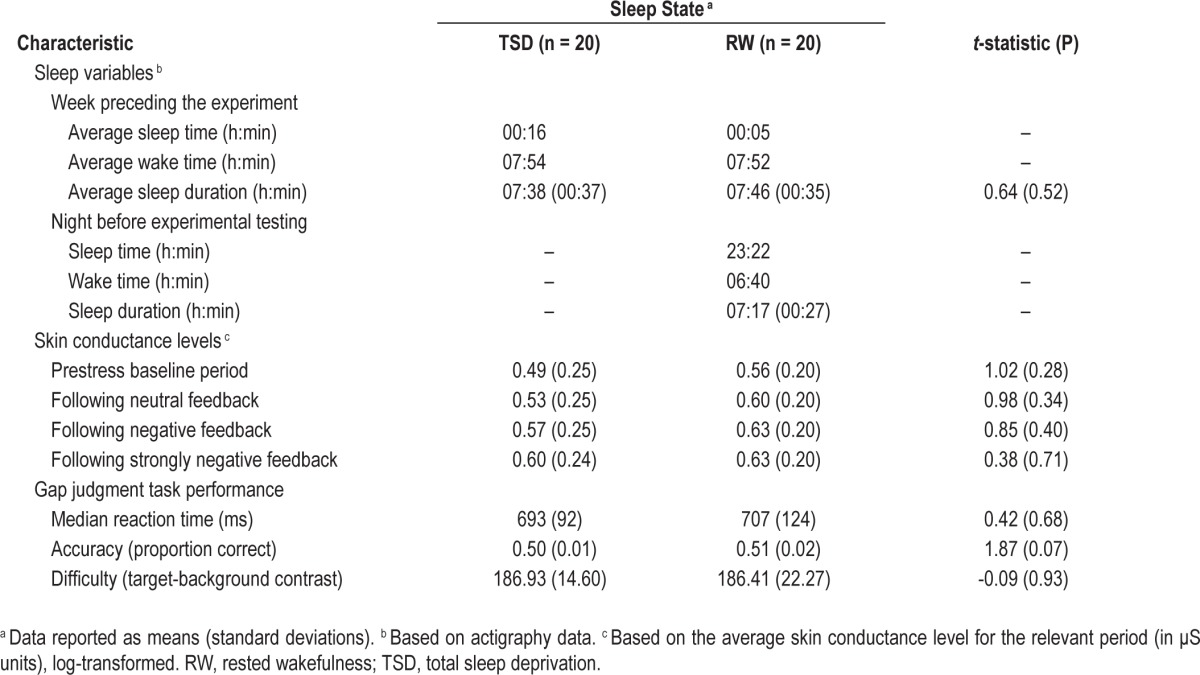
Forty-three healthy young adults were recruited from the National University of Singapore. Participants had to: (1) be aged between 18–35 y, (2) be nonsmokers, (3) have no history of psychiatric or medical disorders, (4) have good habitual sleep (sleep duration of 6.5–9 h daily, sleeping before 00:30, waking before 09:00), and (5) not be of an extreme chronotype.13 Of the 43 participants, two were excluded for noncompliance and one for inability to understand task instructions. The remaining 40 participants were randomly allocated to one of two sleep conditions: 20 participants (10 females; mean age = 22.40 y, standard deviation [SD] = 2.68 y) were assigned to the total sleep deprivation group (TSD), and 20 (nine females; mean age = 21.75 y, SD = 1.41 y) to the rested wakefulness group (RW). Experimental procedures were approved by the National University of Singapore's Institutional Review Board.
Procedure
General Study Procedure
Participants in the TSD group arrived at the laboratory at 21:00 the night before the experiment. Throughout the night, participants were monitored to ensure they kept awake and engaged only in sedentary activities. Participants also completed hourly assessments of vigilance (Psychomotor Vigilance Task; PVT14) and of subjective sleepiness.15,16
Participants in the RW group arrived at the laboratory at 22:30 the night before the experiment and were given 8 h of sleep opportunity (see Table 1 for details of actual sleep obtained). To mitigate possible effects of sleep inertia, participants were given 1 h to wash up upon waking up. RW participants additionally performed one assessment of vigilance and of subjective sleepiness.
Table 1.
Sleep deprived and well-rested participants' baseline characteristics.
On the morning of the experiment, testing commenced between 06:00 to 06:40 (for TSD participants) and between 08:00 and 08:40 (for RW participants). These represent the approximate time when vigilance hits a nadir after a night of sleep deprivation, and the start time of a regular workday17,18; as such, the effects described here represent the interaction of circadian and homeostatic effects.
For all participants, sleep history was monitored through actigraphy for the week preceding the experiment (Table 1).
Experimental Testing
Throughout the experimental testing component, skin conductance data were acquired through a Grass amplifier and skin conductance adaptor (Models CP122 and SCA1; Grass Technologies, Natus Neurology Inc., Warwick, RI) at a sampling rate of 200 Hz. Data were recorded continuously from two silver/silver chloride electrodes (Model F-EGSR) attached to the distal phalanges of the second and third digit of the left hand with skin conductance electrode paste (Type EC33); a constant direct voltage of 0.5 V was applied across the electrodes.
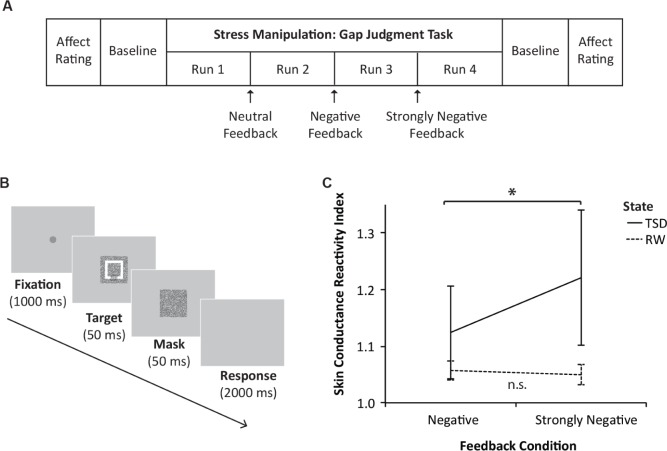
Figure 1A depicts the sequence of events during experimental testing: at the start, participants reported their subjective affect through the Positive and Negative Affect Schedule19 (PANAS; note that subjective stress ratings were not measured to avoid participant suspicions). Thereafter, 2 min of baseline skin conductance data were recorded (Table 1).
Figure 1.
(A) Sequence of events during the experimental testing component. (B) Schematic of a typical trial in the gap judgment task. (C) Sleep deprived and well- rested participants' skin conductance reactivity to increasingly negative feedback; a higher index score indicates higher reactivity, and vertical lines represent 1 standard error of the mean. n.s., not significant; RW, rested wakefulness; TSD, total sleep deprivation.
As the primary stress manipulation, participants performed a difficult perceptual task involving 40 trials per run (gap judgment task; run duration of 2 min 30 sec). On each trial, participants were required to identify the position of a small gap in a rectangular visual target (Figure 1B). Participants were instructed that the experiment could proceed only after they reached a performance criterion; in reality, task difficulty was titrated on a trial-by-trial basis such that each participant would attain only 50% accuracy (Table 1).
To induce increasing levels of stress, participants had to repeat the task for four consecutive runs. After each run, they were told that they had not reached the minimal performance criterion, and were given verbal feedback in increasingly negative wording: (Feedback 1 - Neutral) “Before we proceed to the other tasks, we have to repeat this task one more time.”; (Feedback 2 - Negative) “Your performance is not good enough yet. You have to try harder, because I cannot let you start the other task before this is done.”; and (Feedback 3 - Strongly Negative) “This is taking awhile, are you concentrating? Your performance is much lower than the performance of the other participants, and you cannot proceed before you finish this. Please put in more effort.”
At the end of the task runs, 2 min of baseline skin conductance data were acquired, and participants completed the PANAS scale a second time.
Data Analyses
Skin Conductance
Average skin conductance levels (SCL; in μS units) were computed as the mean SCL for each run of the gap judgment task, and were log-transformed (ln[SCL + 1]).11 Stress reactivity was quantified by comparing the log-transformed SCL during task runs following negative feedback (Runs 3 and 4; see Table 1), divided by SCL during the task run following neutral feedback (Run 2; see Table 1). These indices accounted for baseline differences in electrodermal activity that may arise from homeostatic and circadian processes,20,21 and allowed changes specific to psychosocial stress to be isolated (because the control condition of receiving neutral feedback matched the negative feedback conditions in every other task aspect).
Statistical Analyses
As the primary analysis, a 2 × (2) repeated measures analysis of variance was run with sleep state (TSD versus RW) and feedback condition (negative and strongly negative feedback) as the factors, and skin conductance reactivity scores as dependent variables. All analyses were conducted using SPSS (Version 21, IBM Corp., Armonk, NY), with Type 1 Decision Wise Error Rate controlled at α = 0.05.
RESULTS
Effectiveness of the Sleep Manipulation
Prior to the experimental task, sleep deprived participants showed increased median reaction time on the PVT (mean for TSD group = 487.45 ms, SD = 323.37 ms and mean for RW group = 317.59 ms, SD = 27.12; t(19.31) = -2.34, P = 0.03). This indicates that the sleep manipulation was successful.
Effectiveness of the Stress Manipulation
Subjective Affect Ratings
Participants' affect ratings on the PANAS scale were scored to obtain a subscale score for negative affect. Averaged across the sleep deprived and well-rested groups, participants reported increased negative affect following the stressor than before the stressor (mean before the stressor = 1.49, SD = 0.56 and mean after the stressor = 1.84, SD = 0.61; F(1,36) = 14.07, P = 0.001).
Skin Conductance Levels
Further, collapsed across groups, one-sample t-tests were run to assess whether each skin conductance reactivity score differed from one. (As the scores are ratios, a value > 1 indicates increased SCL following negative relative to neutral feedback.) Reactivity scores were > 1 for negative feedback (mean score = 1.09, SD = 0.26; t(39) = 2.17, P = 0.04); and for strongly negative feedback (mean score = 1.14, SD = 0.39; t(39) = 2.21, P = 0.03).
Together, results from both affect ratings and skin conductance levels indicate that the stress manipulation was successful in sleep deprived and well-rested participants.
Effects of Sleep Condition on Stress Reactivity
Additionally, there was a significant interaction between sleep state and feedback condition on skin conductance reactivity, F(1,38) = 5.71, P = 0.02; Figure 1C. TSD participants showed greater skin conductance reactivity with increasingly negative feedback, t(19) = -2.27, P = 0.04, whereas reactivity in RW participants did not differ as a function of feedback condition, t(19) = 0.80, P = 0.43.
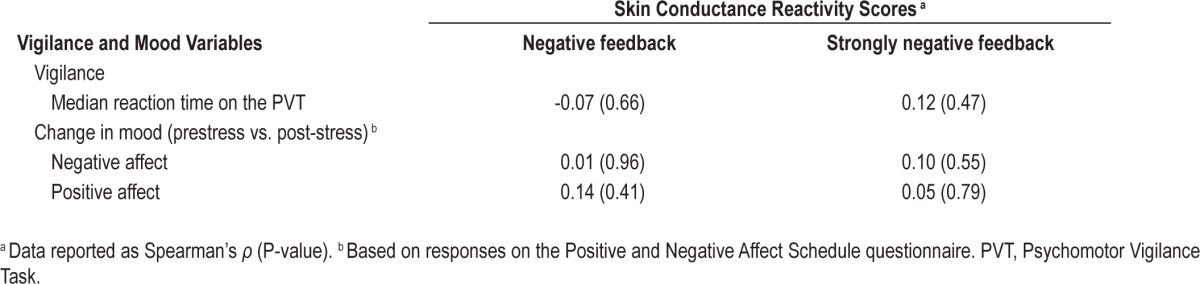
Participants' skin conductance reactivity scores did not correlate with prestress median reaction time on the PVT, nor with the difference between prestress and post-stress negative or positive affect on the PANAS (smallest P = 0.41; Table 2).
Table 2.
Correlations of skin conductance reactivity scores with vigilance and mood variables.
DISCUSSION
In this study, we investigated how sleep deprivation alters skin conductance reactivity to a laboratory stressor. Although participants overall showed increased skin conductance levels following the manipulation, skin conductance in sleep deprived participants continued to rise with increasing stress. Our finding of heightened sudomotor responses concurs with prior studies suggesting that sleep deprivation has a negative effect on stress reactivity (assessed previously in terms of subjective stress8 and systolic blood pressure9).
Together, these findings can be framed within a model of allostasis, which explains disease progression beginning with processes initially engaged to achieve stability through change.22 Chronic sleep deprivation23 and chronic exposure to psychosocial stress22 have both been independently characterized as allostatic loads that predispose a person to illness; each has been studied as a risk factor for cardiovascular disease and for all-cause mortality.2,24–26 Similarly, episodes of total sleep deprivation and acute psychosocial stress have been found to affect dynamic allostatic responses of physiological stress systems.1,6,7 Our findings suggest that the acute allostatic response to psychosocial stress may be altered in sleep deprived persons, constituting a third pathway for allostatic responses when sleep loss and psychosocial stress co-occur.
In our study, we also observed that TSD effects on skin conductance reactivity were uncorrelated with effects on subjective affective ratings and with performance on a psychomotor vigilance task. The lack of correlation between affective and vigilance effects has been reported by others.27 Our findings suggest a further dissociation between the effect of sleep deprivation on vigilance and autonomic stress responses, advocating the use of skin conductance as an independent marker for health risks in sleep deprived individuals.12,28 Skin conductance reactivity to increasing stress appears to be both sensitive to modulation by sleep loss, and is orthogonal to a widely used vigilance measure.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was conducted as partial fulfillment of the second author's Masters program at Utrecht University, and was supported by a grant awarded to Dr. Michael Chee from the National Medical Research Council Singapore (STaR/0004/2008). The authors acknowledge Ong Ju Lynn for sharing task scripts, Zheng Hui for technical advice, and Cher Wei Shan for assistance in data entry. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 2.Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–24. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 3.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1982;2:359–68. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 5.McCabe PM, Gonzales JA, Zaias J, et al. Social environment influences the progression of atherosclerosis in the watanabe heritable hyperlipidemic rabbit. Circulation. 2002;105:354–9. doi: 10.1161/hc0302.102144. [DOI] [PubMed] [Google Scholar]
- 6.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–20. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzen PL, Gianaros PJ, Marsland AL, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–82. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 11.Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2007. pp. 150–81. [Google Scholar]
- 12.Jacobs SC, Friedman R, Parker JD, et al. Use of skin conductance changes during mental stress testing as an index of autonomic arousal in cardiovascular research. Am Heart J. 1994;128:1170–7. doi: 10.1016/0002-8703(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 13.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 14.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 15.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 16.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention perfomance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 18.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 20.Miro E, Cano-Lozano MC, Buela-Casal G. Electrodermal activity during total sleep deprivation and its relationship with other activation and performance measures. J Sleep Res. 2002;11:105–12. doi: 10.1046/j.1365-2869.2002.00286.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller MW, Gronfier C. Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology. 2006;43:297–301. doi: 10.1111/j.1469-8986.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 25.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 27.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MA, Cappuccio FP. Biomarkers of cardiovascular risk in sleep-deprived people. J Hum Hypertens. 2013;27:583–8. doi: 10.1038/jhh.2013.27. [DOI] [PubMed] [Google Scholar]