Abstract
Study Objective:
To study the incidence, remission, and prediction of obstructive sleep apnea (OSA) from middle childhood to late adolescence.
Design:
Longitudinal analysis.
Setting:
The Cleveland Children's Sleep and Health Study, an ethnically mixed, urban, community-based cohort, followed 8 y.
Participants:
There were 490 participants with overnight polysomnography data available at ages 8–11 and 16–19 y.
Measurements and Results:
Baseline participant characteristics and health history were ascertained from parent report and US census data. OSA was defined as an obstructive apnea- hypopnea index ≥ 5 or an obstructive apnea index ≥ 1. OSA prevalence was approximately 4% at each examination, but OSA largely did not persist from middle childhood to late adolescence. Habitual snoring and obesity predicted OSA in cross-sectional analyses at each time point. Residence in a disadvantaged neighborhood, African-American race, and premature birth also predicted OSA in middle childhood, whereas male sex, high body mass index, and history of tonsillectomy or adenoidectomy were risk factors among adolescents. Obesity, but not habitual snoring, in middle childhood predicted adolescent OSA.
Conclusions:
Because OSA in middle childhood usually remitted by adolescence and most adolescent cases were incident cases, criteria other than concern alone over OSA persistence or incidence should be used when making treatment decisions for pediatric OSA. Moreover, OSA's distinct risk factors at each time point underscore the need for alternative risk-factor assessments across pediatric ages. The greater importance of middle childhood obesity compared to snoring in predicting adolescent OSA provides support for screening, preventing, and treating obesity in childhood.
Citation:
Spilsbury JC, Storfer-Isser A, Rosen CL, Redline S. Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence. SLEEP 2015;38(1):23–29.
Keywords: adolescents, children, incidence, obstructive sleep apnea, remission
INTRODUCTION
Obstructive sleep apnea (OSA) affects 1–4% of children and adolescents1–4 and is associated with behavioral, cognitive, and physiological deficits.5–7 Epidemiological data, mainly cross-sectional, have identified several pediatric risk factors: premature birth, African-American race, obesity, and residence in a disadvantaged neighborhood2,8; adenotonsillar hypertrophy9; Hispanic ethnicity10; craniofacial abnormalities11; and history of upper and lower respiratory disease.12
Longitudinal research addressing the incidence and variation of OSA risk factors across pediatric ages is relatively limited.1,7,13–20 Previous longitudinal studies using polysomnography (PSG) reported that approximately 8–10% of children with primary snoring progress to OSA over a 1- to 3-y period,1,15,16 although a recent community-based study of children with a longer follow-up period (4.6 y on average) reported that more than one-third progressed to OSA defined as an obstructive apnea-hypopnea index (OAHI) ≥ 1 event per hour.21 A population-based study of 6- to 12-y-old children followed 5 years reported a sleep disordered breathing (SDB) incidence rate of 10%, defined as a respiratory disturbance index ≥ 1 event per hour.18
Characteristics associated with SDB incidence or remission are not well understood. Male sex has been associated with risk of primary snoring1,19,20 and incident and persistent SDB.18 However, a study of more than 12,000 UK children followed from infancy to early childhood reported that over time, the association of SDB symptoms with child-level factors decreased, whereas factors related to social conditions persisted.17 Furthermore, although SDB predicts future body mass index (BMI),18 only two longitudinal studies have addressed whether BMI predicts future OSA. The first study involved a small, clinic-based sample of primary snorers and reported no significant change in BMI in the sample at the approximately 2-y follow-up.15 The second study, a community-based sample, reported that persistent obesity (obesity at both baseline and 4.6-y follow-up) but not baseline obesity alone predicted OSA at follow-up.21 However, the study sample once again was limited to primary snorers only.
To improve understanding of OSA's epidemiology in children and adolescents, we report the results of a longitudinal study of objectively measured OSA in a community-based child cohort that included a substantial number of African Americans and children born prematurely, two groups with increased risk for OSA during early and middle childhood.2,22 The study's primary purpose was to examine OSA incidence and remission from middle childhood (age 8–11 y) through late adolescence (age 16–19 y) and assess whether risk factors for OSA in middle childhood remained so in adolescence. Because risk factors may change with growth and development, especially as airway size and central body fat increase while lymphoid tissue regresses, we hypothesized that obesity would more strongly associate with OSA in adolescence compared to middle childhood. Secondary objectives were to examine other subject characteristics related to OSA in late adolescence; assess OSA incidence in primary snorers; and explore how alternative OSA definitions affect incidence rates.
METHODS
Sample
The study sample consisted of participants in the longitudinal Cleveland Children's Sleep and Health Study cohort,2 a stratified random sample of 907 term and preterm children born from 1988–1993 at three major Cleveland-area hospitals. African American and former preterm children were intentionally over-represented to increase internal validity and produce stable estimates of associations between OSA and health outcomes for these subgroups. This analysis includes measurements from two key developmental time points: middle childhood (data collected 1998–2002, child age 8–11 y) and late adolescence (data collected from 2006–2010, children age 16–19 y); 517 participated in both examinations (Figure S1, supplemental material). Participant and family characteristics for who did (n = 517) and did not (n = 390) participate in the late adolescent follow-up examination were similar except that a greater proportion of youth participating in both examinations had caregivers with education greater than high school: 59% versus 41%, P = 0.02 (Table S1, supplemental material).
Procedures
Parents (or legal guardians) and adolescents (18 y or older) provided informed, written consent. Children provided assent. The University Hospitals of Cleveland's institutional review board approved the study.
Middle Childhood Assessment
Data collection details have been described previously.2 Assessments occurred in participants' homes. Demographic and medical data were obtained by a parent-completed, standardized questionnaire.23 In-home sleep apnea monitoring was conducted with a Type III sleep monitor recording thoracic and abdominal excursions and estimated tidal volume, pulse oximetry, heart rate, and body position (PT-2 system, SensorMedics, Yorba Linda, CA). Respiratory events were scored if ≥ 8 sec (or two or more missed respiratory cycles). Obstructive apneas were scored when chest and abdominal efforts were asynchronous and estimated tidal volume was absent or nearly absent, irrespective of associated desaturation. Hypopneas were scored when respiratory efforts were accompanied by a 50% reduction in estimated tidal volume and accompanied by ≥ 3% oxyhemoglobin desaturation.
Adolescent Assessment
Overnight PSG and physiological and anthropometric assessments, including a physician-administered physical examination, followed a standardized protocol at the research center, beginning at approximately 17:00 and ending the following day at 11:00.24,25 The PSG recording (Compumedics E-series; Compumedics, Abbotsford, Australia) consisted of measurement of two electroencephalograms (C3/C2 and C4/C1), bilateral electrooculograms, a bipolar submental electro-myogram, thoracic and abdominal respiratory inductance plethysmography, airflow (nasal–oral thermocouple nasal pressure recording), finger-pulse oximetry, electrocardiogram, body position, and bilateral leg movements. Obstructive apneas were scored when a complete or nearly complete absence of airflow occurred on the thermistry channel for ≥ 10 sec in association with respiratory effort. Hypopneas were identified as an approximately 50% reduction in airflow or summed respiratory excursions associated with an oxygen desaturation ≥ 3% (see supplemental methods for additional protocol information).
For both examinations, the OAHI was defined as all obstructive or mixed apneas and hypopneas with ≥ 3% desaturation per sleep hour.
Study Measures
Participant characteristics such as race/ethnicity (African American versus other); history of OSA, tonsillectomy or adenoidectomy; physician-diagnosed asthma; maternal smoking; habitual snoring (≥ 1–2 times per week during the past month); and caregiver education were obtained from parent or adolescent. Preterm status (gestational age < 37 w) was obtained from hospital birth records. Tonsil size was ascertained by physician exam using a five-point scale (see supplemental methods). Residence in a socioeconomically distressed neighborhood was determined using US census data per established procedures (see supplemental methods).8,26 BMI (kg/m2), based on direct height and weight measurement, was converted into age- and sex-adjusted percentiles (http://www.cdc.gov/growthcharts/). Obesity was defined as BMI ≥ 95th percentile for age and sex.
The primary study outcome, OSA, was defined as an OAHI ≥ 5 or an obstructive apnea index (OAI) ≥ 1. Secondary outcomes were: (1) SDB, defined as OSA, habitual snoring, or both; and (2) OSA defined as an OAHI ≥ 1.
Statistical Analyses
Study variables were summarized using means (M) and standard deviations (SD) for normally distributed variables, medians and interquartile ranges for markedly nonnormally distributed variables, counts and proportions for categorical variables, and included two-sample t-tests, Wilcoxon rank-sum tests, and chi-square tests. The concordance of OSA (and SDB) in middle childhood and adolescence were examined using McNemar test. Log-binomial models assessed the relation of previously identified risk factors with OSA in adolescence; relative risk ratios (RR) and 95% confidence intervals (95% CI) are presented. Models were estimated without covariate adjustment and after adjusting for BMI z-score at either age 8–11 y or 16–19 y. Secondary analyses examined associations restricted to full-term births or nonobese participants at age 8–11 y, and association of habitual snoring in middle childhood with incident OSA at age 16–19 y. Logistic regression analyses examined the cross-sectional association of participant characteristics with OSA (see supplemental methods); odds ratios (OR) and 95% CI are reported. Analyses were performed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Sample Characteristics
The analytic sample consisted of 490 participants for whom PSG data were available both at middle childhood (M = 9.5, SD = 0.8 y) and late adolescence (M = 17.7, SD = 0.4 y). The mean time between visits was 8.2 y (SD = 0.7 y). Approximately half of the sample was male, 36.5% were African American, and 44.1% were premature at birth (see supplemental results for details). At the middle childhood examination, 22.1% lived in a distressed neighborhood, 15.1% were obese, 7.1% had a history of a tonsillectomy or adenoidectomy, and 19.6% reported a doctor's diagnosis of asthma. At the late adolescence examination, obesity prevalence increased to 19.4%; tonsillectomy or adenoidectomy to 12.0%, and an asthma diagnosis to 28.8%.
OSA Prevalence, Remission, and Incidence From Middle Childhood to Late Adolescence
OSA prevalence was 4.7% among 8- to 11-y-olds and 4.3% among 16- to 19-y-olds. OSA did not persist from middle childhood to late adolescence. Only two of the 23 participants (8.7%) with OSA at age 8–11 y had OSA at age 16–19 y (P = 0.75) (Figure 1). Five of the 21 children whose OSA remitted by adolescence underwent a tonsillectomy or adenoidectomy between examinations (Figure 1). Of the 467 children without OSA in middle childhood, 19 (4.0%) had incident OSA at late adolescence.
Figure 1.
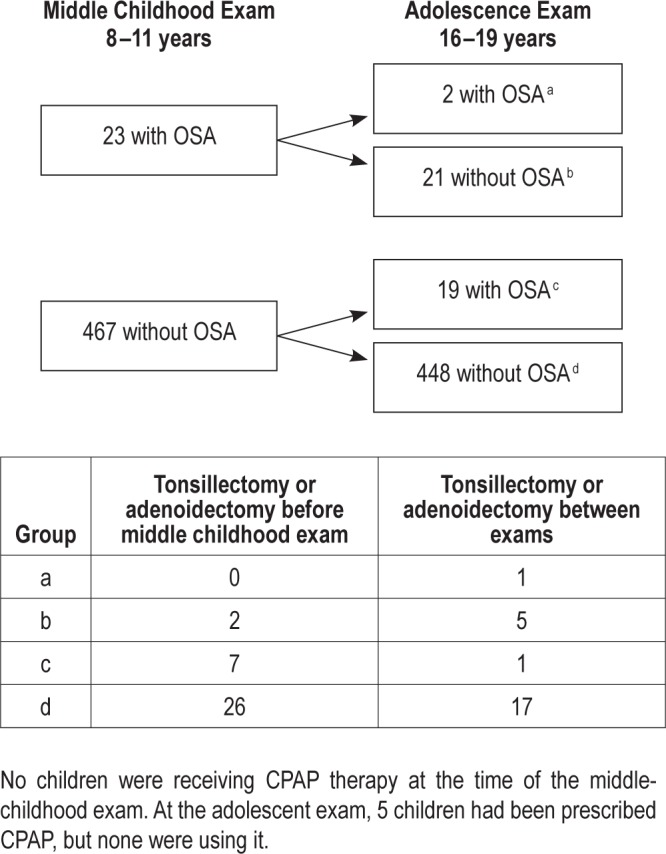
Incidence and remission of OSA from middle childhood to late adolescence.
Predictors of OSA in Middle Childhood and Late Adolescence
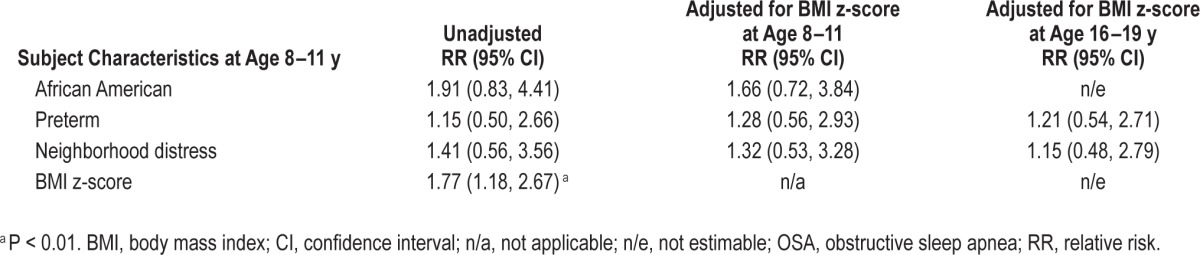
As we reported before,2,8 comparison of children with and without OSA at 8–11 y revealed that a significantly greater proportion of children with OSA were African American, of preterm birth, living in a distressed neighborhood, and habitual snorers (Table 1). However, none of these characteristics predicted OSA at 16–19 y in unadjusted analyses (Tables 1 and 2) or in BMI-adjusted analyses (Table 2). Similar results were observed in secondary analyses stratified by term status (Table S2, supplemental material) and secondary analyses restricted to nonobese participants (Table S3, supplemental material). Child characteristics at age 8–11 y that were associated with OSA at age 16–19 years were male sex, obesity, higher BMI z-score, and history of tonsillectomy or adenoidectomy (Table 1).
Table 1.
Participant characteristics by OSA status, at middle childhood (age 8–11 y) and late adolescent examinations (age 16–19 y) (N = 490); Cleveland Children's Sleep and Health Cohort.
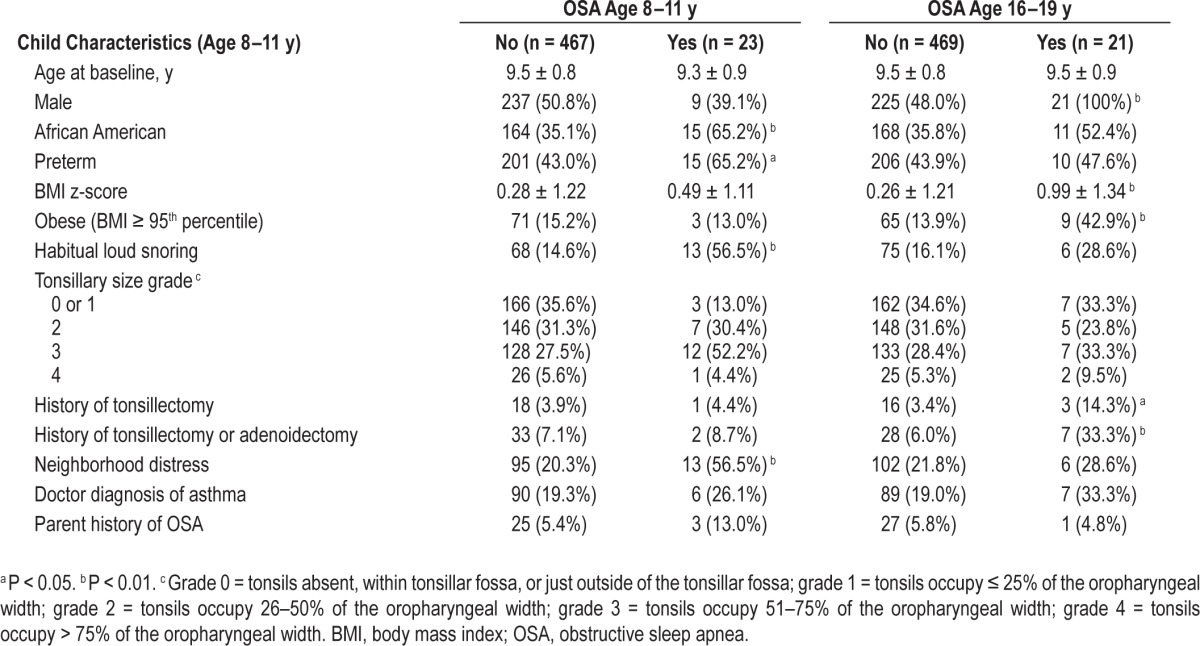
Table 2.
Unadjusted and body mass index-adjusted relative risk of obstructive sleep apnea at age 16–19 y.
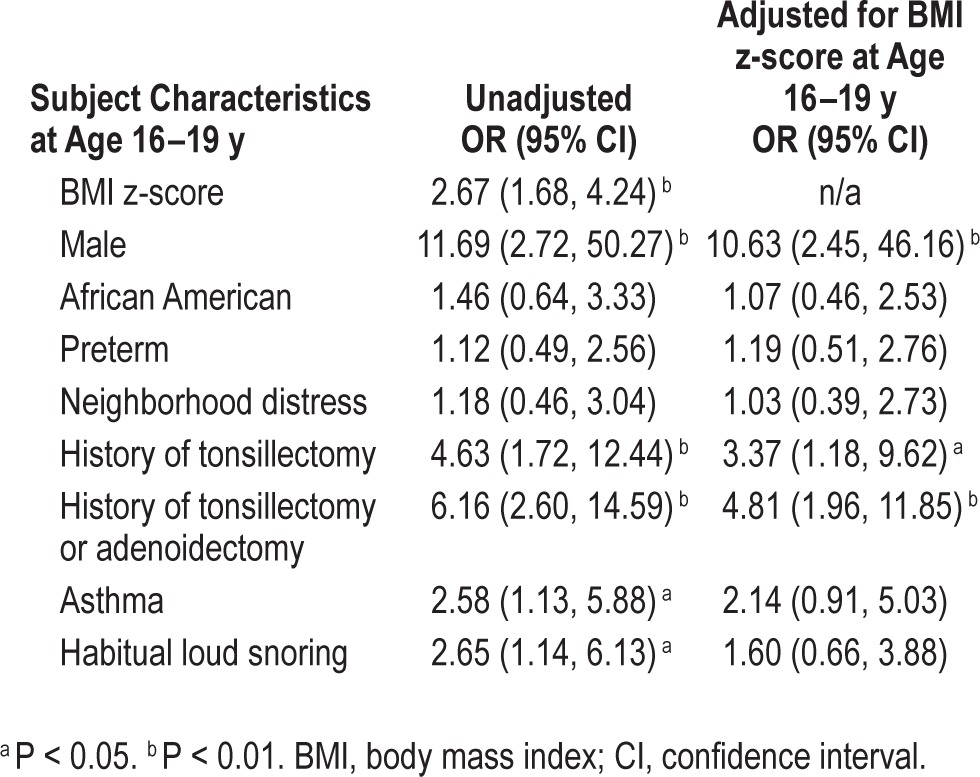
Cross-sectional, unadjusted analyses of characteristics at age 16–19 y revealed that BMI z-score, obesity, male sex, history of tonsillectomy or adenoidectomy, asthma, and habitual snoring were positively associated with adolescent OSA (Tables 3 and 4). After adjusting for BMI z-score, male sex and history of tonsillectomy or adenoidectomy remained significant (Table 4). In secondary BMI-adjusted analyses restricted to full-term participants, males and participants with asthma had significantly increased odds of adolescent OSA (Table S4, supplemental material).
Table 3.
Participant characteristics by obstructive sleep apnea status, at the late adolescent examination (age 16–19 y) (N = 515).
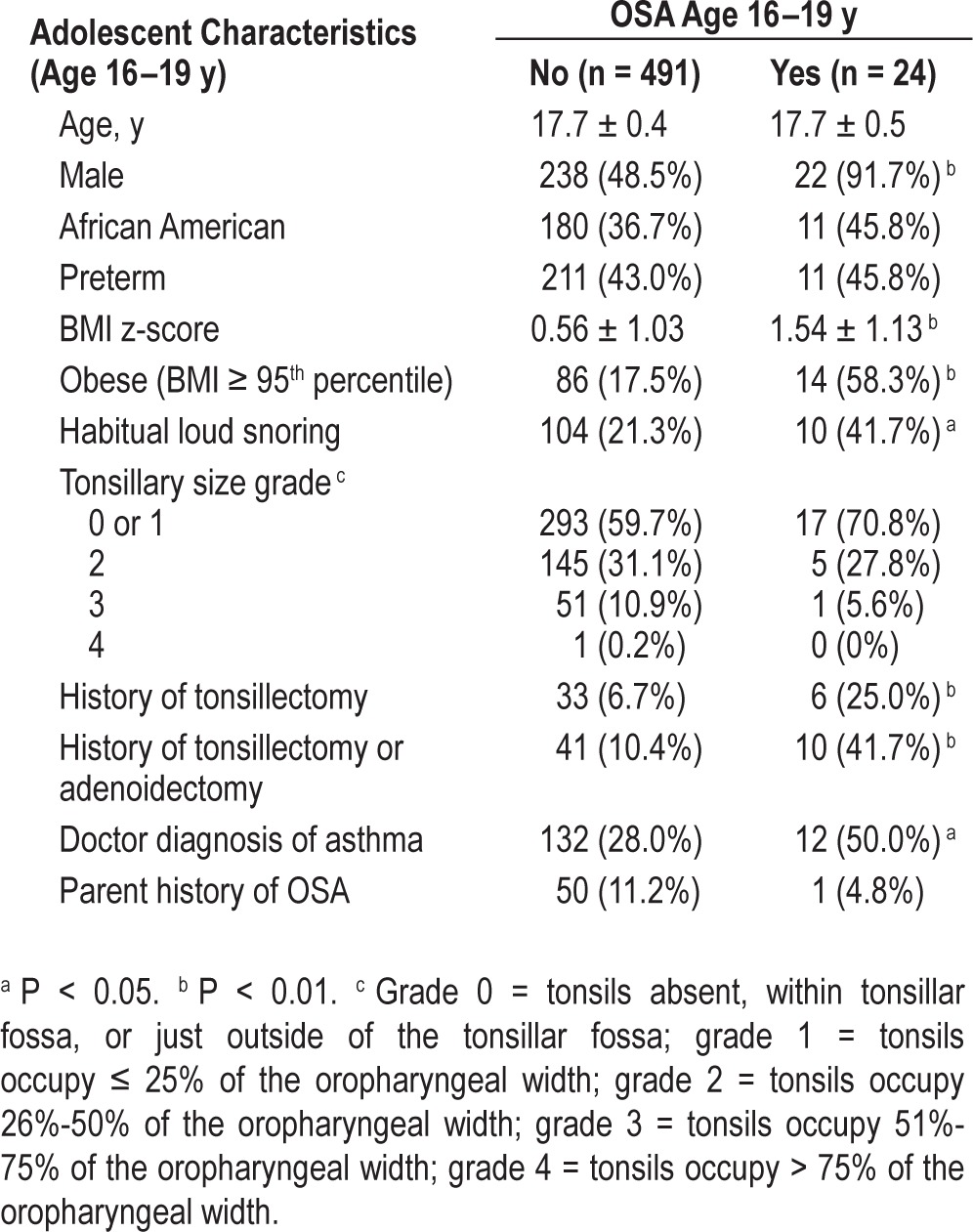
Table 4.
Cross-sectional odds ratios of obstructive sleep apnea at age 16–19 y (N = 515; n = 24 with obstructive sleep apnea).
Habitual Snoring and OSA
Habitual snoring at age 8–11 y was not significantly associated with incident OSA at age 16–19 y. Among the 68 children without OSA who habitually snored at 8–11 y, 5 (7.3%) developed OSA at age 16–19 y. In comparison, 14 of the 397 individuals without OSA who were nonsnorers (3.5%) developed OSA (unadjusted RR = 2.09; 95% CI: 0.78, 5.60; BMI-adjusted RR = 1.55; 95% CI: 0.57, 4.27).
Among the 513 participants with available snoring data (caregiver or self-report) at both time points, 16.2% of the 8- to 11-y-olds and 22.4% of the adolescents were habitual snorers. Half (50.6%) of the habitual snorers at age 8–11 y remained so at age 16–19 y (P = 0.003). Seventeen percent of the 430 nonsnorers at age 8–11 y developed habitual snoring by age 16–19 y.
Alternative OSA and SDB Definitions
Unadjusted analyses using a broader definition of SDB (PSG-measured OSA, habitual snoring, or both) showed that SDB in adolescence was associated with adolescent BMI z-score, male sex, African American background, previous tonsillectomy or adenoidectomy, and asthma (Table S5, supplemental material). All covariates except asthma retained significance in a model adjusted for all characteristics simultaneously.
Sensitivity analyses redefining OSA using a threshold of OAHI ≥ 1 showed that 47.2% of the 53 children now classified as having OSA at age 8–11 y also had OSA at age 16–19 y (P < 0.001), and 27.7% of the 437 children without OSA at 8–11 y developed OSA by 16–19 y. Moreover, 31.7% of the children with habitual snoring but not OSA at 8–11 y developed OSA at age 16–19 y, using the redefinition of OSA at both time points.
DISCUSSION
Knowledge about OSA's natural history during childhood and adolescence is scant, limiting clinical decision-making. Early, aggressive OSA diagnosis in early childhood may be appropriate if OSA persists or progresses over time. However, if OSA usually remits, then early diagnosis may be less critical than ongoing evaluation of OSA-related symptoms and signs across childhood. The Childhood Adenotonsillectomy Trial's recent report estimated that 49% of 5- to 9-y-old tonsillectomy candidates randomized to watchful waiting had remission of PSG evidence for OSA after 7 mo.27 To our knowledge, this is the first report of OSA's natural history from middle childhood to adolescence and its associated risks based on objectively determined OSA status in a community-based cohort with substantial representation of African American and former preterm children, groups previously associated with increased OSA risk.2
In this study, approximately 4% of children and adolescents met a conservative criterion for OSA (OAHI ≥ 5) at each time point. However, different children were affected during middle childhood and adolescence, indicating that OSA identified in a community-based sample rarely persists from middle childhood through late adolescence: During our 8-y period, 91% of middle childhood cases remitted. Most adolescents with OSA were incident cases, occurring in individuals without either OSA or habitual snoring earlier in childhood.
Habitual snoring displayed greater persistence: half of habitual snorers in middle childhood remained so in adolescence, although most of them did not progress to OSA at late adolescence, using our conservative criterion for OSA. Persistence and lack of progression of habitual snoring has been reported elsewhere.14,19 However, a recent report from a Chinese community-based cohort of 6- to 13-y-old children with primary snoring followed approximately 4 y found that 37.1% of the children progressed from primary snoring to OSA, using a liberal OSA definition (OAHI ≥ 1).21 We obtained a similar result (31.7%) when we used this OSA definition in sensitivity analyses. The clinical significance of the temporal concordance of these milder SDB forms is unclear.
Our observed rates for OSA persistence (8.7%) and incidence (4% over 8.2 y) were much lower than those reported from a southwestern US cohort of White and Hispanic children age 6 to 17 y (Tucson Children's Assessment of Sleep Apnea Study, or TuCasa), which found 29% SDB persistence from baseline (M = 8.5 y) to follow-up (M = 14.7 y) and 10% incidence over 5 y.18 The large rate differences between the two longitudinal studies underscore the sensitivity of OSA to the event definitions used (TuCasa used a threshold of one event per hour and included central events). When we explored using an OSA definition similar to TuCasa (OAHI ≥ 1), our OSA incidence increased from 4.0% to 27.7%, and persistence increased from 8.7% to 47.2%.
Similar to findings in children followed from infancy to early childhood,17 our findings revealed little overlap in OSA risk factors in middle childhood compared to late adolescence. Congruent with our previous reports,2,8 African American race, preterm status, and neighborhood distress were risk factors for OSA at age 8–11 y, but not in adolescence. In contrast, new risk factors emerged: male sex and history of tonsillectomy or adenoidectomy. Additionally, adolescents with OSA had a greater BMI compared to their peers without OSA both at adolescence and at middle childhood, even though most of them did not have OSA when younger. The association of obesity with development of OSA has been similarly reported among children with primary snoring.21 Although the association between asthma and OSA was partially confounded with obesity and sex, a higher OSA rate in children with asthma is consistent with prior data.28 Exploratory analyses stratified the sample by obesity or by full-term/preterm status. Although sample sizes were small, observed associations were generally similar to those for the full sample, except for possibly stronger associations between OSA and history of tonsillectomy or adenoidectomy among former preterm participants, and between OSA and asthma among full-term participants (Tables S2–S4).
The identification of male sex as an adolescent OSA risk factor mirrors previous reports.18,20 Sex effects have been reported to be weaker in prepubertal children.17 These findings indicate that sex differences may differentially modulate OSA risk in children in peripubertal and postpubertal periods.29–31
African American race was more strongly associated with OSA in younger compared to older children, for unclear reasons. African American race likely represents a combination of sociocultural, environmental, and genetic risk factors whose influence may vary in association with growth and development. Perhaps the OR = 1.91 for African American race for adolescent OSA, though elevated, failed to reach significance given the relatively small number of children with OSA in this community-based sample. The significant association observed between SDB and African American race among adolescents supports this notion.
Habitual snoring was associated with OSA at each time point. However, snoring during middle childhood did not predict adolescent OSA using our conservative definition of OSA, indicating the greater importance of middle childhood obesity compared to snoring in predicting adolescent OSA, and providing further support for preventing and treating obesity in childhood. Also, snoring, though relatively stable across the ages of 8–18 y and associated with OSA cross-sectionally, did not by itself strongly predict incident OSA, a finding congruent with that reported for a small clinical sample of younger children.15
Anatomical and developmental changes may partially explain shifts in OSA risk factors from childhood through adolescence. Across childhood, large changes occur in upper airway anatomy and pharyngeal airway collapsibility,32 and in body fat distribution, tonsillar size, lung size, and hormonal levels. Younger children may be more sensitive to environmental irritants and to the effects of smaller lung volumes or central ventilatory instability associated with prematurity, whereas older children with larger lung volumes and pharyngeal dimensions may be more susceptible to influences associated with obesity and male sex, reflecting the dynamic complexity of factors influencing airway size and patency across childhood. Of note, a history of tonsillectomy or adenoidectomy was an OSA risk factor at age 16–19 y. OSA in children who have had a tonsil-lectomy has been associated with familial risk of OSA33 and suggests that a history of tonsillectomy may identify children at risk for OSA because of factors extending beyond lymphoid hypertrophy34 (i.e., a “history of tonsillectomy or adenoidectomy” is functioning as a marker of other risk factors). Furthermore, children whose tonsils and/or adenoids were removed may not have been cured, especially if they were obese or African American, two groups in whom symptoms are less likely to resolve after surgery.12,33
Study limitations should be noted. First, certain methodological aspects of the study might have underestimated or overestimated OSA. For example, technological assessments of OSA differed across time points, and results of a recent study comparing overnight polysomnography with respiratory polygraphy (similar to our in-home method) indicate that AHI calculated in home studies may be underestimated because of (1) missed hypopneas causing arousals without desaturation and (2) use of total recording time as the denominator instead of total sleep time, which can be derived from polysomnography.35 Also, approximately 20% of participants at each time point had asthma. Children with asthma are more likely to experience desaturations (especially in rapid eye movement), which are likely caused by their lung condition and underlying sleep neurobiology, not obstruction per se,36 potentially leading to an over-estimation of OSA in this population. Regarding misestimations, we can note that in our study, a sample of children studied with both in-laboratory and in-home studies showed good agreement in AHI measured using both techniques,2 and it was unlikely that use of two techniques resulted in identifying unique groups of children at each examination. Second, using a common definition for OSA in pediatric populations is challenging. Our primary analysis used a conservative definition to maximize comparability and clinical relevance in adolescents. Secondary analyses showed that prevalence and incidence rates increased markedly as thresholds for abnormality were lowered. Third, representation of Hispanic and Asian children was small, limiting generalizability. Fourth, over the 8-y follow-up period, only 57% of the original cohort was studied. However, participants who were and were not followed up were similar, except that caregiver education was lower for those who were not followed up. Finally, the relatively few OSA cases limited power to detect weaker risk factors and precluded adjusting for multiple confounders.
In conclusion, study findings suggest that adolescents present with a more adult-like OSA profile,37 which has important implications for pediatric screening for OSA through clinical history: specifically, screening must be tailored to specific age ranges. Perhaps establishing age-based cutoffs of AHI scores for diagnosis of OSA (e.g., more liberal cutoff for younger ages, and a more conservative cutoff for older ages) might also be appropriate. At this point in time, more research is needed to identify the most appropriate cutoffs for adverse health outcomes in children, teens, and young adults. Findings also underscore the need to prevent obesity in early childhood, which may reduce the likelihood of adolescent OSA. Children who have had tonsillectomy or adenoidectomy are at increased risk for adolescent OSA because they likely have additional risk factors for OSA and should be monitored for OSA symptoms and signs. In the community, untreated OSA in middle childhood usually does not persist. Similarly, habitual snoring usually does not progress to OSA from middle childhood to adolescence. However, this lack of progression does not mean that habitual snoring is unimportant or harmless. Ultimately, concerns about patients' current symptoms and sequelae should guide decisions regarding surgery for the full spectrum of pediatric SDB, not just concerns over persistence or incidence of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by NIH HL07567, HL60957, UL1-RR024989, the Case Western Reserve University Transdisciplinary Research in Energetics and Cancer Center (1U54CA116867) and Harvard Transdisciplinary Research in Energetics and Cancer Center (1U54CA155626). Dr. Spilsbury has received research grant support from the National Institutes of Health and the William T. Grant Foundation. Dr. Redline reports that Brigham and Women's Hospital received a grant from ResMed Foundation and ResMed Inc. and equipment from both ResMed Inc and Philips-Respironics for use in clinical trials. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the families participating in the CCSHS, whose generosity made this study possible.
Footnotes
A commentary on this article appears in this issue on page 11.
SUPPLEMENTAL MATERIAL
SUPPLEMENTAL METHODS
Sleep Study Scoring
Middle-childhood assessment
In-home, sleep-apnea monitoring was conducted with a Type III sleep monitor recording thoracic and abdominal excursions and estimated tidal volume, pulse oximetry, heart rate, and body position (PT-2 system, SensorMedics, Yorba Linda, CA). Respiratory events were scored if ≥ 8 sec long (or two or more missed respiratory cycles). The use of an 8-sec duration accounted for children's faster respiratory rate than in adults (or late adolescents) and lower functional residual capacity, which can lead to more rapid desaturation with short respiratory events. Obstructive apneas were scored when chest and abdominal efforts were asynchronous and estimated tidal volume was absent or nearly absent, irrespective of associated desaturation. Hypopneas were scored when respiratory efforts were accompanied by a 50% reduction in estimated tidal volume and accompanied by ≥ 3% oxyhemoglobin desaturation.
Adolescent assessment
Overnight polysomnography (PSG) and physiological and anthropometric assessments, including a physician-administered physical exam, followed a standardized protocol at the research center, beginning at approximately 17:00 and ending the following day at 11:00.1,2 The PSG recording (Compu-medics E-series; Compumedics, Abbotsford, Australia) consisted of measurement of two electroencephalograms (C3/C2 and C4/C1), bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (nasal–oral thermocouple nasal pressure recording), finger-pulse oximetry, electrocardiogram, body position, and bilateral leg movements. Obstructive apneas were scored when a complete or nearly complete absence of airflow occurred on the thermistry channel for ≥ 10 sec in association with respiratory effort. Hypopneas were identified as an approximately 50% reduction in airflow or summed respiratory excursions associated with an oxygen desaturation of ≥ 3%.
For both examinations, the obstructive apnea-hypopnea index (OAHI) was defined as all obstructive or mixed apneas and hypopneas with a ≥ 3% desaturation per sleep hour.
Comparability of data from the middle childhood to late adolescent examination
A sample of 112 children underwent both in-home sleep apnea testing and full in-laboratory PSG (sleep staging, nasal-oral airflow, respiratory effort, oximetry, and electrocardiography). In a subsample of 55 children who underwent both tests within 3 mo of the other, the mean OAHI index was 2.6 ± 8.0 and 2.9 ± 7.5 for laboratory versus home studies, respectively (intraclass correlation coefficient = 0.85). Furthermore, there was no evidence that inclusion of arousals in the definitions of hypopneas appreciably altered the AHI estimates. In the group of 112 children with in-laboratory PSG at the middle school examination, the mean (paired) difference in AHI for the index derived by scoring hypopneas with corroborating desaturation only compared to hypopneas scored with either desaturation or arousal, was -0.09 (standard deviation = 0.19), and the maximum difference was -1.55. The measures were highly correlated: Spearman r = 0.99 (P < 0.0001), with minimal observed differences between the two measures.
Parental Notification of Sleep Study Results
In both the middle childhood and adolescent examinations, parents of all participants received a letter from the investigators describing the results of the sleep study. In cases where the children exhibited five or more breathing pauses per hour, the letter recommended that parents contact their child's doctor so that the child's breathing could be rechecked.
Measurement of Tonsillary Size
Tonsillar hypertrophy was assessed using a five-point scale3 with scores of 0 to 4: Grade 0 = tonsils absent or within the tonsillar fossa; Grade 1 = tonsils just outside of the tonsillar fossa and occupy ≤ 25% of the oropharyngeal width; Grade 2 = tonsils occupy 26%-50% of the oropharyngeal width; Grade 3 = tonsils occupy 51%-75% of the oropharyngeal width; Grade 4 = tonsils occupy > 75% of the oropharyngeal width. For analytic purposes, Grades 0 and 1 were combined into one category, which represents minimal tissue present in the airway.
Determination of Residence in a Distressed Neighborhood
Residence in a socioeconomically distressed neighborhood was determined by matching participants' residential addresses at the first examination to the corresponding 2000 US Census tract, and then categorizing neighborhood of residence as distressed if the census tract had values ≥ 1 standard deviation above the mean for all US census tracts on at least three of the four following measures: poverty rate, proportion of families with related children headed by single females, high school dropout rate, and proportion of civilian, noninstitutionalized, working age (16–64 y) males unemployed or not in the labor force.4,5
Description of Logistic Regressions
Four sets of logistic regression models were fitted to examine adolescent risk factors associated with sleep disordered breathing (SDB) at age 16–19 y: (1) unadjusted; (2) adjusted for body mass index (BMI) z-score at age 16–19 y; and 3) adjusted for known risk factors: BMI z-score, male sex, African American race, preterm status, neighborhood distress, history of tonsillectomy, physician-diagnosed asthma; (4) same as (3) except adjusted for history of tonsillectomy or adenoidectomy.
SUPPLEMENTAL RESULTS
Race/Ethnicity and Premature Birth Details
The sample was 36.5% African American, and 63.5% other, with other consisting almost entirely of white participants (93%), followed by 4% multiracial/biracial, and 2% Hispanic. The mean gestational age of the former preterm participants was 31 ± 3 w, mean birth weight was 1517 ± 568 g, and 21.3% weighed < 1000 g at birth.
Cleveland Children Sleep and Health Study: participant flowchart between study time points. OSA, obstructive sleep apnea; PSG, polysomnography.
Baseline participant characteristics (age 8–11 y) by participation at follow-up (age 16–19 y).
Unadjusted relative risk of obstructive sleep apnea at age 16–19 y stratified by term status.
Unadjusted relative risk of obstructive sleep apnea at age 16–19 y among nonobese participants only.
Cross-sectional odds ratios of obstructive sleep apnea at age 16–19 y restricted to full-term children (N = 293; n = 13 with obstructive sleep apnea).
Cross-sectional odds ratios of sleep disordered breathing (obstructive sleep apnea or habitual loud snoring) at age 16–19 y (N = 513; n = 128 with sleep disordered breathing).
REFERENCES
- 1.Anuntaseree W, Kuasirikul S, Suntornlohanakul S. Natural history of snoring and obstructive sleep apnea in Thai school-age children. Pediatr Pulmonol. 2005;39:415–20. doi: 10.1002/ppul.20207. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 3.Schlaud M, Urschitz MS, Urschitz-Duprat PM, Poets CF. The German study on sleep-disordered breathing in primary school children: epidemiological approach, representatives of study sample, and preliminary screening results. Paediatr Perinat Epidemiol. 2004;18:431–40. doi: 10.1111/j.1365-3016.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 4.Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3-11-year old Turkish children. Pediatr Pulmonol. 2005;39:251–6. doi: 10.1002/ppul.20179. [DOI] [PubMed] [Google Scholar]
- 5.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44:417–22. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RB, Kelly J. Behavior, neurocognition, and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2006;70:395–406. doi: 10.1016/j.ijporl.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Bonuck KA, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediat Otorhinolaryngol. 2006;70:769–78. doi: 10.1016/j.ijporl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Brooks LJ, Draper KR, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin JL, Babar SI, Kaemingk KL, et al. Symptoms related to sleep-disordered breathing in White and Hispanic children: the Tucson children's assessment of sleep apnea study. Chest. 2003;124:196–203. doi: 10.1378/chest.124.1.196. [DOI] [PubMed] [Google Scholar]
- 11.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in Whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 12.Redline SS, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 13.Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71:74–6. doi: 10.1136/adc.71.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–90. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Hamer A, Loughlin GM. Natural history of primary snoring in children. Pediatr Pulmonol. 1998;26:6–11. doi: 10.1002/(sici)1099-0496(199807)26:1<6::aid-ppul3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Topol HI, Brooks LJ. Follow-up of primary snoring in children. J Pediatr. 2001;138:291–3. doi: 10.1067/mpd.2001.110122. [DOI] [PubMed] [Google Scholar]
- 17.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34:875–84. doi: 10.5665/SLEEP.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6- to 17-year old children--the Tucson children's assessment of sleep apnea study. J Pediatr. 2010;157:57–61. doi: 10.1016/j.jpeds.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urschitz MS, Guenther A, Eitner S, et al. Risk factors and natural history of habitual snoring. Chest. 2004;126:790–800. doi: 10.1378/chest.126.3.790. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Armengol A, Ruiz-Garcia A, Carmona-Bernal C, et al. Clinical and polygraphic evolution of sleep-related breathing disorders in adolescents. Eur Respir J. 2008;32:1016–22. doi: 10.1183/09031936.00133907. [DOI] [PubMed] [Google Scholar]
- 21.Li AM, Zhu Y, Au CT, et al. Natural history of primary snoring in school-aged children: a 4-year follow-up study. Chest. 2013;143:729–35. doi: 10.1378/chest.12-1224. [DOI] [PubMed] [Google Scholar]
- 22.Weinstock TG, Marcus CL, Amin RS, et al. Obstructive sleep apnea severity and associated co-morbidities in candidates for adenotonsillectomy: the Childhood Adenotonsillectomy (CHAT) Study. Am J Respir Crit Care Med. 2012:A1055. [Google Scholar]
- 23.Kump K, Whalen C, Tishler PV, et al. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respir Crit Care Med. 1994;150:735–41. doi: 10.1164/ajrccm.150.3.8087345. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents' fat and carbohydrate consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hare W, Mather M. Baltimore, MD: the Annie E. Casey Foundation and Population Reference Bureau; 2003. [Accessed June 24, 2014]. The growing number of kids in severely distressed neighborhoods: evidence from the 2000 Census. Available at: http://www.colorado.edu/journals/cye/13_2/Reprints13_2/KidsInDistressedNeighborhoods/SeverelyDistressedNeighborhoods.pdf. [Google Scholar]
- 27.Marcus C, Moore R, Rosen C, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea (CHAT) N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross KR, Hart MA, Storfer-Isser A, et al. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatr Pulmonol. 2009;44:877–84. doi: 10.1002/ppul.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cistulli P, Grunstein R, Sullivan C. Effect of testosterone administration on upper airway collapsibility during sleep. Am J Respir Crit Care Med. 1994;149:530–2. doi: 10.1164/ajrccm.149.2.8306057. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes-Pradera MA, Sanchez-Armengol A, Capote-Gil F, et al. Effects of sex on sleep-disordered breathing in adolescents. Eur Respir J. 2004;23:250–4. doi: 10.1183/09031936.03.00022003. [DOI] [PubMed] [Google Scholar]
- 31.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–96. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 33.Morton S, Rosen C, Larkin E, Tishler P, Aylor J, Redline S. Predictors of sleep-disordered breathing in children with a history of tonsillectomy and/or adenoidectomy. Sleep. 2001;54:823–9. doi: 10.1093/sleep/24.7.823. [DOI] [PubMed] [Google Scholar]
- 34.Guilleminault C, Huang Y, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg. 2007;136:169–75. doi: 10.1016/j.otohns.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Tan HL, Gozal D, Ramirez HM, Bandla HPR, Kheirandish-Gozal L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep. 2014;37:255–60. doi: 10.5665/sleep.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez GF, Gutierrez MJ, Shahlanoor H, et al. Oximetry signal processing identifies REM sleep-related vulnerability trait in asthmatic children. Sleep Disord. 2013. 2013:Article ID 406157, 6 pages, http://dx.doi.org/10.1155/2013/406157. [DOI] [PMC free article] [PubMed]
- 37.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin. 2007;2:433–44. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
SUPPLEMENTAL REFERENCES
- 1.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents' fat and carbohydrate consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–69. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 4.O'Hare W, Mather M. Baltimore, MD: the Annie E. Casey Foundation and Population Reference Bureau; 2003. The growing number of kids in severely distressed neighborhoods: evidence from the 2000 Census. [Google Scholar]
- 5.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cleveland Children Sleep and Health Study: participant flowchart between study time points. OSA, obstructive sleep apnea; PSG, polysomnography.
Baseline participant characteristics (age 8–11 y) by participation at follow-up (age 16–19 y).
Unadjusted relative risk of obstructive sleep apnea at age 16–19 y stratified by term status.
Unadjusted relative risk of obstructive sleep apnea at age 16–19 y among nonobese participants only.
Cross-sectional odds ratios of obstructive sleep apnea at age 16–19 y restricted to full-term children (N = 293; n = 13 with obstructive sleep apnea).
Cross-sectional odds ratios of sleep disordered breathing (obstructive sleep apnea or habitual loud snoring) at age 16–19 y (N = 513; n = 128 with sleep disordered breathing).


