Abstract
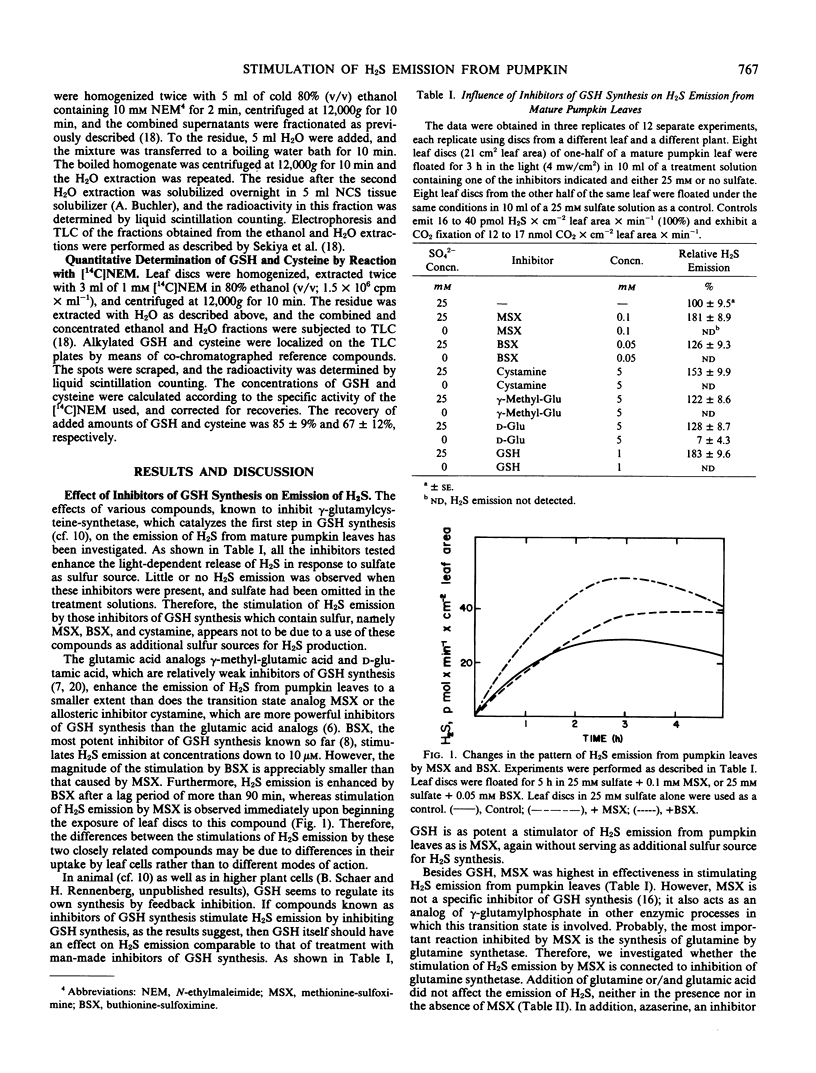
The effect of inhibitors of glutathione (GSH) synthesis, namely γ-methyl glutamic acid, d-glutamic acid, cystamine, methionine-S-sulfoximine (MSX), buthionine-S-sulfoximine, and GSH itself, on the emission of H2S was investigated. All these compounds stimulated H2S emission from pumpkin (Cucurbita pepo L. cv Small Sugar Pumpkin) leaf discs in response to sulfate. MSX and GSH were the most effective compounds, stimulating H2S emission from leaf discs of mature pumpkin leaves by about 80% in response to sulfate. Both inhibitors did not appreciably enhance H2S emission in response to l-cysteine and inhibited H2S emission in response to sulfite.
Treatment with MSX or GSH enhanced the uptake of sulfate by pumpkin leaf discs, but did not affect the incorporation of sulfate into reduced sulfur compounds. Inhibition of GSH synthesis by MSX or GSH caused an increase in the pool size of cysteine, and, simultaneously, reduced the incorporation of labeled sulfate into cysteine. The incorporation of labeled sulfate into the sulfite and sulfide pools of the cells are stimulated under these conditions.
These observations are consistent with the idea that inhibition of GSH synthesis leads to an elevated cysteine pool that inhibits further cysteine synthesis. The H2S emitted under these conditions appears to arise from diversion of a precursor of the sulfur moiety of l-cysteine. Therefore, stimulation of H2S emission in response to sulfate upon inhibition of GSH synthesis may reflect a role of H2S emission in keeping the cysteine concentration below a critical level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressan R. A., Wilson L. G., Filner P. Mechanisms of resistance to sulfur dioxide in the Cucurbitaceae. Plant Physiol. 1978 May;61(5):761–767. doi: 10.1104/pp.61.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Larsson A., Meister A. Inhibition of gamma-glutamylcysteine synthetase by cystamine: an approach to a therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem Biophys Res Commun. 1977 Dec 7;79(3):919–925. doi: 10.1016/0006-291x(77)91198-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Selective inhibition of gamma-glutamyl-cycle enzymes by substrate analogs. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3330–3334. doi: 10.1073/pnas.74.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980 Jan;65(1):151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Sekura R., Van Der Werf P., Meister A. Mechanism and significance of the mammalian pathway for elimination of D-glutamate; inhibition of glutathione synthesis by D-glutamate. Biochem Biophys Res Commun. 1976 Jul 12;71(1):11–18. doi: 10.1016/0006-291x(76)90242-4. [DOI] [PubMed] [Google Scholar]
- Wilson L. G., Bressan R. A., Filner P. Light-dependent Emission of Hydrogen Sulfide from Plants. Plant Physiol. 1978 Feb;61(2):184–189. doi: 10.1104/pp.61.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]


