Abstract
Study Objectives:
D. melanogaster is an excellent animal model to study how the circadian (≅ 24-h) timing system and sleep regulate daily wake-sleep cycles. Splicing of a temperature-sensitive 3'-terminal intron (termed dmpi8) from the circadian clock gene period (per) regulates the distribution of daily activity in Drosophila. The role of dmpi8 splicing on daily behavior was further evaluated by analyzing sleep.
Design:
Transgenic flies of the same genetic background but expressing either a wild-type recombinant per gene or one where the efficiency of dmpi8 splicing was increased were exposed to different temperatures in daily light-dark cycles and sleep parameters measured. In addition, transgenic flies were briefly exposed to a variety of sensory-mediated stimuli to measure arousal responses.
Results:
Surprisingly, we show that the effect of dmpi8 splicing on daytime activity levels does not involve a circadian role for per but is linked to adjustments in sensory-dependent arousal and sleep behavior. Genetically altered flies with high dmpi8 splicing efficiency remain aroused longer following short treatments with light and non-photic cues such as mechanical stimulation.
Conclusions:
We propose that the thermal regulation of dmpi8 splicing acts as a temperature-calibrated rheostat in a novel arousal mechanism, so that on warm days the inefficient splicing of the dmpi8 intron triggers an increase in quiescence by decreasing sensory-mediated arousal, thus ensuring flies minimize being active during the hot midday sun despite the presence of light in the environment, which is usually a strong arousal cue for diurnal animals.
Citation:
Cao W, Edery I. A novel pathway for sensory-mediated arousal involves splicing of an intron in the period clock gene. SLEEP 2015;38(1):41–51.
Keywords: circadian rhythms, Drosophila, period gene, pre-mRNA splicing, sleep, arousal, light, temperature
INTRODUCTION
Animals exhibit daily wake-sleep cycles that are partially controlled by interacting networks of cell-based circadian (≅ 24-h) “clocks” or pacemakers located in the brain.1–3 Drosophila melanogaster is an excellent animal model system to study the mechanisms underlying circadian rhythms and sleep.4–6 Like many diurnal animals, the daily distribution of activity in D. melanogaster is largely bimodal with clock-controlled morning and evening peaks separated by a period of relative inactivity generally referred to as the midday “siesta.”7 Temperature is a potent environmental signal that modulates the daily distribution of activity in animals.8,9 In D. melanogaster, increases in daily average temperature are accompanied by a gradual delay in the onset of the evening bout of activity, a more robust midday siesta, and although less significant in magnitude, an earlier offset in morning activity.8,10,11 This thermally regulated behavioral plasticity likely endows D. melanogaster with the ability to gate activity to times in the day when the temperature is more optimal. For example, the shift in activity towards the cooler nighttime hours on warm days likely minimizes the risks associated with being active during the hot midday sun.
We showed that this temperature-dependent behavioral adaptation in the distribution of daily activity is partially controlled by thermosensitive splicing of a short intron found in the 3' untranslated region (UTR) from the Drosophila melanogaster period (dper) transcript,8,10,11 a fundamental circadian “clock gene” in this species.12 Expression of dper is under circadian regulation via a negative transcriptional-translational feedback loop (TTFL), which contributes to daily cycles in dper RNA and protein levels, molecular oscillations that are central to setting the pace of the clock and hence timing of daily activity.2 Splicing of the dper 3'-terminal intron, named dmpi8 (D. melanogaster per intron 8), becomes progressively less efficient as temperature increases.8 Although temperature has the most potent effects on dmpi8 splicing efficiency, light induces moderate reductions in dmpi8 intron removal.13,14 Thus, on warm days the combination of heat and light results in daytime levels of dmpi8 splicing that are relatively low. Numerous lines of evidence indicate that splicing of the dmpi8 intron adjusts the levels of dper transcripts, which in turn modulates the accumulation phase of these transcripts during the mid-to-late day.8,10,11 For example, on warm days the inefficient splicing of dmpi8 reduces overall dper mRNA levels and delays its daily upswing. Although it is not clear how dmpi8 regulates dper mRNA levels, splicing is required as opposed to the presence or absence of intronic sequences in the transcript, suggesting spliceosome assembly at the dmpi8 intron enhances the production of mature dper mRNAs.8
The mechanism underlying the thermal sensitivity of dmpi8 splicing is based on suboptimal 5' and 3' splicing signals (ss), suggesting that splice site recognition/binding by the spliceosome to dmpi8 is inefficient at higher temperatures.11 To mechanistically link changes in dmpi8 splicing with alterations in the distribution of daily activity, we generated transgenic flies where the efficiency of dmpi8 intron removal was altered.11 Most notably, transgenic flies wherein the strengths of the dmpi8 5' and 3'ss were increased (termed M2M1) exhibit higher dper mRNA levels, earlier evening activity, slightly later morning activity and shorter midday siesta. Together, the findings suggested a clock-based model wherein dmpi8 splicing plays a prominent role in the seasonal adaptation of behavioral programs in D. melanogaster by primary effects on the clock mechanism, which ultimately controls the timing of the morning and evening bouts of activity. For example, on warm days because both heat and light act in sync to reduce splicing of dmpi8 this leads to a later timing in the daily accumulation of dper transcripts, which we proposed contributes to the delay in the onset of evening activity and as a result a concomitant lengthening in the duration of the midday siesta.
This aforementioned model was based on the well-established role of per genes as the key components regulating the pace of circadian clocks in animals.15,16 However, the daily wake/sleep patterns of animals result from a balance of several interacting systems, including circadian, sleep homeostatic, and arousal pathways that are themselves influenced by external environmental stimuli, such as light and temperature.17,18 While the circadian system regulates the timing of sleep, its role in sleep duration or quality, parameters normally more associated with mechanisms governing sleep homeostasis, is less clear. In this study we made the surprising discovery that splicing of the dmpi8 intron has strong effects on midday activity levels via a non-circadian mechanism that involves changes in sensory-dependent arousal and sleep quality. Intriguingly, genetically altered flies with high dmpi8 splicing efficiency (e.g., M2M1) are more responsive to the wake-promoting effects of brief light exposure, a phenomenon also observed with non-photic cues, such as mechanical stimulation. We propose that on warm days the weak splicing of dmpi8 increases thresholds for sensory-dependent arousal, tipping the wake/sleep balance towards extended periods of daytime quiescence, thus ensuring flies are less active during the hot midday sun despite the presence of light in the environment, which normally acts as an arousal cue. If on the other hand, flies encounter a cold day, the more efficient splicing of dmpi8 favors wakefulness during the midday, enabling flies to take advantage of the warmer daytime hours without the deleterious risks associated from intense heat.
METHODS
Fly Strains and General Handling
All flies were routinely reared at room temperature (22–25°C) and maintained in vials or bottles containing standard agar-cornmeal-sugar-yeast-Tegosept-media. The w per01; p{dmper/ M2M1} and w per01; p{dmper/8:8} flies used in this study were as previously described; for p{dmper/8:8} we used lines f9, f46; for p{dmper/M2M1} we used lines m17, m32.11 In addition, we generated additional lines using the same transgenes, transgenic facility, and crossing scheme as before.11 This was done to increase the number of independent lines with per transgenes in different genetic loci, increasing the strength of the results by pooling data from multiple fly strains. Briefly, new independent lines of p{dmper/M2M1} and p{dmper/8:8} flies were produced by Genetic Services, Inc. (Sudbury, MA, USA) in a w1118 background and subsequently crossed into a w per01 background with a double balancer line (w per01;Sco/CyO;MKRS/TM6B). We used the following lines from this new batch; p{dmper/8:8}, m38-K; p{dmper/M2M1}, f13. For each genotype, similar results were obtained when assaying behavior or molecular phenotypes (data not shown).
Behavioral Assays
Locomotor activity rhythms and sleep
Individual adult male or female flies (1–5 day old) were placed in 65 mm × 5 mm glass tubes containing 5% sucrose with 2% Bacto agar. Locomotor activity was continuously monitored and recorded by using the Trikinetics (Waltham, MA, USA) system, as previously reported.11,19 Briefly, throughout the testing period flies were maintained at the indicated temperature (18°, 25°, or 29°C) and subjected to ≥ 5 days of 12-h light: 12-h dark cycles [LD; where zeitgeber time 0 (ZT0) is defined as lights-on]. Cool white fluorescent light (∼1000 lux) was used during LD and the temperature did not vary by more than 0.5°C between the light and dark periods. In general, after 5 days in LD, flies were kept in constant darkness (DD) or constant light (LL) for 7 days. Data analysis of either loco-motor activity or sleep parameters was done with the FaasX and Matlab programs, as previously described.11 Sleep was defined as no detection of locomotor activity movement for any period of 5 contiguous min, which is routinely used in the field.4 The values were based on pooling data from multiple individual flies of the same genotype, sex, and treatment. In general, for measuring sleep values or locomotor activity, the data for LD was an average of the last 3 LD days. For DD or LL, the values were from single days. Free-running periods of locomotor activity rhythms were based on the data collected during 6 consecutive days in DD and using the FaasX program (kindly provided by F. Rouyer, France), as previously described.11 For the arousal experiments, the data were from the 3 consecutive days when the stimulations were given (described below).20 P-values of significance were calculated by using Student t-test or ANOVA.
Arousal
We used two different stimuli for arousal; either a slight mechanical stimulation by scraping the tubes or a brief light pulse, based on a recent study measuring arousal in D. melanogaster.20 For experiments involving scraping, we used the older version of the Trikinetics activity monitors where the tubes housing flies are aligned next to each other on the same plane. Beginning on the third day of LD, one set of flies was rubbed at ZT6 for 3 consecutive days, whereas another group received the same treatment but was rubbed at ZT18; a third group served as controls and received no treatment. The slight mechanical stimulation was delivered by the back-and-forth scraping (3–4 times) of the tubes housing individual flies with a plastic rod that was hand held. Other methods of delivering a slight mechanical stimulation were also tried (including using more mechanized methods such as shaking the entire monitor) but we found the hand-held method of scraping fly tubes to be easy to deliver, reproducible and sub-saturating (i.e., not all the sleeping flies were awakened by this treatment) (data not shown). We tried several different temperatures for the mechanical stimulation and show results obtained at 25°C because they yielded the most consistent differences between the p{dmper/M2M1} and p{dmper/8:8} flies (data not shown). For the light pulse experiments, the p{dmper/M2M1} and p{dmper/8:8} flies were en-trained at 18°C for 5 days then placed in DD. Beginning on the first day of DD and for the next 2 days, one set of flies was exposed to 5 min of light at CT6, whereas another group was exposed to 5 min of light at CT18; a third group served as controls and was not exposed to light. For the light-pulse experiments, we mainly tried 5 min of light, which was saturating (i.e., the majority of sleeping flies, if not all, were awake during the light phase). In addition, although similar results were obtained at other temperatures, the largest consistent differences between the p{dmper/M2M1} and p{dmper/8:8} flies were obtained at 18°C (data not shown).
RESULTS
Splicing of the dmpi8 intron regulates daytime sleep but has little to no effect on nighttime sleep during a daily light-dark cycle
In a prior study we examined the role of dmpi8 splicing on daily behavior by generating transgenic flies wherein the strengths of the 5' and 3' splice sites (ss) of dmpi8 were increased (termed p{dmper/M2M1}) and compared their daily locomotor activity profiles to control transgenics with the wild-type copy of dmpi8 (termed p{dmper/8:8}).11 The transgenes are in the per01 genetic background and hence supply the only functional copies of dper. To further investigate the effects of dmpi8 splicing efficiency on the daily wake-sleep cycles in D. melanogaster we measured sleep behavior. D. melanogaster is a well-established model to study sleep, which in this organism is routinely measured as no detectable locomotor activity for a contiguous period of (usually) 5 min.4,21 For each genotype, ≥ 3 independent transgenic lines of male and female adult flies were synchronized (entrained) to standard 12-h light/12-h dark cycles [LD; where zeitgeber time (ZT) 0 is lights-on] at different temperatures (18°, 25°, and 29°C), and then maintained for a week under constant dark conditions (DD) to measure free-running activity rhythms.
Analysis of locomotor activity rhythms verified that the p{dmper/8:8} and p{dmper/M2M1} flies used in this study behaved as previously reported (Figure S1, supplemental material).11 For example, compared to their wild-type controls, p{dmper/M2M1} flies exhibit later offset of morning activity, earlier onset of evening activity and a shorter duration midday siesta (Figure S1). Finally, in agreement with earlier findings, differences in the distribution of daily activity between p{dmper/M2M1} and p{dmper/8:8} flies are less apparent at higher temperatures (i.e., 29°C; Figure S1C and S1I),8,10,11 presumably because in combination with light, high temperatures have potent direct effects on reducing activity levels (termed “paradoxical” or negative masking; see Discussion).9,22
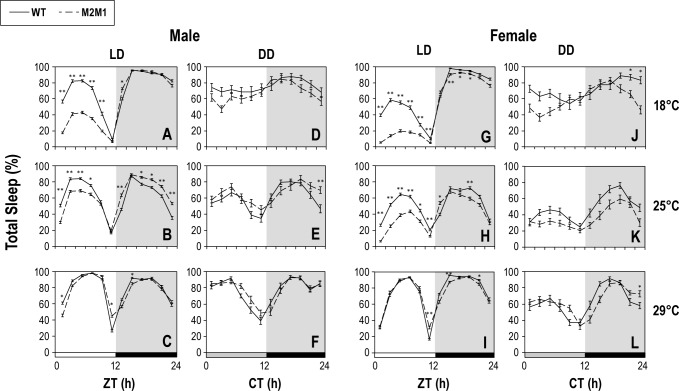
With regards to sleep in D. melanogaster, the majority occurs during the night, but some sleep is also observed during the day.4,23 Daytime sleep is usually limited to the middle of the day and is more fragmented (e.g., shorter sleep bout durations) compared to sleep at night.23 In addition, the proportion of flies sleeping during the midday increases as temperature rises and males exhibit more midday sleep compared to females.4,23 As expected, the control p{dmper/8:8} transgenics exhibit sleep patterns very similar to those previously described for wild-type strains, including the temperature and gender differences on daytime sleep (Figure 1A–1C and 1G–1I). Intriguingly, at 18° and 25°C, both sexes of p{dmper/ M2M1} flies show significantly reduced daytime sleep, whereas nighttime sleep is more similar to that observed in p{dmper/8:8} flies (Figure 1A, 1B, 1G, and 1H). We noted that in some cases the p{dmper/M2M1} flies actually show higher levels of sleep during some nighttime hours compared to the control wild-type p{dmper/8:8} flies (e.g., Figure 1B). However, this was not consistent for all temperatures, times of day, or sexes, and might be a consequence of a slight sleep rebound in the night due to strongly reduced sleep during the day.24,25 At the elevated temperature of 29°C, daytime sleep was similar for p{dmper/M2M1} and p{dmper/8:8} flies (Figure 1C and 1I), as might be expected based on previous reports showing that high temperatures strongly inhibit daytime activity in a manner that can override the status of dmpi8 splicing efficiency.8–11 Thus, the splicing efficiency of dmpi8 regulates daytime sleep, especially at lower temperatures, with little to no effect on nighttime sleep levels.
Figure 1.
The splicing efficiency of dmpi8 regulates daytime but not nighttime sleep in daily light-dark cycles. (A-L) Shown are group averages for the percentage of flies sleeping during 4-h time-windows throughout a 24-h day for either p{dmper/8:8} [indicated as wild-type (WT)] (black line) or p{dmper/ M2M1} (dashed black line) adult flies maintained at the indicated temperatures (right of panels) and light/dark conditions (top of panels). Results shown are for either male (A-F) or female (G-L) flies. For each genotype, gender, and entrainment condition, data from at least 16 flies was used to generate the sleep profiles shown. LD, 12-h light/12-h dark; DD, constant darkness. For LD, sleep data from the last 3 days were pooled, whereas for DD, only data from the first day were used. White, black, and dark gray horizontal bars denote periods of light, dark, and “subjective daytime” in DD, respectively. Values for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are significantly different using student's t-test; * P < 0.05; ** P < 0.005.
Daytime sleep levels in p{dmper/M2M1} and p{dmper/8:8} flies are similar in the absence of light
Because per is a critical gene involved in circadian regulation, we reasoned that the differential day/night sleep pattern between p{dmper/M2M1} and WT flies (i.e., p{dmper/8:8}) would continue in constant darkness (DD). Surprisingly, the daily sleep profiles are virtually identical for both genotypes in DD (Figure 1D–1F and 1J–1L). A circadian effect on the proportion of flies sleeping was observed in constant darkness, especially as temperature increases, but this was the case for both genotypes. Most notably, in constant darkness at the higher temperatures tested, both genotypes exhibit minimal levels of sleep just prior to what would have been lights-off (i.e., CT12), coincident with the clock-controlled evening bout of activity (e.g., Figure S1). Indeed, it is thought that the amplitude of the clock increases as ambient temperature rises.26 Thus, the clock-controlled evening bout of activity is strongly wake-promoting irrespective of the status of dmpi8 splicing, especially around peak levels of activity. Because the circadian effects on the temporal regulation of sleep observed in constant dark conditions are independent of dmpi8 splicing efficiency, this suggests a novel non-circadian role for this splicing event in regulating wake/sleep behavior in a manner dependent on the presence of light (see below).
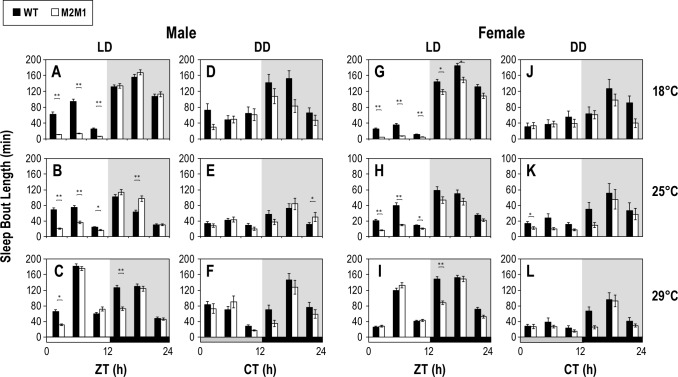
Role for dmpi8 splicing in regulating the duration of sleep bouts
To better probe the architecture of sleep we measured the average duration of sleep bouts and the number of sleep episodes, which are measures of sleep consolidation or quality. At 18° and 25°C, the median duration of a sleep bout during the day in a daily light-dark cycle is approximately 3–5 fold shorter in p{dmper/M2M1} flies, whereas during the night it is of similar duration to that observed in p{dmper/8:8} flies (Figure 2A, 2B, 2G and 2H). At 29°C, p{dmper/M2M1} male flies showed a consistently lower sleep duration compared to p{dmper/8:8} males (Figure 2C), but this was restricted to the early day (i.e., ZT0-4). Moreover, during the first half of the day in LD, p{dmper/M2M1} male flies also manifested more sleep episodes compared to male p{dmper/8:8} flies (data not shown). Consolidation of daytime sleep might be less affected in p{dmper/M2M1} females because of a ceiling effect as females already sleep much less during the midday.23 Importantly, even at 18° and 25°C, the difference in sleep bout length (and number of sleep episodes) between p{dmper/M2M1} and p{dmper/8:8} was smaller towards the end of the daytime hours (i.e., ZT8-12). As noted above, during the late day the clock acts in a strong wake-promoting manner by driving the evening bout of activity, further suggesting that during peak levels of evening activity dmpi8 splicing is not rate-limiting with regards to controlling activity levels. Both genotypes exhibit a similar temporal pattern of sleep bout length throughout a daily cycle in constant darkness (Figure 2D–2F and 2J–2L). Because sleep during the subjective night in DD (i.e., between CT12-24) is similar for both genotypes, this further supports the contention that any mild differences in nighttime sleep that we observed in LD cycles are due to secondary effects arising from more substantial alterations in daytime sleep behavior. As earlier work showed that light also suppresses dmpi8 splicing,13,14 this might contribute to the generally longer “daytime” sleep bout duration of p{dmper/8:8} flies in LD compared to DD (e.g., compare Figure 2B and 2E; see Discussion).
Figure 2.
Duration of sleep bouts during the early-to-mid day is much shorter in flies genetically altered to have highly efficient dmpi8 splicing. (A-L) The results are based on the flies used in Figure 1 and show group averages for median durations of sleep bouts for either p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) adult flies maintained at the indicated temperatures (right of panels) and light/dark conditions (top of panels). Results shown are for either male (A-F) or female (G-L) flies. For each genotype, gender, and entrainment condition, data from at least 16 flies were used to generate the sleep profiles shown. LD, 12-h light/12-h dark; DD, constant darkness. For LD, activity data from the last 3 days was pooled, whereas for DD, only data from the first day was used. White, black, and dark gray horizontal bars denote periods of light, dark, and “subjective daytime” in DD, respectively. Values for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are significantly different using student's t-test; * P < 0.05; ** P < 0.005.
During wake periods both p{dmper/M2M1} and p{dmper/8:8} flies showed the same activity levels (Figure S2A–S2R, supplemental material), indicating that dmpi8 splicing is unlikely to have direct effects on locomotion or health status that could be inadvertently diagnosed as changes in sleep/arousal. Finally, the timing of the first sleep episode (sleep onset or latency) following lights-on was generally earlier for p{dmper/8:8} flies compared to p{dmper/ M2M1} flies (Figure S2S–S2X), whereas the timing of sleep onset after dark was similar in both genotypes (data not shown). Thus, the splicing efficiency of dmpi8 has a large effect on total daytime sleep in both males and females by modulating the onset of sleep and the duration/maintenance of sleep episodes, especially at lower temperatures and during the early-to-mid day prior to the start of the evening bout of activity.
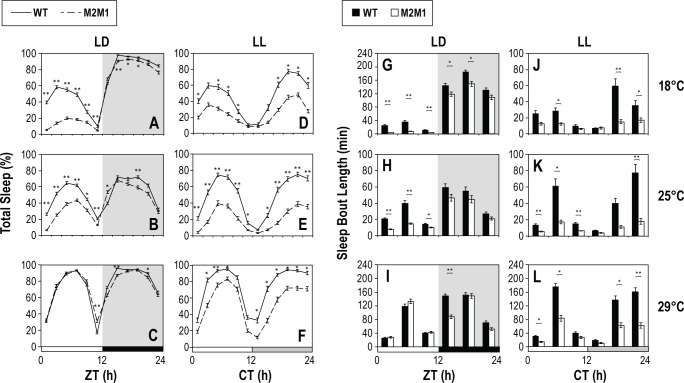
p{dmper/M2M1} flies sleep less during the nighttime in the presence of light
To further explore the role of light in mediating the day/night differences in sleep behavior between the p{dmper/M2M1} and p{dmper/8:8} flies, we exposed flies to 24 h of constant light (LL) following entrainment to a daily light-dark cycle (Figure 3). Much larger differences in sleep between p{dmper/ M2M1} and p{dmper/8:8} flies were observed during the “subjective” night (i.e., CT12-24) in the presence of light then compared to the same 12-h period in LD cycles (Figure 3). This was the case for total sleep (e.g., compare Figure 3D–3F to Figure 3A–3C) and average sleep bout duration (e.g., compare values between CT12-24 for Figure 3J–3L to 3G–3I). Again, differences in sleep parameters between p{dmper/M2M1} and p{dmper/8:8} flies were more apparent at the lower temperatures. Thus, even nighttime sleep can be more fragmented in p{dmper/M2M1} flies if exposed to light, indicating the effect of dmpi8 splicing efficiency on sleep behavior is not time-of-day restricted. Nonetheless, during the first day of LL differences in sleep behavior between p{dmper/M2M1} and p{dmper/8:8} flies are absent or reduced during the subjective late day/early night, coincident with the major clock-controlled evening bout of activity. This again reinforces the notion that the clock regulation of evening activity, especially during peak levels, involves a very potent wake-promoting signal that can override intrinsic differences in sleep behavior caused by variations in dmpi8 splicing efficiency.
Figure 3.
Exposure to light during the night strongly reduces sleep consolidation in p{dmper/M2M1} flies compared to wild-type controls. (A-F) Shown are group averages for the percentage of female flies sleeping during 4-h time-windows throughout a 24-h day for either p{dmper/8:8} (WT; black line) or p{dmper/ M2M1} (dashed black line) adult flies maintained at the indicated temperatures (right of panels) and light/dark conditions (top of panels). LD, 12-h light/12-h dark; LL, constant light. For LD, sleep data from the last 3 days were pooled (see Figure 1G–1I), whereas for LL, only data from the first day were used. White, black, and light gray horizontal bars denote periods of light, dark, and “subjective nighttime” in LL, respectively. (G-L) The results are based on the flies used in panels A-F [p{dmper/8:8} (WT; black rectangle) or p{dmper/M2M1} (white rectangle)] and show group averages for median durations of sleep bouts. For each genotype and entrainment condition, data from at least 16 flies were used to generate the profiles shown. Values for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are significantly different using student's t-test; * P < 0.05; ** P < 0.005. Male flies showed similar results as females (data not shown).
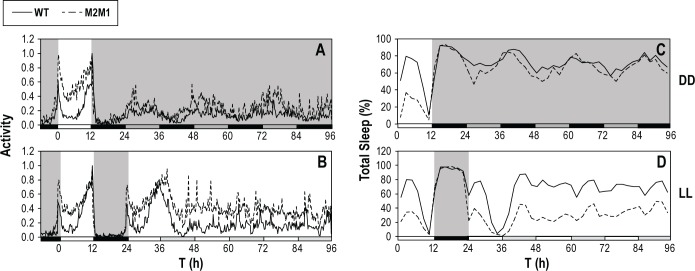
Remarkably, differences in the sleep behavior of p{dmper/ M2M1} and p{dmper/8:8} flies still occur after the third and even fourth day in LL, when circadian regulation in molecular and behavioral rhythms is abolished (Figure 4).27,28 This was clearly observed at 18°C for both males and females (Figure 4 and data not shown). Although sleep levels in p{dmper/M2M1} and p{dmper/8:8} flies were more similar during extended LL at higher temperatures (data not shown), the p{dmper/M2M1} flies still manifest significantly shorter sleep bout durations and a greater number of sleep episodes, indicative of more fragmented sleep (Figure S3, supplemental material).
Figure 4.
Reduced sleep in p{dmper/M2M1} flies continues even during extended constant light conditions where circadian clock function is abolished. Shown are group averages of locomotor activity (A,B) and sleep profiles (C,D) for either p{dmper/8:8} (WT; black line) or p{dmper/M2M1} (black dashed line) male flies at 18°C. Flies were entrained for 5 days in LD. Subsequently, one group was placed in constant darkness (DD), whereas another group was placed in constant light (LL). For each genotype and entrainment condition, data from at least 16 flies was used to generate the profiles shown. White, black, dark gray, and light gray horizontal bars denote periods of light, dark, “subjective daytime” in DD, and “subjective nighttime” in LL, respectively.
Enhanced arousal in p{dmper/M2M1} flies following brief exposure to light and non-photic cues
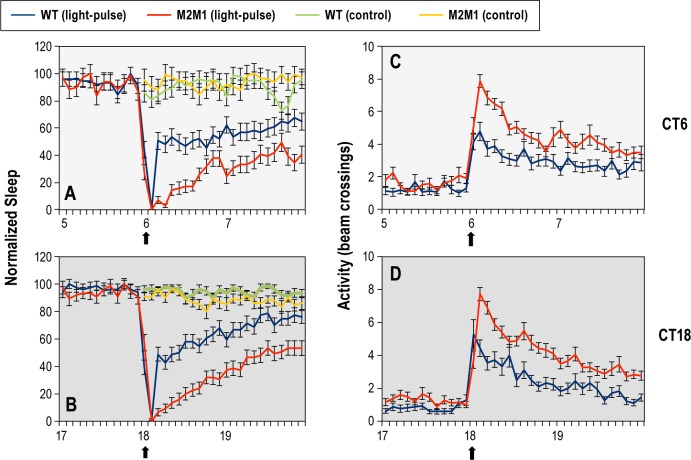
Short duration nocturnal light pulses can evoke arousal in sleeping flies and is used as a standard approach to compare arousal behavior between different flies and or conditions.29 To determine if dmpi8 splicing efficiency can modulate the ability of light to act as an acute arousal cue, we entrained p{dmper/ M2M1} and p{dmper/8:8} flies to standard LD cycles at 18°C, followed by several days of DD whereby we exposed them to 5 min of light on 3 successive days at either CT6 or CT18 (Figure 5), similar in design to a recent study measuring arousal in D. melanogaster.20 A well-established approach to determine arousal thresholds in Drosophila is to measure the proportion of sleeping flies that awake following a brief stimulation.29 However, in our experimental design, the majority of flies were awake by the end of the 5 min exposure to light, indicating the light treatment was saturating and hence less informative with regards to arousal thresholds. Nonetheless, a larger proportion of p{dmper/M2M1} flies remained awake during the recovery phase following acute photic stimulation at either CT6 or CT18 (Figure 5A and 5B). This was also reflected in the shorter duration of sleep bouts for p{dmper/M2M1} flies following light exposure (data not shown). Similar results were obtained for both sexes and at 25°C (data not shown), although genotypespecific differences were more readily observed at 18°C, consistent with results obtained in LD and LL (Figures 1–4).
Figure 5.
Prolonged wakefulness in flies with high dmpi8 splicing efficiency following brief light stimulation. (A-D) Flies were at kept 18°C in LD for 5 days and subsequently placed in DD. During the first 3 days of DD, flies were exposed to 5 min of light at either CT6 or CT18 (as indicated by vertical arrows and right of panels) and returned to the dark; a third group served as non-treated controls. From the collected activity recordings we generated graphs that show either sleep (A,B) or locomotor activity; i.e., beam-crossings (C,D). Results are only shown for female flies but similar findings were obtained with male flies (data not shown). (A,B) Shown are normalized group averages of total sleep for light-pulsed p{dmper/8:8} (WT; blue line) or p{dmper/M2M1} (red line) flies; also shown are the control values, beginning at either CT6 or CT18, for flies that were not exposed to light-pulses; p{dmper/8:8} (WT; green line) or p{dmper/ M2M1} (yellow line) flies. To facilitate comparison (especially at ZT6), the highest value for total sleep prior to the light treatment was set to 100 and all other values were normalized. (C,D) Shown are group averages of activity levels for light-pulsed p{dmper/8:8} (WT) (blue line) or p{dmper/M2M1} (red line) flies. For each value shown, data from at least 16 flies were used to generate the sleep and activity profiles shown.
Although the majority of flies were aroused by nocturnal exposure to 5 min of light, we also compared activity levels to further examine the arousal state of the flies. The p{dmper/M2M1} flies exhibited significantly more locomotor activity during and immediately after the light-pulse compared to the p{dmper/8:8} flies (Figure 5C and 5D). Because both genotypes showed similar activity levels during normal daily wake periods (Figure S2), this suggests that once awakened from sleep by unexpected photic cues, p{dmper/M2M1} flies are in a more aroused state that persists for longer compared to wild-type controls. Thus, even though we did not establish sub-saturating conditions or determine dose-response relationships between light exposure and arousal, the results clearly indicate that acute exposure to photic cues is more effective in evoking longer-lasting transitions from sleep to wakefulness in p{dmper/M2M1} flies.
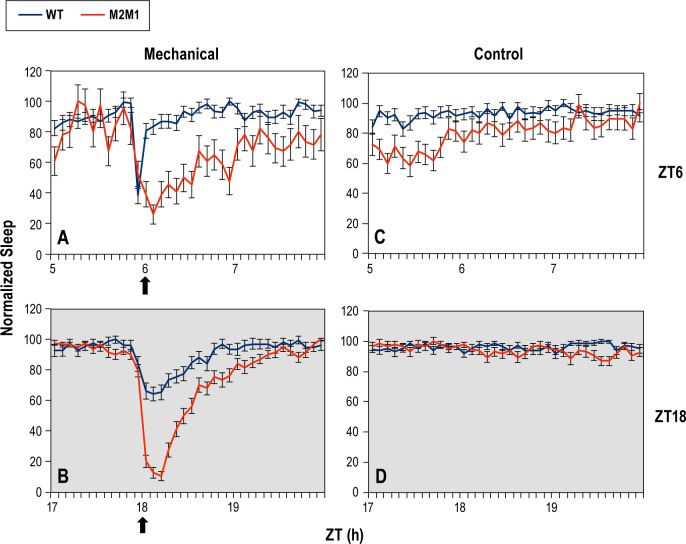
To determine if the differences in arousal between p{dmper/ M2M1} and p{dmper/8:8} are solely restricted to light as an external wake-promoting stimulus, we also subjected the flies to short duration mechanical stimulation, which is another standard approach to study arousal/sleep in flies.20 In this case, we kept flies at 25°C and subjected them to a very brief mechanical cue by scraping the tubes housing the flies (see Materials and Methods) at either ZT6 or ZT18 for 3 consecutive days.20 Remarkably, we noted a similar increase in arousal for p{dmper/ M2M1} flies compared to p{dmper/8:8} following mechanical stimulation (Figure 6). In contrast to the 5-min photic stimulation, the mechanical treatment did not arouse all the flies (Figure 5), indicating that the stimulus was sub-saturating. Although slightly more p{dmper/M2M1} flies were awake immediately after application of the mechanical stimulus at ZT6, a dramatic difference was noted during the posttreatment phase whereby p{dmper/8:8} flies returned to pretreatment sleep levels within 15 min, whereas p{dmper/M2M1} flies did not return to baseline sleep values even after 2 h (Figure 6A and 6C). An even greater effect was observed when the mechanical stimulation was applied at ZT18, resulting in an approximately 2–3 fold increase in the number of p{dmper/M2M1} flies awakened by brief mechanical stimulation compared to p{dmper/8:8} flies (Figure 6B and 6D). In addition, following scraping of the fly tubes at ZT18, p{dmper/M2M1} flies remained awake longer before returning to baselines sleep levels, a trend similar to that observed at ZT6.
Figure 6.
The splicing efficiency of dmpi8 also modulates arousal to non-photic sensory stimulation. (A-D) Flies were kept in LD at 25°C for 5 days, and during the last 3 days of LD, flies were subjected to a brief mechanical stimulation at either ZT6 or ZT18 (as indicated by vertical arrows and right of panels); a third group served as non-treated controls. Shown are normalized group averages of total sleep for p{dmper/8:8} (WT; blue line) or p{dmper/M2M1} (red line) flies that were either treated (A,B) or not treated (C,D) with a brief mechanical stimulation. Results are shown for females but similar results were obtained with males (data not shown). For each value shown, data from at least 16 flies were used to generate the sleep profiles shown. To facilitate comparison, the highest value for total sleep was set to 100 and all other values were normalized.
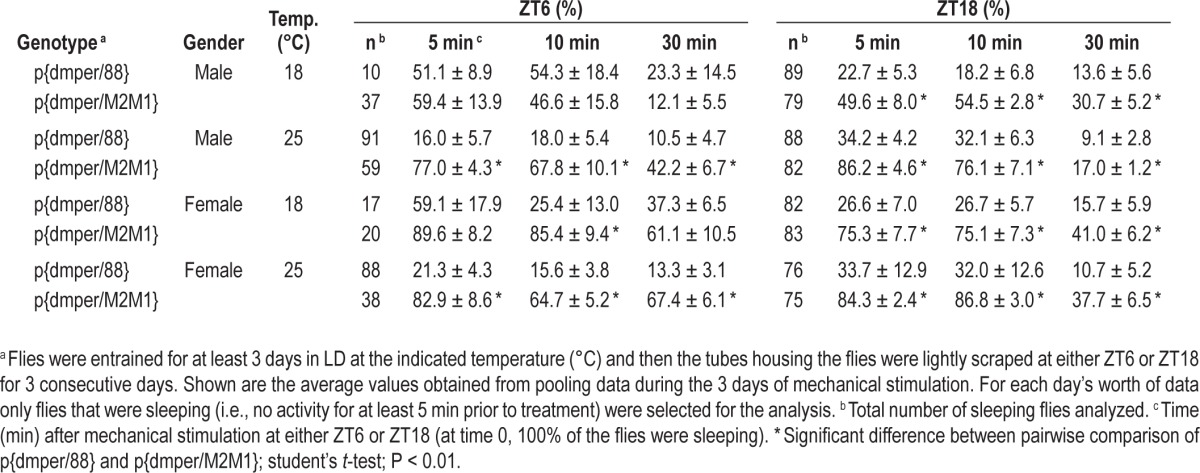
Because our procedure for mechanical stimulation did not wake up all the flies, we also did an analysis of only those flies that were asleep prior to the treatment and measured the proportion of that were awake shortly after the treatment was delivered, a more definitive assessment of arousal thresholds. When the mechanical stimulus was applied at ZT18, a significantly higher proportion of p{dmper/M2M1} flies were aroused from sleep compared to the wild-type control flies, a result observed with both sexes and at 18° and 25°C (Table 1; see values at 5 min posttreatment). In addition, p{dmper/M2M1} flies remained awake for longer times posttreatment (Table 1; see values at 30 min posttreatment). At ZT6 p{dmper/M2M1} flies were generally more aroused compared to the wild-type control following mechanical stimulation but this was mainly observed at 25°C. The temperature effect at ZT6 is not surprising because on cold days, wild-type flies are already quite active during the daytime hours (e.g., Figure S1), minimizing the ability to detect genotypic differences based on procedures aimed at evoking increases in arousal. In summary, although we only examined a limited number of arousal conditions, the results clearly indicate that flies with inefficient splicing of dmpi8 (as found in wild-type flies) are less responsive to the wake-promoting effects of a variety of sensory-mediated external cues.
Table 1.
Percentage of sleeping flies that were awake following mechanical stimulation at either ZT6 or ZT18
DISCUSSION
In this report we made the surprising discovery that splicing of the 3'-terminal intron in dper has a potent non-circadian role in modulating the daily distribution of activity in D. melanogaster by regulating sleep behavior via a mechanism that involves sensory-dependent arousal. Daily changes in wake-sleep levels are governed by a complex web of interactions between circadian timing systems, sleep homoeostatic functions and arousal pathways that differentially respond to environmental cues, such as light and temperature.17,18 In constant dark conditions, D. melanogaster still exhibits morning and evening bouts of activity, suggesting that those times in the day are most associated with strong wake-promoting signals. Although the majority of sleep occurs during the night, daytime sleep, which mainly occurs in the midday, is genetically controlled by components that can modulate baseline or endogenous sleep levels.23 However, a major difference between daytime and nighttime sleep is the presence of substantial light intensity in the environment, which is normally an effective arousal cue for diurnal animals. In addition, daytime hours can be associated with hot temperatures and levels of incoming solar radiation peak at midday, posing serious health risks that demand a strategy to reduce exposure. It is with respect to minimizing activity during the hot midday sun despite the presence of light that our findings provide an attractive model for how dmpi8 splicing might contribute to the adaptation of daily behavior in natural conditions.
A key observation is that the main effect of dmpi8 splicing efficiency on sleep occurs during the first half of the day, a phenomena that is readily observed even at colder temperatures (Figures 1 and 2). This suggests that a major role for dmpi8 splicing is to track ambient temperature and photic signals during the early day and integrate the environmental information to evoke a scaled response in activity levels that begins prior to midday. Moreover, because temperatures normally increase throughout the first half of the day, this implies that the real-world default function for dmpi8 splicing is to steadily increase the propensity for sleep between sunrise and midday. On warm days because dmpi8 splicing is strongly inhibited, the early transition to higher thresholds for sensory mediated arousal favors sleep despite the presence of light which normally functions as an arousal cue. As a result, flies enter a more robust and extended quiescent state following the morning bout of activity that lasts through midday and until the cooler evening hours when the clock once again promotes wakefulness. On the other hand, during cold days the daytime splicing efficiency of dmpi8 remains relatively high and the threshold for arousal is lower, allowing flies to take advantage of the warmer midday hours on cold days.
We view the early morning start of dmpi8 splicing on adjusting daytime activity levels as an adaptive “early-warning” rheostat that helps flies anticipate midday temperatures and adopt appropriate activity levels and corresponding behavioral programs. In this context it should be noted that elevated but physiologically relevant temperatures (e.g., 30°C) in combination with high light intensity is very effective in directly suppressing activity, which is termed paradoxical or negative masking in diurnal animals.9,22,30–33 Although the mechanism evoking the ability of light and heat to acutely suppress activity is not known, this response does not require the per gene,9 and indeed we show that at high temperatures (29°C) the status of dmpi8 splicing has minimal effects on daytime sleep levels (e.g., Figure 2). While this paradoxical masking response supports the notion that flies seek to avoid extended periods of activity when exposed to daytime heat, this behavioral reaction lacks predictive value as it depends on direct exposure to adverse environmental conditions.
While the concept of non-circadian roles for core clock genes is not new,34 per has a unique position in the hierarchy of animal circadian timing systems by functioning as the key circadian factor regulating the pace of the clock.15,16 Indeed, the daily cycles in the levels of per mRNA and protein are inextricably linked to progression of the clock.15 As such, conventional wisdom would suggest that effects of per gene function on daily activity patterns, which are strongly influenced by the circadian timing system, should reflect a circadian role(s). Perhaps the most striking evidence that the effect of dmpi8 splicing is not circadian in nature is that the sleep differences between p{dmper/M2M1} and p{dmper/8:8} flies persist for many days in constant light exposure (Figure 4D), conditions that severely dampen and eventually abolish the clockworks.27,28,35 This result is also surprising because light evokes the rapid degradation of TIMELESS (TIM), which is a key partner of dPER.2 In the absence of TIM, dPER is very unstable, does not accumulate to high levels and mainly resides in the cytoplasm.35,36 Within the context of clock function, the major biochemical role of PER is in the nucleus where it contributes to cyclic gene expression by acting as the key component in timing the daily repression of the main clock transcription factors, CLOCK (dCLK) and CYCLE (CYC).2 Thus, it is not clear how the p{dmper/M2M1} and p{dmper/8:8} flies exhibit such large differences in sleep quality during extended exposure to light when dPER protein levels are likely to remain low and mainly reside in the cytoplasm. While elucidating the mode-of-action by which changes in dmpi8 splicing efficiency and presumably dPER levels modulate sleep behavior is of utmost importance, our findings raise the intriguing possibility that the mechanism is different from the well-known circadian function of dPER in transcriptional feedback.
When considering the role(s) of dPER on wake-sleep behavior, it is also important to note that although activity rhythms are driven by a complex circuit of pacemaker neurons located in the brain, not all dper-expressing neurons have the same function in governing daily behavior. There are about 150 clock neurons (or better, dper-expressing cells) in the adult Drosophila brain that can be divided into several major subgroups based on their location, shape and function.37 A conceivable site of action for mediating the effects of dmpi8 splicing on arousal are the l-LNvs, identified as wake-promoting cells whose firing rate increases in response to acute light exposure.38–41 It is possible that reductions in the splicing efficiency of dmpi8 enhance midday sleep by lowering the wake promoting effects of the l-LNvs and/or other arousal cells. In addition, besides the strong effects of the l-LNvs on morning activity,42 these cells are also important in timing evening activity.43 Thus modulating the function of the l-LNvs by dmpi8 splicing could also contribute to network-based circadian regulation in the timing of evening activity as a function of temperature. Finally, dmpi8 splicing might also affect arousal and sleep by modulating the visual system where per is expressed and has been shown to affect a myriad of clock-dependent and clock-independent effects of light on Drosophila behavior.32
In summary, we have identified a novel arousal mechanism in Drosophila that plays an important role in regulating light-dependent arousal, especially during the early-to-mid day. This splicing event provides behavioral flexibility by its graded response as a function of temperature, ensuring that on warm days flies initiate an extended quiescent period prior to the heat of the midday and until the cooler evening hours where the clock once again promotes wake, but still allowing for increased daytime activity on cold days, presumably enabling flies to take advantage of the warmer daytime hours. In addition, we recently showed that naturally occurring polymorphisms in the dper 3' UTR that affect dmpi8 splicing efficiency also lead to changes in the duration of midday siesta,10 strongly suggesting that this mechanism contributes to inherited variability in the sleep behavior of D. melanogaster in the wild. Indeed, our prior work showed that the thermally regulated splicing of the 3'-terminal intron and its ability to adjust the distribution of activity as a function of temperature are not manifested by all Drosophila species.11 Thus, the behavioral plasticity resulting from the ability to anticipate midday temperatures and hence make appropriate early adjustments in the balance between quiescence and wakefulness might have facilitated the successful colonization of D. melanogaster to a wide variety of climates. Recent findings using semi-natural conditions have suggested that flies do not rest in the midday on warm days but rather exhibit elevated levels of activity,44 whereas others have suggested this is an “escape” response seeking to find shade.45 More natural field observations where flies can freely roam and have more enriched social contexts are needed to better address these important issues. Clearly, per occupies a unique position in bridging how circadian, sleep and arousal pathways interact with external cues to govern daily wake-sleep patterns in D. melanogaster. An important future challenge is to determine the molecular and cellular bases for how dmpi8 splicing contributes to setting thresholds for sensory-mediated arousal.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by a grant from the NIH (NS042088) to Dr. Edery. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Kwang Low and George Ghanim for help with the early experiments on sleep and arousal.
SUPPLEMENTAL MATERIAL
Daily locomotor activity rhythms of p{dmper/8:8} and p{dmper/M2M1} flies at different temperatures. (A-L) Flies were entrained to 12:12LD for 5 days at the indicated temperature (right of panels) and locomotor activity recorded. Shown are group averages of daily activity rhythms for adult male (left) and female (right) p{dmper/8:8} (WT; black line) and p{dmper/M2M1} (dashed black line) flies at the indicated temperatures (right of panels). To facilitate comparisons, the peak value in daily activity for each genotype was set to 1.0 and the normalized profiles superimposed. For LD, the last 3 days' worth of data was pooled; for DD, the first day is shown. For each genotype and condition, data from at least 40 flies were used to generate the activity profiles shown. Black horizontal bar (dark period); white horizontal bar (light period); gray horizontal bar (subjective day); ZT, zeitgeber time (h); CT, circadian time (h). The results show that the p{dmper/8:8} and p{dmper/M2M1} transgenic flies used in this study behave as previously reported (Low et al., 2008). Essentially, both male and female p{dper/M2M1} flies exhibit shorter, less robust midday troughs in activity, earlier onsets of evening activity and later offsets of morning activity, especially at 18° and 25°C.
The splicing efficiency of dmpi8 does not affect activity levels during wake episodes but does modulate the onset of sleep after lights-on. (A-R) Results show group averages for wake activity per wake period for p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) flies maintained at the indicated temperatures (right of panels) and light/dark conditions (top of panels). Results shown are for either male (A-I) or female (J-R) flies. LD, 12-h light/12-h dark; DD, constant darkness; LL, constant light. For LD, activity data from the last three days was pooled, whereas for DD and LL, only data from the first 24-h day was used. White, black, dark gray and light gray horizontal bars denote periods of light, dark, “subjective daytime” in DD, and “subjective nighttime” in LL, respectively. Note that there are little to no differences in activity levels during wake episodes between the two genotypes, indicating that the large variations in sleep behavior are not because p{dmper/M2M1} flies are “hyperactive.” (S-X) Shown are sleep onsets in LD (time to first sleep episodes measured from lights-on at ZT0) for either p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) flies at the indicated temperatures (below panels). Male flies (S-U); female flies (V-X). The data show that the onset of sleep after lights-on at ZT0 is later for p{dmper/M2M1} flies. (A-X) For each genotype, gender, and entrainment condition, data from at least 16 flies was used to generate the values shown. Values for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are significantly different using student's t-test; * P < 0.05; ** P < 0.005.
p{dmper/M2M1} flies have more fragmented sleep during extended LL, even at higher temperatures where total sleep levels are similar to the wild-type control. Shown are group averages of average sleep bout duration (A-C) and number of sleep bouts (D-F) for either p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) male flies at the indicated temperature (right of panels). On the fifth day of LD, the lights were left on at ZT12, and flies continued to be exposed to constant light (LL) for 3-4 more days. Time 0 denotes the end of the last 12 h dark period of the LD cycles (LD5) prior to placing the flies in constant light. For each genotype, data from at least 16 flies was used to generate the profiles shown. White and light gray horizontal bars denote “subjective daytime” and “subjective nighttime” in LL, respectively. Even though total sleep levels for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are similar at 29°C (Figure 3 and data not shown), p{dmper/M2M1} flies manifest less consolidated sleep as shown by the overall shorter sleep bout lengths (C) and more numerous sleep episodes (F).
REFERENCES
- 1.Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–24. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–73. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirelli C. Searching for sleep mutants of Drosophila melanogaster. Bioessays. 2003;25:940–9. doi: 10.1002/bies.10333. [DOI] [PubMed] [Google Scholar]
- 5.Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 2009;25:262–9. doi: 10.1016/j.tig.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks JC, Sehgal A. Why a fly? Using Drosophila to understand the genetics of circadian rhythms and sleep. Sleep. 2004;27:334–42. doi: 10.1093/sleep/27.2.334. [DOI] [PubMed] [Google Scholar]
- 7.Rosato E, Kyriacou CP. Analysis of locomotor activity rhythms in Drosophila. Nat Protoc. 2006;1:559–68. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- 8.Majercak J, Sidote D, Hardin PE, Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron. 1999;24:219–30. doi: 10.1016/s0896-6273(00)80834-x. [DOI] [PubMed] [Google Scholar]
- 9.Tomioka K, Sakamoto M, Harui Y, Matsumoto N, Matsumoto A. Light and temperature cooperate to regulate the circadian locomotor rhythm of wild type and period mutants of Drosophila melanogaster. J Insect Physiol. 1998;44:587–96. doi: 10.1016/s0022-1910(98)00046-8. [DOI] [PubMed] [Google Scholar]
- 10.Low KH, Chen WF, Yildirim E, Edery I. Natural variation in the Drosophila melanogaster clock gene period modulates splicing of its 3'-terminal intron and mid-day siesta. PloS One. 2012;7:e49536. doi: 10.1371/journal.pone.0049536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low KH, Lim C, Ko HW, Edery I. Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron. 2008;60:1054–67. doi: 10.1016/j.neuron.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins BH, Rosato E, Kyriacou CP. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc Natl Acad Sci U S A. 2004;101:1945–50. doi: 10.1073/pnas.0308240100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majercak J, Chen WF, Edery I. Splicing of the period gene 3'-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol Cell Biol. 2004;24:3359–72. doi: 10.1128/MCB.24.8.3359-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu JC, Vanselow JT, Kramer A, Edery I. The phospho-occupancy of an atypical SLIMB-binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev. 2008;22:1758–72. doi: 10.1101/gad.1682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng X, Sehgal A. Speed control: cogs and gears that drive the circadian clock. Trends Neurosci. 2012;35:574–85. doi: 10.1016/j.tins.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7:e1000145. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 19.Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J Vis Exp. 2010;28:2157. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Chen D, Sehgal A. Dopamine acts through Cryptochrome to promote acute arousal in Drosophila. Genes Dev. 2012;26:1224–34. doi: 10.1101/gad.186338.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushey D, Cirelli C. From genetics to structure to function: exploring sleep in Drosophila. Int Rev Neurobiol. 2011;99:213–44. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Thakurdas P, Sinam B, Joshi D. Paradoxical masking effects of bright photophase and high temperature in Drosophila malerkotliana. Chronobiol Int. 2012;29:157–65. doi: 10.3109/07420528.2011.644875. [DOI] [PubMed] [Google Scholar]
- 23.Ishimoto H, Lark A, Kitamoto T. Factors that differentially affect daytime and nighttime sleep in Drosophila melanogaster. Front Neurol. 2012;3:24. doi: 10.3389/fneur.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 25.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 26.Rensing L, Ruoff P. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol Int. 2002;19:807–64. doi: 10.1081/cbi-120014569. [DOI] [PubMed] [Google Scholar]
- 27.Marrus SB, Zeng H, Rosbash M. Effect of constant light and circadian entrainment of perS flies: evidence for light-mediated delay of the negative feedback loop in Drosophila. EMBO J. 1996;15:6877–86. [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, Hardin PE. per mRNA cycling is locked to lights-off under photoperiodic conditions that support circadian feedback loop function. Mol Cell Biol. 1996;16:4182–8. doi: 10.1128/mcb.16.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Swinderen B, Andretic R. Arousal in Drosophila. Behav Processes. 2003;64:133–44. doi: 10.1016/s0376-6357(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 30.Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–61. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Liu W, Guo F, Guo A. Circadian modulation of light-induced locomotion responses in Drosophila melanogaster. Genes Brain Behav. 2008;7:730–9. doi: 10.1111/j.1601-183X.2008.00411.x. [DOI] [PubMed] [Google Scholar]
- 32.Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–91. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 33.Yoshii T, Sakamoto M, Tomioka K. A temperature-dependent timing mechanism is involved in the circadian system that drives locomotor rhythms in the fruit fly Drosophila melanogaster. Zoolog Sci. 2002;19:841–50. doi: 10.2108/zsj.19.841. [DOI] [PubMed] [Google Scholar]
- 34.Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev. 2010;34:1249–55. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Price JL, Dembinska ME, Young MW, Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995;14:4044–9. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vosshall LB, Price JL, Sehgal A, Saez L, Young MW. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–9. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- 37.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 38.Parisky KM, Agosto J, Pulver SR, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–94. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheeba V, Gu H, Sharma VK, O'Dowd DK, Holmes TC. Circadianand light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–88. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheeba V, Kaneko M, Sharma VK, Holmes TC. The Drosophila circadian pacemaker circuit: Pas De Deux or Tarantella? Crit Rev Biochem Mol Biol. 2008;43:37–61. doi: 10.1080/10409230701829128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheeba V, Fogle KJ, Holmes TC. Persistence of morning anticipation behavior and high amplitude morning startle response following functional loss of small ventral lateral neurons in Drosophila. PloS One. 2010;5:e11628. doi: 10.1371/journal.pone.0011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potdar S, Sheeba V. Large ventral lateral neurons determine the phase of evening activity peak across photoperiods in Drosophila melanogaster. J Biol Rhythms. 2012;27:267–79. doi: 10.1177/0748730412449820. [DOI] [PubMed] [Google Scholar]
- 44.Vanin S, Bhutani S, Montelli S, et al. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–5. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 45.De J, Varma V, Saha S, Sheeba V, Sharma VK. Significance of activity peaks in fruit flies, Drosophila melanogaster, under seminatural conditions. Proc Natl Acad Sci U S A. 2013;110:8984–9. doi: 10.1073/pnas.1220960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Daily locomotor activity rhythms of p{dmper/8:8} and p{dmper/M2M1} flies at different temperatures. (A-L) Flies were entrained to 12:12LD for 5 days at the indicated temperature (right of panels) and locomotor activity recorded. Shown are group averages of daily activity rhythms for adult male (left) and female (right) p{dmper/8:8} (WT; black line) and p{dmper/M2M1} (dashed black line) flies at the indicated temperatures (right of panels). To facilitate comparisons, the peak value in daily activity for each genotype was set to 1.0 and the normalized profiles superimposed. For LD, the last 3 days' worth of data was pooled; for DD, the first day is shown. For each genotype and condition, data from at least 40 flies were used to generate the activity profiles shown. Black horizontal bar (dark period); white horizontal bar (light period); gray horizontal bar (subjective day); ZT, zeitgeber time (h); CT, circadian time (h). The results show that the p{dmper/8:8} and p{dmper/M2M1} transgenic flies used in this study behave as previously reported (Low et al., 2008). Essentially, both male and female p{dper/M2M1} flies exhibit shorter, less robust midday troughs in activity, earlier onsets of evening activity and later offsets of morning activity, especially at 18° and 25°C.
The splicing efficiency of dmpi8 does not affect activity levels during wake episodes but does modulate the onset of sleep after lights-on. (A-R) Results show group averages for wake activity per wake period for p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) flies maintained at the indicated temperatures (right of panels) and light/dark conditions (top of panels). Results shown are for either male (A-I) or female (J-R) flies. LD, 12-h light/12-h dark; DD, constant darkness; LL, constant light. For LD, activity data from the last three days was pooled, whereas for DD and LL, only data from the first 24-h day was used. White, black, dark gray and light gray horizontal bars denote periods of light, dark, “subjective daytime” in DD, and “subjective nighttime” in LL, respectively. Note that there are little to no differences in activity levels during wake episodes between the two genotypes, indicating that the large variations in sleep behavior are not because p{dmper/M2M1} flies are “hyperactive.” (S-X) Shown are sleep onsets in LD (time to first sleep episodes measured from lights-on at ZT0) for either p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) flies at the indicated temperatures (below panels). Male flies (S-U); female flies (V-X). The data show that the onset of sleep after lights-on at ZT0 is later for p{dmper/M2M1} flies. (A-X) For each genotype, gender, and entrainment condition, data from at least 16 flies was used to generate the values shown. Values for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are significantly different using student's t-test; * P < 0.05; ** P < 0.005.
p{dmper/M2M1} flies have more fragmented sleep during extended LL, even at higher temperatures where total sleep levels are similar to the wild-type control. Shown are group averages of average sleep bout duration (A-C) and number of sleep bouts (D-F) for either p{dmper/8:8} (WT; black bar) or p{dmper/M2M1} (white bar) male flies at the indicated temperature (right of panels). On the fifth day of LD, the lights were left on at ZT12, and flies continued to be exposed to constant light (LL) for 3-4 more days. Time 0 denotes the end of the last 12 h dark period of the LD cycles (LD5) prior to placing the flies in constant light. For each genotype, data from at least 16 flies was used to generate the profiles shown. White and light gray horizontal bars denote “subjective daytime” and “subjective nighttime” in LL, respectively. Even though total sleep levels for p{dmper/8:8} (WT) and p{dmper/M2M1} flies are similar at 29°C (Figure 3 and data not shown), p{dmper/M2M1} flies manifest less consolidated sleep as shown by the overall shorter sleep bout lengths (C) and more numerous sleep episodes (F).