Abstract
Background
Describing the undiagnosed HIV-infected population is essential for guiding HIV screening policy, implementing interventions and resource planning.
Methods
We used French national HIV surveillance data and a back-calculation approach to estimate the number of undiagnosed HIV-infected individuals in France and the distribution of time since HIV infection among undiagnosed individuals. We also used data on CD4 cell count decline to assess the CD4 count distribution among undiagnosed individuals.
Results
We estimated that 29,000 (95% confidence interval (CI): 24,200–33,900) individuals were living with undiagnosed HIV infection at the end of 2010. Of these, 28.7% (95% CI: 27.1–30.4) were infected less than a year ago, 16.4% (95% CI: 15.0–17.8) more than 5 years ago, and 59.6% (95% CI: 59.2–59.8) were eligible for anti-retroviral treatment (CD4<500/μL) according to the 2010 French guidelines. Men represented 70.0% of the undiagnosed HIV-infected individuals and had lower CD4 cell counts than women. The numbers of undiagnosed infections in men who have sex with men (MSM), non French-national heterosexuals and French-national heterosexuals were similar (9,200, 9,300, 10,000, respectively). However, due to differences in group size, undiagnosed HIV prevalence varied significantly between these groups (2.95%, 0.36%, 0.03%, respectively; p<0.001).
Conclusions
Our findings suggest that i) many undiagnosed HIV-infected individuals were eligible for treatment and thus lack of HIV diagnosis is a lost chance for them; ii) many more heterosexuals than MSM will need to be tested to find those undiagnosed; iii) universal screening of men may be cost-effective, especially in the areas most affected by the epidemic, such as the Paris region.
Keywords: HIV, undiagnosed infection, hidden epidemic, mathematical modeling, distribution of time since HIV infection, CD4, screening strategy
Introduction
Despite the advent of effective combination anti-retroviral treatment (cART), the HIV epidemic continues to spread [1]. Studies have shown that the majority of new HIV transmissions may originate from individuals who are unaware of their HIV infection [2, 3]. This may be due to higher infectiousness, because of elevated viral load at the time of HIV seroconversion [4], and more frequent high-risk practices among undiagnosed than diagnosed HIV-infected individuals [5]. Undiagnosed individuals are also at risk of delayed diagnosis and thus might not benefit from timely initiation of cART and experience HIV-related morbidity and premature mortality [6, 7]. Therefore, screening for the undiagnosed HIV-positive individuals is key to turning the tide on HIV transmission and reduce mortality [8].
Until recently, in most settings, including France, voluntary testing was the mainstay of HIV screening [9]. This involves individuals actively seeking HIV testing at a health or community-based facility; only pregnant women are systematically offered an HIV test during their initial prenatal medical visit. To reduce undiagnosed HIV infection, many countries have revised or consider revising their screening guidelines toward universal testing [10–12]. Particularly, the French National Authority for Health recommends [11] at least one HIV test in a lifetime for everyone aged 15 to 70, in addition to annual testing for men who have sex with men (MSM), intravenous drug users (IDUs), and heterosexuals from sub-Saharan Africa and the Caribbean who have multiple partners. HIV testing tools and strategies are numerous. To choose the most appropriate and cost-effective strategy, health care planners need to know the number and characteristics of individuals living with undiagnosed HIV. However, these data cannot be directly observed.
Mathematical modeling, such as back-calculation models, can be used to get insights on the undiagnosed HIV-infected population (also called hidden epidemic in the following) [13–15]. The back-calculation method uses surveillance data on new HIV diagnoses together with the distribution of time from infection to diagnosis, to work backwards and infer the number of HIV infections that occurred over time to reproduce the observed number of new HIV diagnoses [16]. The estimated number of HIV infections can then be projected forward to unravel the size of the hidden epidemic. Here we used this method to estimate the size of the hidden epidemic in France at the end of 2010 as well as the distribution of time since HIV infection among undiagnosed individuals. Then, we combined these estimates with data on the natural history of CD4 cell counts among HIV-infected patients [17] to estimate the distribution of CD4 counts among undiagnosed individuals.
Methods
In France, mandatory nationwide reporting of new HIV diagnoses was implemented in 2003. Data on new HIV diagnoses, including date of diagnosis, demographic information (sex and nationality), HIV exposure group, and clinical status at diagnosis (primary infection, asymptomatic or AIDS) have since been recorded in a national database [18]. In a previous study [19], we used data on new HIV diagnoses and a new back-calculation model to estimate both HIV incidence and distribution of time from infection to diagnosis among each HIV exposure group in France over the 2004–2007 period. Specifically, we used the numbers of reported new HIV diagnoses, adjusted for both delay in reporting and under-reporting, and data on the clinical status at HIV diagnosis to disentangle the contributions to observed new HIV diagnoses made by HIV incidence and time-varying rates of diagnosis.
Estimation of the number of undiagnosed HIV-infected individuals
In this study, we projected forward the HIV incidence (shown in Figure S1 of the Supplementary Material) according to the distribution of time from infection to diagnosis to estimate the number of undiagnosed HIV-infected individuals at the end of 2010. Specifically, from the number of newly HIV-infected individuals at each point in time, we estimated those who were still undiagnosed at the end of 2010, using the cumulative probabilities of not being diagnosed with HIV over time; these probabilities were calculated from the distribution of time between infection and diagnosis (Figure S2). To estimate the number of undiagnosed individuals at the end of 2010, we made the following assumptions. First, we only projected forward the incidence between 2000 and 2010, because the cumulative probabilities of not being diagnosed eleven years after being infected were small (<0.05) (Figure S2). Hence, we assumed that all individuals who acquired HIV before 2000 were already diagnosed by the end of 2010.
Second, the HIV incidence between 2000 and 2010 was determined by extrapolating our findings for the 2004–2007 period. In the baseline scenario, we assumed that yearly HIV incidence between 2000 and 2003 was equal to its level in 2004 and yearly HIV incidence between 2008 and 2010 was equal to its level in 2007 (Figure S1). We also assumed that the distribution of time from infection to diagnosis for individuals infected between 2000 and 2003 (respectively 2008 and 2010) was equal to that of individuals infected in 2004 (respectively 2007). These assumptions are plausible since incidence and time from infection to diagnosis were shown to remain stable between 2004 and 2007 [19]. Nevertheless, we explored other scenarios in sensitivity analyses. We considered midpoint, best-case, and worst-case scenarios. In the midpoint scenario, values for the HIV incidence were extrapolated from a linear regression of the incidence data for the 2004–2007 period. In the best-case scenario, we assumed that yearly HIV incidence between 2008 and 2010 was 20% lower than its level in 2007, while in the worst-case scenario, we assumed it was 20% higher; in both scenarios yearly HIV incidence between 2000 and 2003 was assumed to be equal to its level in 2004. We considered these two last scenarios as extreme situations, since there is no data to support that, in France, HIV incidence has either decreased or increased after 2007. We also considered a fourth scenario to study the sensitivity of the results to assumptions made on incidence levels before 2004. In this scenario, we assumed that yearly HIV incidence between 2000 and 2003 was 20% higher than its level in 2004 and yearly HIV incidence between 2008 and 2010 was equal to its level in 2007.
Upper and lower bounds of the 95% confidence intervals (CI) of the number of HIV-infected individuals still undiagnosed by the end of 2010 were obtained by projecting forward two thousand bootstrap estimates of the HIV incidence curve using the corresponding distributions of time from infection to diagnosis, obtained in our previous study [19].
All numbers were rounded to the nearest hundred. We then calculated HIV undiagnosed prevalence by gender and HIV exposure group at the end of 2010. The denominators used for prevalence calculations were based on population size (aged 18–64) estimates in 2010 from the National Institute of Statistics and Economic Studies [20], the latest national survey on sexual behavior in France [21] and a report on the prevalence of drug use in France [22].
Distribution of time since HIV infection among undiagnosed individuals
From these projections, we obtained the numbers of undiagnosed individuals at the end of 2010 stratified by time since HIV infection. These numbers was then used to calculate the proportion of undiagnosed individuals infected for less than a year, 1 to 5 years and more than 5 years; we defined recent infection as being infected for less than a year and old infection as being infected for more than 5 years. Ninety-five percent CI for these proportions were obtained from the two thousand projections.
Distribution of CD4 cell counts among undiagnosed individuals
We then estimated the distribution of CD4 cell counts among undiagnosed individuals using the numbers of undiagnosed individuals stratified by time since HIV infection and data on CD4 cell count decline from cART-naïve HIV-infected individuals [17]. CD4 cell count distributions at 1, 2, and 5 years after seroconversion were previously estimated using CASCADE data from cART-naive individuals with well-estimated dates of HIV seroconversion from Europe, Australia, and Canada [17]. For the present study, proportions of individuals with CD4 i) <200, ii) 200–350, iii) 350–500 and iv) ≥500 cells/mm3 (and 95% corresponding CI) at 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 years after HIV seroconversion were estimated using the same methodology and dataset (personal communication SL; Figure S3).
To estimate the distribution of CD4 cell counts among undiagnosed individuals, we proceeded as follows. First, for each stratum of time since HIV infection, we randomly sampled a set of proportions of individuals having CD4 counts i) <200, ii) 200–350, iii) 350–500 and iv) ≥500 cells/mm3, based on their mean estimates and 95% CI (Figure S3). Second, we multiplied the random sample by the number of undiagnosed individuals in the corresponding stratum. Third, we aggregated the results over the time since infection and obtained the proportions of undiagnosed individuals with CD4 counts i) <200, ii) 200–350, iii) 350–500 and iv) ≥500 cells/mm3 at the end of 2010. Ninety-five percent CI for these proportions were derived from the two thousand projections.
Chi-square tests were used to detect differences between proportions. Data analyses were performed using Matlab, R2010b.
Results
Under the baseline scenario, we estimated that 83,800 (95% CI: 76,000–91,300) individuals were newly infected with HIV between 2000 and 2010 in France (Figure S1). Of these individuals, 29,000 (24,200–33,900) remained undiagnosed by the end of 2010 (Table 1); 70.0% were men. MSM made up 31.7% of all undiagnosed individuals, heterosexuals with French nationality 34.5%, non French-national heterosexuals 32.1%, and IDUs 1.7%. Undiagnosed HIV prevalence (Table 1) varied significantly by gender and exposure group (p<0.001). The prevalence among men was 10 per 10,000, more than twice the prevalence for women (4 per 10,000). Among exposure groups, MSM had the highest prevalence (295 per 10,000), followed by IDUs (62 per 10,000). Undiagnosed HIV prevalence among non French-national heterosexuals and French-national heterosexuals were 36 per 10,000 and 3 per 10,000, respectively, 8 and 98 times lower than that among MSM.
Table 1.
Estimated number and prevalence (per 10,000 population) of persons living with undiagnosed HIV infection in France in 2010.
| Number of persons living with undiagnosed HIV (95% CI)a |
Estimated size of the population aged 18–64 yearsb |
Undiagnosed HIV prevalence (95% CI) |
|
|---|---|---|---|
| Overall | 29,000 (24,200–33,900) |
39,566,800 | 7 (6–9) |
| Menc | 20,300 (16,600–24,500) |
19,517,600 | 10 (9–13) |
| Women | 8700 (6,100–11,000) |
20,049,200 | 4 (3–5) |
| MSM | 9,200 (7,800–11,200) |
312,300d | 295 (250–359) |
| IDUs | 500 (100–1,100) |
81,000 | 62 (12–136) |
| French-national heterosexuals | 10,000 (6,400–13,900) |
36,564,200 | 3 (2–4) |
| French-national heterosexual women | 3,600 (1,700–5,300) |
18,752,800 | 2 (1–3) |
| French-national heterosexual men | 6,400 (3,700–9,800) |
17,811,400 | 4 (2–6) |
| Non French-national heterosexuals | 9,300 (7,000–11,900) |
2,609,300 | 36 (27–46) |
| Non French-national heterosexual women | 4,900 (3,600–6,600) |
1,296,400 | 38 (28–51) |
| Non French-national heterosexual men | 4,400 (2,400–6,600) |
1,312,900 | 34 (18–50) |
MSM: men who have sex with men; IDUs: injecting drug users; CI: confidence intervals;
Assuming that HIV incidence between 2000 and 2003 (respectively 2008 and 2010) was equal to its level in 2004 (respectively 2007);
Population size (aged 18–64) estimates were obtained from the National Institute of Statistics and Economic Studies [20], the latest national survey on sexual behavior in France [21] and a report on the prevalence of drug use in France [22];
We assumed that 2/3 of undiagnosed IDUs are men, since 2/3 of newly diagnoses IDUs are men;
The number of MSM was evaluated using the proportion of men (1.6%) who reported having had sex with another man in the last 12 months in the survey on sexual behavior [21].
We estimated that 28.7% (27.1–30.4) of undiagnosed individuals had been infected for less than a year and�16.4% (15.0–17.8) for more than 5 years (Figure 1). The distribution of time since HIV infection among undiagnosed individuals varied significantly by gender and exposure group (p<0.001). Compared to women, men had a higher proportion of individuals with old infections (i.e., acquired >5 years ago), 18.2% (16.5–19.8) for men versus 12.2% (11.2–13.3) for women. Proportions of individuals with old infections were 21.4% (18.1–24.4) among French-national heterosexual men, 19.2% (16.8–21.4) among non French-national heterosexuals, 19.1% (13.7–24.6) among IDUs, 15.3% (12.3–16.8) among MSM, 13.7% (12.1–15.6) among French-national heterosexual women and 10.9% (9.8–12.4) among non French-national heterosexual women. Proportions of individuals infected for less than a year were higher for non French-national heterosexual women (32.4% (29.6–35.7)) and MSM (31.5% (28.1–33.9)) compared to heterosexual men (26.2% (23.3–30.2) among non French-nationals and 25.0% (21.7–29.0) among French-nationals).
Figure 1.
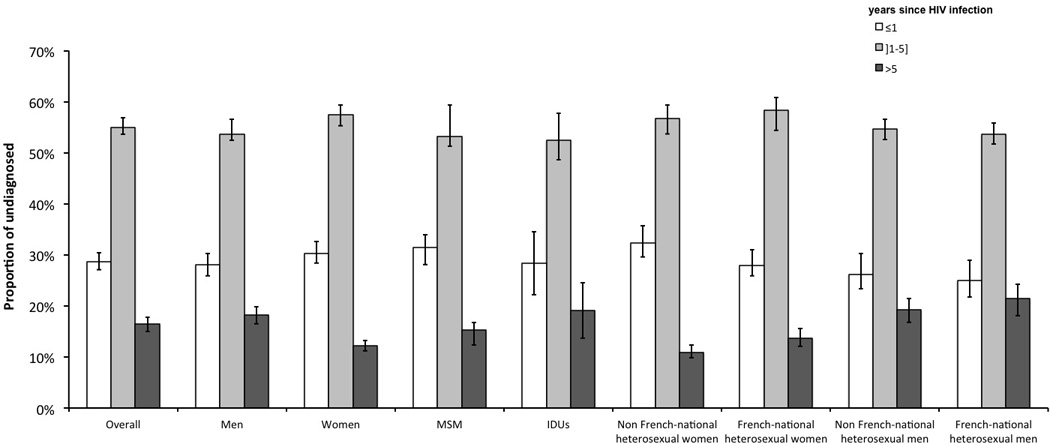
Estimated distributions of time since HIV infection among undiagnosed HIV-infected individuals at the end of 2010 in France. Vertical lines within bars represent 95% confidence intervals. MSM: men who have sex with men; IDUs: injecting drug users.
The CD4 cell count distribution of undiagnosed individuals is depicted in Figure 2. We estimated that 59.6% (59.2–59.8) of undiagnosed individuals had CD4 <500 cells/μL. Among them, a third had CD4 counts between 350 and 500 cells/μL, another third between 200 and 350, and the rest had CD4 <200 cells/μL. Distributions of CD4 counts varied by gender and exposure group (p<0.001). The main differences were found in the proportion of individuals with high CD4 counts (≥500 cells/μL) and low CD4 counts (<200 cells/μL). Compared to women, men had a lower proportion of individuals with high CD4 counts (38.2% (37.4–38.9) versus 45.8% (45.3–46.2)) and a higher proportion of individuals with low CD4 counts (21.4% (20.6–22.3) versus 14.5% (14.2–14.8)). Among exposure groups, heterosexual men, whether French or non French-nationals, had the lowest proportion of individuals with high CD4 counts (35.2% (34.2–36.2) and 35.6% (34.7–36.5), respectively) and the highest proportion of individuals with low CD4 counts (24.1% (20.8–25.3) and 23.4% (22.3–24.4), respectively), while non French-national heterosexual women combined both the highest proportion of individuals with high CD4 counts (46.5% (45.6–47.2)) and the lowest proportion of individuals with low CD4 counts (13.8% (13.3–14.4)).
Figure 2.
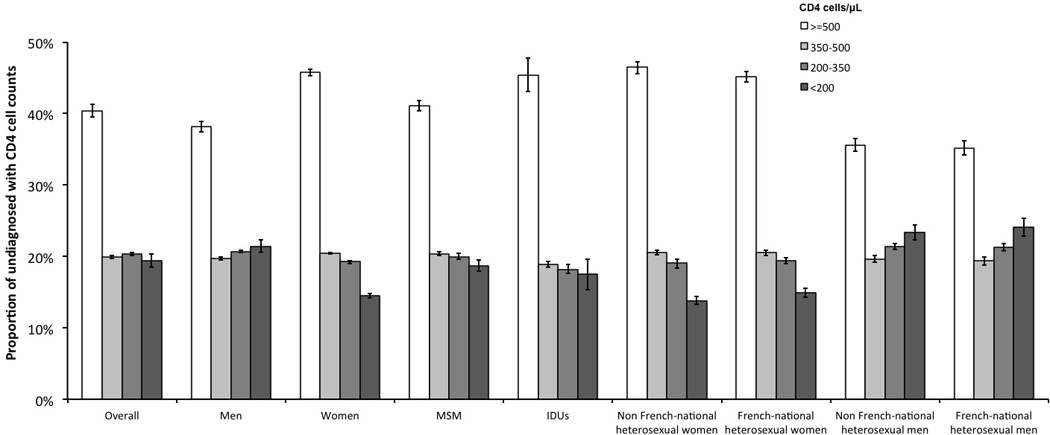
Estimated distributions of CD4 cell counts among undiagnosed HIV-infected individuals at the end of 2010 in France. Vertical lines within bars represent 95% confidence intervals. MSM: men who have sex with men; IDUs: injecting drug users.
The results from our sensitivity analysis are reported in Table 2. Assuming a linear trend for HIV incidence outside the 2004–2007 period (midpoint scenario) or assuming that yearly HIV incidence between 2000 and 2003 was 20% higher than that in 2004 and constant between 2007 and 2010 (scenario 4) led to very similar estimates of the size of the hidden epidemic (Table 2) than those obtained under the baseline scenario (Table 1). Assuming that HIV incidence was constant between 2000 and 2004 and either 20% lower or 20% higher between 2008 and 2010 than its level in 2007 (best-case and worst-case scenarios, respectively) changed the estimated number of undiagnosed individuals by 11% (Tables 1 and 2). The percentage of undiagnosed individuals infected for less than a year varied from 26.0% (24.5–27.7) (best-case scenario) to 30.8% (29.1–32.5) (worst-case scenario), while the percentage of undiagnosed individuals infected for more than 5 years varied from 14.6% (13.3–15.9) (worst-case scenario) to 18.5% (17.0–20.0) (best-case scenario). The percentage of undiagnosed individuals with CD4 counts <500 cells/μL varied from 58.8% (58.3–58.9) (worst-case scenario) to 60.6% (60.1–60.8) (best-case scenario).
Table 2.
Sensitivity analyses – changes in the estimated number of persons living with undiagnosed HIV infection in France in 2010 resulting from changes in assumptions for incidence levels outside the 2004–2007 period.
| Number of persons living with undiagnosed HIV (95% CI) |
|||||
|---|---|---|---|---|---|
| Midpoint scenario | Best-case scenario |
Worst-case scenario | Scenario 4 | ||
| Overall | 29,700 (24,700–34,600) |
25,700 (21,400–30,200) |
32,600 (27,300–38,100) |
29,400 (24,500–34,400) |
|
| Mena | 20,800 (16,900–25,100) |
18,200 (14,700–22,100) |
22,900 (18,700–27,700) |
20,600 (16,800–24,900) |
|
| Women | 8,800 (6,200–11,300) |
7,500 (5,300–9,600) |
9,700 (6,800–12,300) |
8,700 (6,200–11,100) |
|
| MSM | 9,500 (8,000–11,400) |
8,400 (7,100–10,200) |
10,700 (9,100–13,000) |
9,300 (7,900–11,300) |
|
| IDUs | 500 (100–1,100) |
400 (100–900) |
500 (100–1,200) |
500 (100–1,100) |
|
| French-national heterosexuals | 10,200 (6,500–14,100) |
8,800 (5,600–12,400) |
11,000 (7,000–15,200) |
10,100 (6,500–14,100) |
|
| French-national heterosexual women | 3,600 (1,700–5,300) |
3,100 (1,500–4,600) |
4,000 (1,900–5,800) |
3,600 (1,700–5,300) |
|
| French-national heterosexual men | 6,500 (3,700–10,000) |
5,700 (3,300–8,800) |
7,000 (4,000–10,800) |
6,500 (3,700–10,000) |
|
| Non French-national heterosexuals | 9,500 (7,000–12,200) |
8,100 (6,000–10,400) |
10,400 (7,800–13,200) |
9,400 (7,000–12,100) |
|
| Non French-national heterosexual women | 5,100 (3,500–6,800) |
4,900 (3,600–6,700) |
5,500 (4,000–7,400) |
4,200 (3,100–5,700) |
|
| Non French-national heterosexual men | 4,500 (2,300–6,800) |
4,500 (2,300–6,700) |
4,900 (2,600–7,200) |
3,900 (2,100–5,800) |
|
MSM: men who have sex with men; IDUs: injecting drug users; CI: confidence intervals; in the midpoint scenario we extrapolated values of HIV incidence before 2004 and after 2007 from a linear regression of the incidence data for the 2004–2007 period; in the best-case scenario, we assumed that HIV incidence between 2000 and 2003 was equal to its level in 2004 and HIV incidence between 2008 and 2010 was 20% lower than its level in 2007; in the worst-case scenario, we assumed that HIV incidence between 2000 and 2003 was equal to its level in 2004 and HIV incidence between 2008 and 2010 was 20% higher than its level in 2007; in scenario 4, we assumed that HIV incidence between 2000 and 2003 was 20% higher than its level in 2004 and HIV incidence between 2008 and 2010 was equal to its level in 2007;
We assumed that 2/3 of undiagnosed IDUs are men, since 2/3 of newly diagnoses IDUs are men.
Discussion
Using mathematical modeling, we estimated that 29,000 (24,200–33,900) individuals were living with undiagnosed HIV infection at the end of 2010 in France. Of these, 70% were men. The numbers of MSM, non French-national heterosexuals and French-national heterosexuals with undiagnosed HIV infection were similar (9,200, 9,300 and 10,000, respectively). However, due to differences in the size of the HIV exposure groups, undiagnosed HIV prevalence varied significantly between exposure groups (295 per 10,000 for MSM, 36 per 10,000 for non French-national heterosexuals and 3 per 10,000 for French-national heterosexuals). This implies that many more heterosexuals than MSM will need to be tested to find those undiagnosed. IDUs made up only 1.7% of the estimated size of the hidden epidemic but had the second highest HIV undiagnosed prevalence (62 per 10,000).
We found that more than 70% of individuals had lived with undiagnosed HIV infection for more than a year and 16% for more than five years. The proportion of old infections (>5 years) was remarkably high among heterosexual men and IDUs. We also found, that almost 60% of undiagnosed HIV-infected individuals had CD4 counts below 500 cells/μL, and were thus eligible for cART according to the 2010 French guidelines [23], and around 20% had advanced HIV disease (CD4 count <200 cells/μL) [24]. This latter proportion is alarming since a recent study showed that, in France, patients presenting for care with CD4 counts below 200 cells/μL were thirteen times more likely to die from HIV within the next 6 months after enrolment than patients presenting with CD4 counts above 200 cells/μL [7].
The strengths of our back-calculation approach are that i) it requires only routinely collected data from HIV/AIDS surveillance systems, ii) it can be easily updated as new data become available, and iii) it provides estimates of the size of the hidden epidemic, stratified by HIV exposure group, together with characteristics of individuals living with undiagnosed HIV infection, such as the time since infection. Furthermore combining these estimates with data on the natural history of CD4 cell counts among HIV-infected patients allows estimating the distribution of CD4 cell counts among undiagnosed individuals.
Our approach makes several modeling assumptions subject to limitations. To estimate the number of undiagnosed HIV infections at the end of 2010, we assumed that all individuals who acquired HIV before 2000 were diagnosed by the end of 2010 and neglected the pre-HIV diagnosis mortality. These assumptions may have led to underestimating the size of the hidden epidemic, especially among IDUs for whom overdose remains a main cause of death. In addition, we assumed that HIV incidence and time from infection to diagnosis were stable over the periods 2000–2004 and 2007–2010. These assumptions were based on previous findings [19], showing that both incidence and time from infection to diagnosis were stable over the 2004–2007 period, and supported by HIV surveillance data and screening policies; both the number of newly diagnosed HIV cases and their clinical stage at diagnosis (i.e. the proportion of primary infection, asymptomatic and AIDS cases among newly diagnosed HIV cases) remained stable between 2007 and 2010 [25] and French screening policies remained unchanged until November 2010, when the National HIV and STI Plan for 2010–2014 was released [26]. Although these assumptions may be arguable, we showed that our estimates are quite robust to these assumptions via a sensitivity analysis. Furthermore, our overall estimate of the size of the hidden epidemic is in agreement with the estimates of 29,008 (95% CI: 11,603–70,958) undiagnosed HIV infections recently reported by Cazein et al. [27], who used different data and methodology. This also suggests that our hypotheses are reasonable.
To determine CD4 cell counts of undiagnosed HIV-infected individuals, we used longitudinal CD4 count data from a cohort of diagnosed HIV-infected patients with well-estimated dates of HIV seroconversion [17]. This may have led to some bias since seroconverters cohorts may not be representative of the HIV infected population due to the circumstances resulting in repeated testing and increased perception of their risk of HIV infection. Nevertheless, a recent study from CASCADE [28] has reported no evidence of a difference in CD4 count decline between seroconverters and seroprevalent HIV patients (i.e., patients with unknown date of seroconversion). Therefore, we believe that this potential bias is unlikely to invalidate our results. In addition, we assumed that CD4 cell count decline was similar for non French-national and French-national heterosexuals. However, it has recently been showed [29] that African HIV-infected migrants reach CD4≤350 cells/mm3 approximately 1.5 years earlier than non-African European. Therefore, we might have overestimated CD4 cell counts among non French-national heterosexuals.
Findings from our study may help interpreting HIV screening surveys. For example, in a recent French survey evaluating the relevance of non-targeted screening in emergency departments [30], all individuals newly diagnosed with HIV were fond to belong to a high-risk group (i.e. MSM and non French-nationals heterosexuals) and no new HIV-positive cases were discovered among the 8,430 French heterosexuals tested. The investigators of this survey found their results unexpected and concluded that that there was no hidden epidemic among French heterosexuals and thus non-targeted screening had modest public health impact [30]. However finding zero cases out of 8,430 tested individuals results in a 95% CI for the undiagnosed HIV prevalence between 0 and 4 per 10,000. This is in agreement with our estimated prevalence ranging from 2 to 4 per 10,000 for French heterosexuals and thus compatible with a number of undiagnosed HIV infections among French heterosexuals between 0 and 13,900. Therefore, according to our estimates, the survey did not screen enough French heterosexuals to support the conclusion of the authors.
Our estimates may also help guiding the implementation of new HIV screening strategies. We found that the overall undiagnosed HIV prevalence was 0.07% (0.06–0.09), which is below the 0.10% threshold for a HIV screening strategy to be cost-effective in France [31]. Therefore, according to current estimates, universal screening of the whole French population would not be cost-effective. This finding will, however, need to be re-evaluated in the future for two reasons. First, the threshold value of 10%, for a screening strategy to be cost-effective in France, was estimated before 2010 and under conservative assumptions (e.g. high cost of HIV screening) [31]. Second, the size of the hidden epidemic may have changed since 2010. Indeed, the estimated annual number of individuals newly infected with HIV [19, 32] was always higher than the observed annual number of individuals newly diagnosed with HIV [25] by at least 500 between 2003 and 2008, the last time period where HIV incidence was estimated. Therefore, if similar trends in HIV incidence and newly diagnosed HIV cases had occurred after 2010, the number of undiagnosed HIV-infected individuals could have increased since 2010.
As expected, we found that screening strategies targeted toward specific high-risk group would be cost-effective. Interestingly, universal screening of men, who represent 70% of the hidden epidemic, could also be cost-effective since undiagnosed HIV prevalence among men was estimated at 0.10% (0.09–0.13). Universal screening of men could have the additional benefit to reduce stigma associated with targeted screening and improve the acceptability of HIV testing of migrant men, as it was shown to be the case for migrant women in antenatal care [33]. While targeted screening of MSM have shown promising results in France [34, 35], targeted screening may be more difficult to implement toward migrants, who may be more difficult to reach, because of language and cultural barriers [33]. Finally, universal screening of men is likely to be more cost-effective if implemented in high HIV prevalence settings such as the metropolitan area of Paris [30, 36].
In summary, our study suggests that 29,000 individuals living with HIV remained undiagnosed in France in 2010 and the vast majority of them were eligible for treatment. New screening strategies to reach out undiagnosed individuals are needed in order to reduce HIV transmission [3] and ensure timely access to HIV care for these individuals [7]. Finally, universal screening of men could be cost-effective in the areas most affected by the epidemic, such as the metropolitan area of Paris, and thus should be further evaluated.
Supplementary Material
Acknowledgments
VS thanks Romulus Breban for fruitful discussions. VS is grateful for the financial support of Sidaction and ANRS, in the form of postdoctoral research fellowships. NJDA is grateful for financial support from the French Ministry of Education, Research and Technology, in the form of a MENRT PhD fellowship. The sponsors had no role in the study.
Footnotes
Contributions: VS and DC conceived and designed the study; VS, JDAN and SL acquired the data; VS performed the statistical analysis; VS, JDAN, SL and DC analyzed and interpreted the data; VS drafted the manuscript; VS, JDAN, SL and DC critically revised the manuscript for important intellectual content.
Conflicts of interest: The authors declare that they have no conflict of interest.
References
- 1.UNAIDS. Report on the global AIDS epidemic. Available from http://www.has-sante.fr/portail/upload/docs/application/pdf/2009-10/synthese_depistage_vih_volet_2_vfv_2009-10-21_16-48-3_460.pdf. [Google Scholar]
- 2.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–896. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 6.Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montlahuc C, Guiguet M, Abgrall S, Daneluzzi V, de Salvaldor F, Launay O, et al. Impact of late presentation on the risk of death among HIV-infected people in France (2003–2009) J Acquir Immune Defic Syndr. 2013;64:197–203. doi: 10.1097/QAI.0b013e31829cfbfa. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounier-Jack S, Nielsen S, Coker RJ. HIV testing strategies across European countries. HIV medicine. 2008;9(Suppl 2):13–19. doi: 10.1111/j.1468-1293.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for disease Control and Prevention. Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR. 2006;55 [PubMed] [Google Scholar]
- 11.Haute Autorité de Santé. Recommendations de Santé Publique : Dépistage de l’infection par le VIH en France. Stratégies et dispositif de dépistage. Synthèse et recommandations. 2009 Octobre; Available from http://www.has-sante.fr/portail/upload/docs/application/pdf/2009-10/synthese_depistage_vih_volet_2_vfv_2009-10-21_16-48-3_460.pdf. [Google Scholar]
- 12.Health Protection Agency. Time to test for HIV: Expanding HIV testing in healthcare and community services in England. 2011 Available from http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1316424799217. [Google Scholar]
- 13.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 14.HIV in hiding: methods and data requirements for the estimation of the number of people living with undiagnosed HIV. AIDS. 2011;25:1017–1023. doi: 10.1097/QAD.0b013e3283467087. [DOI] [PubMed] [Google Scholar]
- 15.Birrell PJ, Gill ON, Delpech VC, Brown AE, Desai S, Chadborn TR, et al. HIV incidence in men who have sex with men in England and Wales 2001-10: a nationwide population study. Lancet Infect Dis. 2013;13:313–318. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookmeyer R. Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol Rev. 2010;32:26–37. doi: 10.1093/epirev/mxq002. [DOI] [PubMed] [Google Scholar]
- 17.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53:817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 18.Semaille C, Cazein F, Pillonel J, Lot F, Le Vu S, Pinget R, et al. Four years of surveillance of recent HIV infections at country level, France, mid 2003 – 2006: experience and perspectives. Euro Surveill. 2008;13 [PubMed] [Google Scholar]
- 19.Ndawinz JD, Costagliola D, Supervie V. New method for estimating HIV incidence and time from infection to diagnosis using HIV surveillance data: results for France. AIDS. 2011;25:1905–1913. doi: 10.1097/QAD.0b013e32834af619. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Statistics and Economic Studies (INSEE) [Google Scholar]
- 21.Bajos N, Bozon M. Enquête sur la sexualité en France : pratiques, genre et santé. Paris: Editions La Découverte; 2008. [Google Scholar]
- 22.Costes JM, Vaissade L, Colasante E, Palle C, Legleye S, Janssen E, et al. Prévalence de l’usage problématique de drogues en France—estimations 2006. Saint-Denis: Observatoire Français des Drogues et des Toxicomanies; 2009. [Google Scholar]
- 23.Yeni P. Prise en charge médicale des personnes infectées par le VIH (Rapport 2010) Available from http://www.sante.gouv.fr/IMG/pdf/Rapport_2010_sur_la_prise_en_charge_medicale_des_personnes_infectees_par_le_VIH_sous_la_direction_du_Pr-_Patrick_Yeni.pdf. [Google Scholar]
- 24.Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12:61–64. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 25.Cazein F, Pinget R, Lot F, Pillonel J, Le Strat Y, Sommen C, et al. New HIV and AIDS diagnoses � France, 2003–2011. Bulletin Epidémiologique Hebdomadaire. 2013;28–29:333–340. [Google Scholar]
- 26.Ministère de la santé et des Sports. Plan national de lutte contre le VIH/SIDA et les IST 2010–2014. 2010 Nov; Available from http://www.sante.gouv.fr/IMG/pdf/plan_national_lutte_contre_le_VIH-SIDA_et_les_IST_2010-2014.pdf. [Google Scholar]
- 27.Cazein F, Barin F, Le Strat Y, Pillonel J, Le Vu S, Lot F, et al. Prevalence and Characteristics of Individuals With Undiagnosed HIV Infection in France: Evidence From a Survey on Hepatitis B and C Seroprevalence. J Acquir Immune Defic Syndr. 2012;60:e116–e118. doi: 10.1097/QAI.0b013e318256b3fd. [DOI] [PubMed] [Google Scholar]
- 28.Lodi S, Phillips A, Touloumi G, Pantazis N, Bucher HC, Babiker A, et al. CD4 decline in seroconverter and seroprevalent individuals in the precombination of antiretroviral therapy era. AIDS. 2010;24:2697–2704. doi: 10.1097/QAD.0b013e32833ef6c4. [DOI] [PubMed] [Google Scholar]
- 29.Pantazis N, Morrison C, Amornkul PN, Lewden C, Salata RA, Minga A, et al. Differences in HIV Natural History among African and Non-African Seroconverters in Europe and Seroconverters in Sub-Saharan Africa. PLoS One. 2012;7:e32369. doi: 10.1371/journal.pone.0032369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.d'Almeida KW, Kierzek G, de Truchis P, Le Vu S, Pateron D, Renaud B, et al. Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch Intern Med. 2012;172:12–20. doi: 10.1001/archinternmed.2011.535. [DOI] [PubMed] [Google Scholar]
- 31.Yazdanpanah Y, Sloan CE, Charlois-Ou C, Le Vu S, Semaille C, Costagliola D, et al. Routine HIV screening in France: clinical impact and cost-effectiveness. PLoS One. 2010;5:e13132. doi: 10.1371/journal.pone.0013132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Vu S, Le Strat Y, Barin F, Pillonel J, Cazein F, Bousquet V, et al. Population-based HIV-1 incidence in France, 2003–08: a modelling analysis. Lancet Infect Dis. 2010;10:682–687. doi: 10.1016/S1473-3099(10)70167-5. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Del Arco D, Monge S, Azcoaga A, Rio I, Hernando V, Gonzalez C, et al. HIV testing and counselling for migrant populations living in high-income countries: a systematic review. Eur J Public Health. 2013;23:1039–1045. doi: 10.1093/eurpub/cks130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champenois K, Le Gall JM, Jacquemin C, Jean S, Martin C, Rios L, et al. ANRS-COM'TEST: description of a community-based HIV testing intervention in non-medical settings for men who have sex with men. BMJ Open. 2012;2:e000693. doi: 10.1136/bmjopen-2011-000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouzioux C, Le Talec JY, Kreplak G, Tessier P, Sebbah G, Tellier G, et al. Frequent HIV testing in a community setting improves detectection of acute and recent infections, among MSM in Paris, France (Checkpoint study). International AIDS Conference; Rome, Italy. 2011. [Google Scholar]
- 36.Casalino E, Bernot B, Bouchaud O, Alloui C, Choquet C, Bouvet E, et al. Twelve months of routine HIV screening in 6 emergency departments in the Paris area: results from the ANRS URDEP study. PloS One. 2012;7:e46437. doi: 10.1371/journal.pone.0046437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


