Abstract
Somatostatin receptors (SSTr) are overexpressed in a wide range of neuroendocrine tumors, making them excellent targets for nuclear imaging and therapy, and radiolabeled somatostatin analogues have been investigated for positron emission tomography imaging and radionuclide therapy of SSTr-positive tumors, especially of the subtype-2 (SSTr2). The aim of this study was to develop a somatostatin analogue, Tyr3-octreotate (Y3-TATE), conjugated to a novel cross-bridged macrocyclic chelator, 11-carboxymethyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4-methanephosphonic acid (CB-TE1A1P). Unlike traditional cross-bridged macrocycles, such as 4, 11 - bis (carboxymethyl) - 1, 4, 8, 11 -etraazabicyclo[6.6.2]hexadecane (CB-TE2A), CB-TE1A1P-Y3-TATE was radiolabeled with 64Cu in high purity and high specific activity using mild conditions. Saturation binding assays revealed that 64Cu-CB-TE1A1P-Y3-TATE had comparable binding affinity but bound to more binding sites in AR42J rat pancreatic tumor cell membranes than 64Cu-CB-TE2A-Y3-TATE. Both radiopharmaceuticals showed comparable uptake in SSTr2 positive tissues in AR42J tumor-bearing rats. 64Cu-CB-TE1A1PY3- TATE demonstrated improved blood clearance compared to 64Cu-CB-TE2A-Y3-TATE, as the tumor/blood ratios of 64Cu-CB-TE1A1P-Y3-TATE were shown to be significantly higher than those of 64Cu-CB-TE2A-Y3-TATE at 4 and 24 h postinjection. 64Cu-CB-TE1A1P-Y3-TATE, in spite of a relatively high kidney uptake, accumulated less in nontarget organs such as liver, lung, and bone. Small animal PET/CT imaging of 64Cu-CB-TE1A1P-Y3-TATE in AR42J tumor bearing rats validated significant uptake and good contrast in the tumor. This study suggests that CB-TE1A1P is a promising bifunctional chelator for 64Cu-labeled for Y3-TATE, owing to high binding affinity and target tissue uptake, the ability to radiolabel the agent at lower temperatures, and improved tumor/nontarget organ ratios over 64Cu-CB-TE2A-Y3-TATE.
INTRODUCTION
Copper-64 (T1/2 = 12.7 h) is an attractive radiometal for positron emission tomography, as it decays by β+ at 656 keV (17.8% abundance), which provides high-quality PET images at relatively low radiation dose to patients and radiochemistry personnel. Moreover, 64Cu is a promising therapeutic radiometal owing to its emission of β− at 573 keV (39.6% abundance). Thus, 64Cu holds great promise for combined cancer imaging and targeted radiotherapy.1 We previously reported that the cross-bridged macrocycle 4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A) was a superior bifunctional chelator for 64Cu compared with 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid (TETA) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) due to the exceptional kinetic inertness of its Cu (II) complexes.2 CB-TE2A stably chelated 64Cu in vivo and significantly reduced the amount of 64Cu being transchelated to superoxide dismutase in the liver.3−5 The advantage could be retained after CB-TE2A was conjugated to the somatostatin analogue Y3-TATE, as 64Cu-CB-TE2A-Y3-TATE demonstrated improved tumor uptake, as well as liver and blood clearance compared to 64Cu-TETA-Y3-TATE, resulting in higher tumor to tissue ratios.6 Though CB-TE2A is a very promising bifunctional chelator for 64Cu, a significant disadvantage is that it cannot be applied in radiolabeling antibodies or other heat-sensitive compounds, due to the fact that radiolabeling with 64Cu requires heating to 95 °C for at least 1 h.
It has been reported that the metal-binding kinetics of cyclamderived macrocycles can be accelerated by substituting the carboxylate pendant arms with phosphonate pendant arms.7 The thermodynamic stability of the Cu(II) complexes of certain phosphonate chelators was proven to be favorable compared to their acetate analogues.8 The radiochemistry and biodistribution of a diphosphonate cross-bridged macrocycle, 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-bismethanephosphonic acid (CB-TE2P) labeled with 64Cu, was recently communicated.7 Although CB-TE2P could be radiolabeled with 64Cu at room temperature, there were challenges in conjugating the diphosphonate to primary amine side chains of peptides. Ferdani et al. recently reported the synthesis and 64Cu radiolabeling of a monocarboxylate monophosphonate chelator, CB-TE1A1P (11-carboxymethyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4-methanephosphonic acid).9 64Cu-CB-TE1A1P can be prepared at room temperature and was shown to have high in vivo stability in normal rats.
We report here the synthesis of CB-TE1A1P conjugated to the somatostatin analogue Y3-TATE radiolabeled with 64Cu for PET imaging and targeted radiotherapy for SSTr2 positive tumors. SSTr2-positive rat pancreatic AR42J cells and tumor-bearing rats are used to evaluate the in vitro and in vivo behavior of 64Cu-CBTE1A1P-Y3-TATE. The biological behavior of 64Cu-CBTE1A1P-Y3-TATE is compared to previously published data with 64Cu-CB-TE2A-Y3-TATE.12
MATERIALS AND METHODS
Copper-64 was produced on a CS-15 biomedical cyclotron at Washington University School of Medicine as previously described.10 Unless specified, all chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Aqueous solutions were prepared using ultrapure water (resistivity, 18 mΩ). The peptide Y3-TATE was purchased from CS Bio (Madera, CA) and Tianma Pharma (Sichuan, China). Analytical and semipreparative reversed-phase high-performance liquid chromatography (HPLC) was performed on a Waters 600E (Milford, MA) chromatography system with a Waters 991 photodiode array detector and an Ortec model 661 (EG&G Instruments, Oak Ridge, TN) radioactivity detector. Nonradioactive HPLC samples were analyzed on an analytical C18 column (Vydac, Deerfield, IL) and purified on a semipreparative C18 column (Waters, Milford, MA). Radiochemistry reaction progress and purity were monitored on a rocket C18 column (Alltima, Deerfield, IL). The mobile phase was H2O (0.1% TFA; solvent A) and acetonitrile (0.1% TFA; solvent B). Radioactive samples were counted using an automated well-type γ-counter (8000; Beckman, Irvine, CA) or a β-plate reader (1450 Micro Beta; Perkin-Elmer, Waltham, MA). The PET data were acquired using an Inveon small animal PET/CT scanner (Siemens Medical Solutions; Knoxville, TN). Electrospray mass spectrometry was accomplished using a Waters Micromass ZQ (Milford, MA).
Animal Model
Male Lewis rats (age, 21 d; weight, 40−50 g) were purchased from Charles River Laboratories (Boston, MA) and handled according to the procedures outlined by the Washington University Animal Studies Committee. AR42J pancreatic tumors were implanted into the hind limbs by serial passage as previously described and were allowed to grow 10−14 d.6
Synthesis of CB-TE1A1P-Y3-TATE
The conjugation of Y3-TATE to CB-TE1A1P was performed based on the method by Sprague et al.6 side-chain protected Y3-TATE on resin (50.3 mg, 6.2 µmol of Y3-TATE peptide) was washed sequentially with dichloromethane (DCM) (2 × 3 mL), methanol (2 × 3 mL), and DCM (2 × 3 mL), and dried under vacuum. The resin was swollen in DCM (3 mL) for 45 min and washed with DMF (3 mL) several times. To a solution of CB-TE1A1P (32.2 mg, 85 µmol) in DMF (1.5 mL) and DMSO (1 mL), DIC (20.4 mg, 161 µmol), and DIEA (37.1 mg, 288 µmol) was added. The mixture was stirred for 25 min before being added to the peptide resin. After end-to-end rotation at room temperature for 10 d, the resin was filtered, washed with DCM (3 × 3 mL) to remove residual DMF, and dried under vacuum. The peptide conjugate was cleaved from the resin by stirring with a mixture of TFA/water/ thioanisol/phenol (85:5:5:5) for 4 h followed by precipitation in cold t-butyl methyl ether. HPLC purification of CB-TE1A1P-Y3-TATE was performed on a semipreparative C18 column (10 × 250 mm, 10 µm; Alltima) using two solvent systems with the gradient elution method at a flow rate of 3 mL/min. Solvent A was 0.1% TFA in water, and solvent B was 0.1% TFA in acetonitrile. The elution method started with a linear gradient from 90% to 80% A over 10 min, followed by 80% to 70% A over 10 min, and from 70% to 40% over 3 min. The elution profile was monitored by UV absorbance at 254 nm.
Purified CB-TE1A1P-Y3-TATE (~7% yield) was obtained as a white powder after lyophilization in water/acetonitrile mixture. The compound was characterized by analytical HPLC and electrospray mass spectroscopy. Calculated mass for C64H92N14O16P1S2 = 1409.6 (ESI + MS) m/z (% relative intensity, ion), (M+H)+ = 1409.1399, (M+2H)2+ = 705.0614, and (M+3H)3+ = 470.3873.
Radiochemistry of 64Cu-CB-TE1A1P-Y3-TATE
Radiolabeling of CB-TE1A1P-Y3-TATE with 64Cu was achieved by reacting 1−1.5 mCi (37−55.5 MBq) of 64Cu with 1 µg (7.09 × 10−4 µmol) of CB-TE1A1P-Y3-TATE in 100 µL of 0.1 M ammonium acetate (pH 8.0). The reaction was incubated for various times at 95 °C, 40 °C or room temperature. Radiochemical purity was determined by radio-HPLC with a rocket C18 column (7 × 53 mm, 3 µm; Alltima). For reactions of less than 95% purity, ethylenediaminetetraacetic acid (EDTA) and a C-18 SepPak Light cartridge (Waters) were used to remove nonchelated 64Cu as described by Wadas et al.11 Briefly, buffered EDTA was added to chelate free 64Cu and the mixed solution was then loaded onto a SepPak cartridge (Waters, Milford, MA). After washing the SepPak cartridge with 5 mL water, eluting with absolute ethanol containing 5% acetic acid recovered 70−80% of the labeled peptide. The radiopharmaceutical was evaporated and rediluted in buffer or saline before being used for further cell or animal studies.
In Vitro Binding Affinity
The affinity of 64Cu-CB-TE1A1PY3-TATE for SSTr2 was determined by a saturation binding assay based on previously published methods.12 AR42J cell membrane preparations were diluted in binding buffer [0.1% bovine serum albumin, 50 mM Tris-HCl (pH 7.4), 5.0 mmol/L MgCl2, 0.5 µg/mL aprotinin, 200 µg/mL bacitracin, 10 µg/mL leupeptin, and 10 µg/mL pepstatin A], and 20 µg of membrane was added to each well of a 96-well filtration plate (Multiscreen Durapore; Millipore; Billerica, MA). Membranes were incubated with increasing concentrations of 64Cu-CB-TE1A1P-Y3-TATE for 2 h at room temperature. Nonspecific binding was determined by saturating receptors with excess Y3-TATE. Upon reaching equilibrium, unbound radioactivity was filtered, and the membranes were washed twice with 200 µL binding buffer. Bound radioactivity was measured with a liquid scintillation and luminescence plate reader (1450 Microbeta; Perkin-Elmer; Waltham, MA). Total binding sites (Bmax) and binding affinity (Kd) were determined by a nonlinear regression fit of bound peptides per milligram of protein versus concentration of radioligand using GraphPad Prism v 5.0 (San Diego, CA).
Cellular Internalization
AR42J cells were seeded in 6-well plates and cultured with DMEM medium supplemented with 20% fetal bovine serum and 0.1% gentamicin (3 mL/well) at 37 °C in a humidified atmosphere containing 5% CO2 until 90% confluence was reached. Cells were incubated with 4 nM 64Cu-CB-TE1A1P-Y3-TATE (37 MBq/µg), with or without the addition of excess Y3-TATE (2 µg/well) to block nonspecific internalization. At each time point, the surface-bound fractions were collected as previously described.13 Internalized activity was collected by lysing the cells with 0.5% SDS. Total protein content in the cell lysates was determined by BCA protein assay (Pierce Biotechnology; Rockford, IL). Surface-bound and internalized fractions were expressed as the percentage of administered activity per milligram of protein.
In Vivo Biodistribution
The biodistribution studies were carried out as previously described.14 64Cu-CB-TE1A1P-Y3-TATE (0.74 MBq, 20 ng in 150 µL) was injected into AR42J tumor-bearing male Lewis rats (5 weeks old) via tail vein. At 1, 4, and 24 h after injection, rats were sacrificed and selected organs were taken, weighed, and counted on a γ counter (Beckman 8000; Irvine, CA). A blocking study was also conducted to examine the specificity of in vivo uptake where 64Cu-CBTE1A1P-Y3-TATE (0.74 MBq, 20 ng in 150 µL) was coinjected with 200 µg of Y3-TATE into AR42J tumor-bearing male Lewis rats (5 weeks old) via tail vein. Tissue uptake was measured at 4 h after injection.
Small Animal PET/CT Imaging
AR42J tumor-bearing male Lewis rats (5 weeks old) were injected with 64Cu-CB-TE1A1PY3- TATE (7.4 MBq, 200 ng in 150 µL). At 2 h after injection, rats were anesthetized with 1−2% isoflurane and imaged. A blocking study was conducted in two AR42J tumor-bearing male Lewis rats (5 weeks old) by coinjecting 64Cu-CB-TE1A1P-Y3-TATE (7.4 MBq, 200 ng in 150 µL) with 200 µg of Y3-TATE. The blocking group was also imaged at 2 h postinjection. Tumor standard uptake values (SUV) were generated by measuring regions of interest from PET/CT images and calculated with the formula: SUV = [nCi/mL] × [animal weight (g)]/injected dose [nCi].
Statistical Methods
All of the data are presented as mean ± standard error. To determine statistical significance, two-tailed unpaired t-tests were performed using GraphPad Prism, with p < 0.05 considered statistically significant.
RESULTS
Synthesis of CB-TE1A1P-Y3-TATE
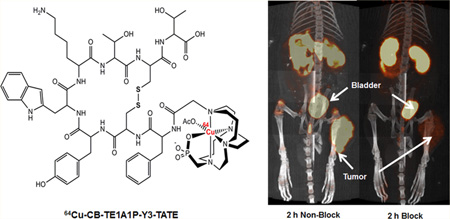
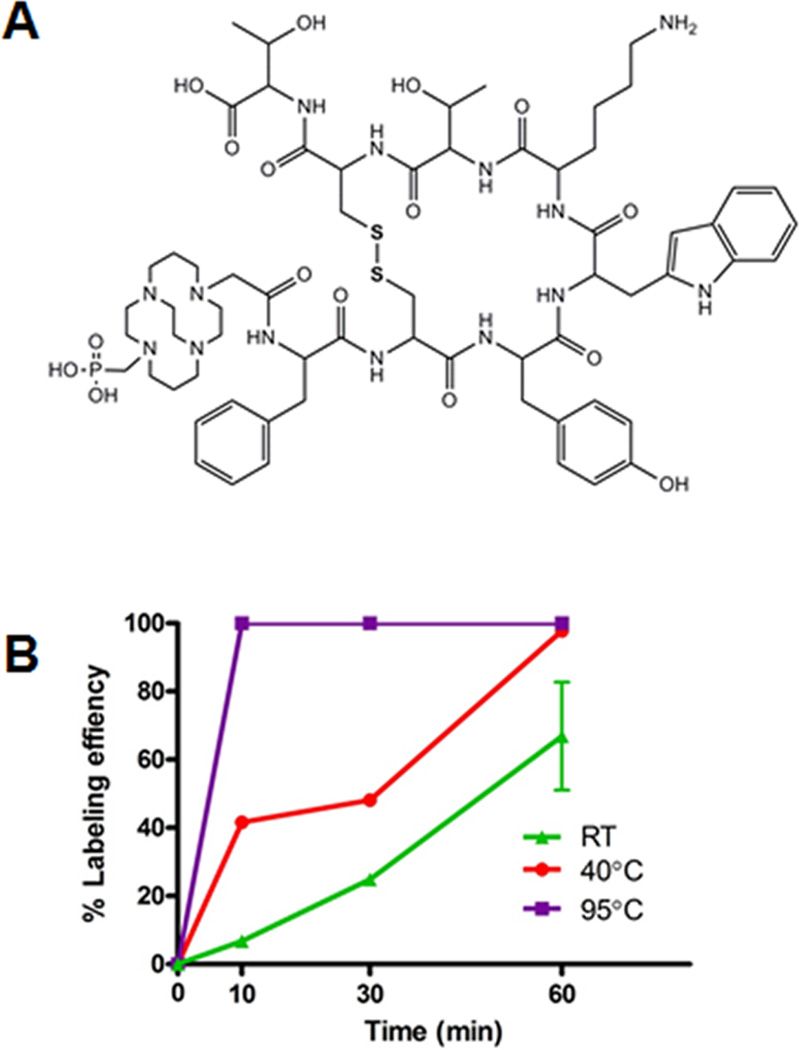
The chemical structure of CB-TE1A1P-Y3-TATE is shown in Figure 1A. The carboxylic group of CB-TE1A1P first reacts with dicyclohexylcarbodiimide to form an O-acylisourea or an intermolecular acid anhydride. Both activated intermediates then react with N-terminal amino groups of the peptides on solid support to produce amide linkages. The presence of phosphoramidates was not observed, indicating that the phosphonic group of CB-TE1A1P does not form stable conjugates with peptides. Previous studies have shown that the reaction between phosphonic groups and amines can occur, but the products hydrolyze and are stable only under strongly basic conditions.15,16 We postulate that the hydrolysis of phosphoramidates leads to a lowered conjugation yield (~7%) and necessitates HPLC purification to separate desired carboxylate-conjugated species from the unconjugated Y3-TATE peptide. The yield can be further improved by more cycles of HPLC purification, if needed.
Figure 1.
Chemical structure of CB-TE1A1P-Y3-TATE (A). Labeling efficiency of CBTE1A1P-Y3-TATE with 64Cu in in 0.1 M NH4OAc (pH 8.0) at different temperatures (B).
Radiochemistry of 64Cu-CB-TE1A1P-Y3-TATE
As shown in Figure 1B, radiolabeling of CB-TE1A1P-Y3-TATE with 64Cu in 0.1 M NH4OAc (pH 8.0) at room temperature resulted in ~60% labeling efficiency after 1 h, while raising the temperature to 40 °C increased the radiochemical yield to over 90%. Heating to 95 °C reduced the reaction time to less than 10 min with a labeling efficiency over 95% and improved specific activity from 1 mCi/µg to 1.5 mCi/µg. The radiochemical purity of 64Cu-CBTE1A1P-Y3-TATE was validated by radio-HPLC on a rocket C18 column.
In Vitro Binding Affinity
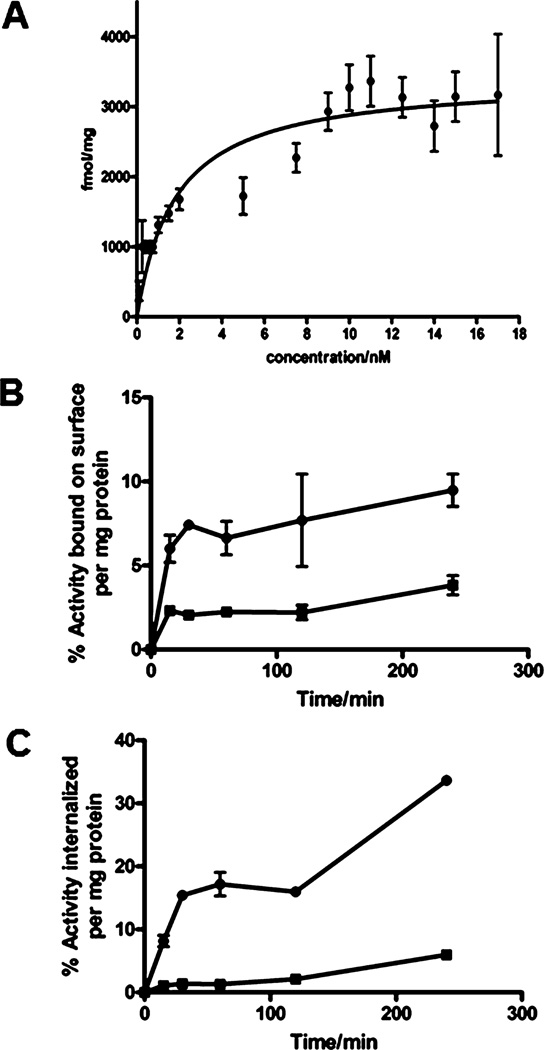
Saturation binding assays were performed with AR42J cell membranes bearing SSTr2 based on previously described methods (Figure 2A).15 The total number of binding sites (Bmax) for 64Cu-CB-TE1A1P-Y3-TATE was 3410 ± 220 fmol/mg, which is 1.5−3-fold higher than that observed for 64Cu-CB-TE2A-Y3-TATE (Bmax = 1090−2100 fmol/mg).6 The Kd for 64Cu-CB-TE1A1P-Y3-TATE was 1.8 ± 0.5 nM, suggesting that the CB-TE1A1P and CB-TE2A conjugates (Kd of 64Cu-CB-TE2A-Y3-TATE = 1.7 nM) have similar binding affinities for SSTr2 in AR42J membranes.
Figure 2.
In vitro binding affinity of 64Cu-CB-TE1A1P-Y3-TATE for SSTr2 was determined by a saturation binding assay in AR42J rat pancreatic tumor cell membranes (n = 3 for each data point; mean value ± SE) (A). Internalization studies of 64Cu-CB-TE1A1P-Y3-TATE were conducted in AR42J whole cells. Radioactivity bound on cell surface (B) and internalized (C) were determined. Specific uptake was blocked by addition of 2 µg Y3-TATE to the incubation media (64Cu-CB-TE1A1PY3-TATE; blocked 64Cu-CB-TE1A1P-Y3-TATE; n = 3 for each data point; mean value ± SE).
Cellular Internalization
Internalization studies of 64Cu-CB-TE1A1P-Y3-TATE were also performed with AR42J cells based on previously described methods (Figure 2B).20 All data are presented as the percentage of administrated radioactivity per milligram of protein. Rapid early stage internalization was observed for 64Cu-CB-TE1A1P-Y3-TATE during the initial 30 min, which was followed by two slower phases at 60 and 120 min. The amount of radiotracer internalized reached 34 ± 0.4% by 240 min. Specific receptor-mediated internalization could be readily blocked by an excess of Y3-TATE in the medium. The surface-bound fraction of radiotracer was also collected and quantified. The amount of 64Cu-CB-TE1A1P-Y3-TATE bound to the cell surface reached 5.2 ± 0.03% within the first 15 min, and increased slightly over the next 225 min. Surface-bound activity was blocked with excess Y3-TATE in the culture medium.
In Vivo Biodistribution
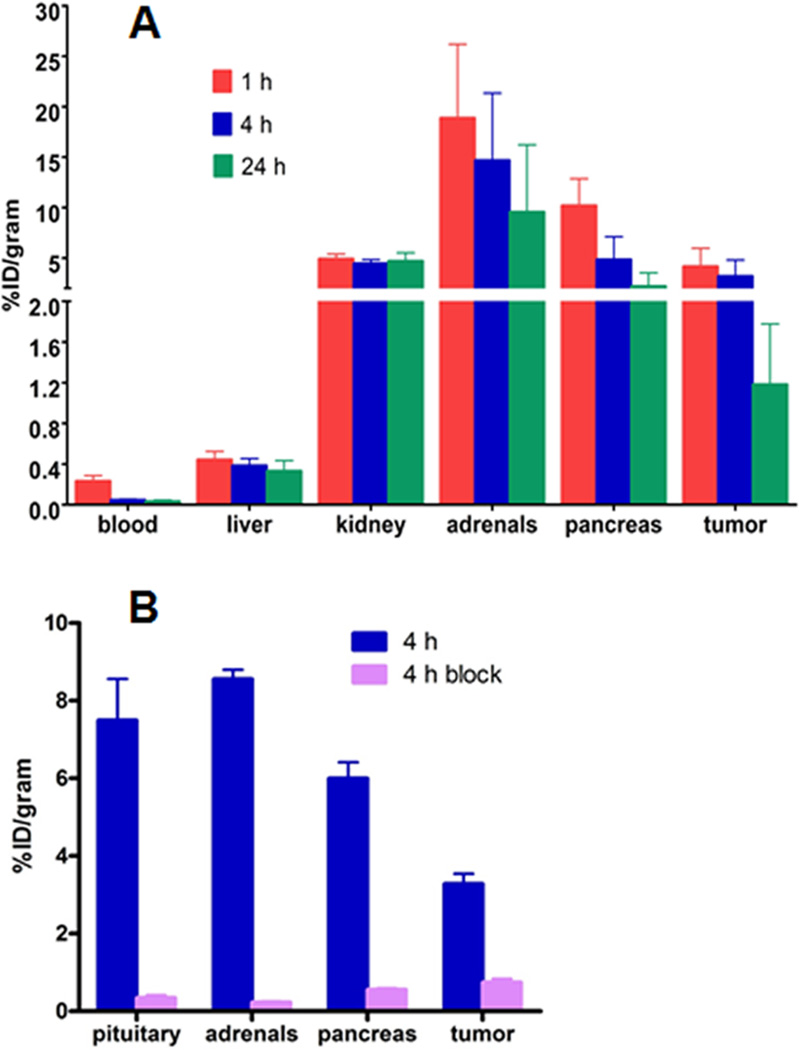
The biodistribution study of 64Cu-CB-TE1A1P-Y3-TATE was conducted in 42-day-old AR42J tumor-bearing male Lewis rats (Figure 3). The data are compared to previously published data with 64Cu-CB-TE2A-Y3-TATE.12 Both 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CBTE2A-Y3-TATE demonstrated rapid blood clearance at 1 h (0.2 ± 0.05 vs 0.2 ± 0.01%ID/g for 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE, respectively). By 24 h, 64Cu-CB-TE1A1P-Y3-TATE cleared from the blood to 14.6% of the 1 h 64Cu activity, compared to 26.3% for 64Cu-CB-TE2A-Y3-TATE. At 1 h, liver uptake of 64Cu-CB-TE2A-Y3-TATE was significantly higher than of 64Cu-CB-TE1A1P-Y3-TATE (p < 0.03). However, there was higher uptake of 64Cu-CB-TE1A1P-Y3-TATE in the kidneys at every time point compared to 64Cu-CBTE2A-Y3-TATE (p < 0.0001). From 1 to 24 h, 75% of the 64Cu-B-TE1A1P-Y3-TATE activity was cleared from liver, whereas less than 10% was excreted from the kidneys. The 64Cu-CB-TE2A-Y3-TATE activity in the liver and kidneys fell by 36% and 50% at 24 h, respectively.
Figure 3.
Biodistribution of 64Cu-CB-TE1A1P-Y3-TATE was conducted in AR42J tumor-bearing male lewis rats (A). A separate blocking study (B) was performed to confirm the specificity of 64Cu-CB-TE1A1P-Y3-TATE for SSTr2. Data are presented as percent injected dose per gram (n = 3−5 for each data point; bars ± SE).
Lower accumulation of 64Cu activity in other nontarget organs was observed for the 64Cu labeled CB-TE1A1P conjugate as compared to CB-TE2A. Bone uptake at every time point was significantly higher for 64Cu-CB-TE2A-Y3-TATE than for 64Cu-CB-TE1A1P-Y3-TATE (p < 0.03). In addition, there was about 3-fold greater activity in the lung for 64Cu-CB-TE2A-Y3-TATE than for 64Cu-CB-TE1A1P-Y3-TATE at 1 h after injection (p = 0.001), increasing to 5.6 times greater at 4 h (p < 0.0001). Activity levels in the lung were reduced by 76% and 85% of the 1 h activity for 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CB-TE2AY3- TATE at 24 h, respectively, resulting in comparable uptake for the two radiopharmaceuticals.
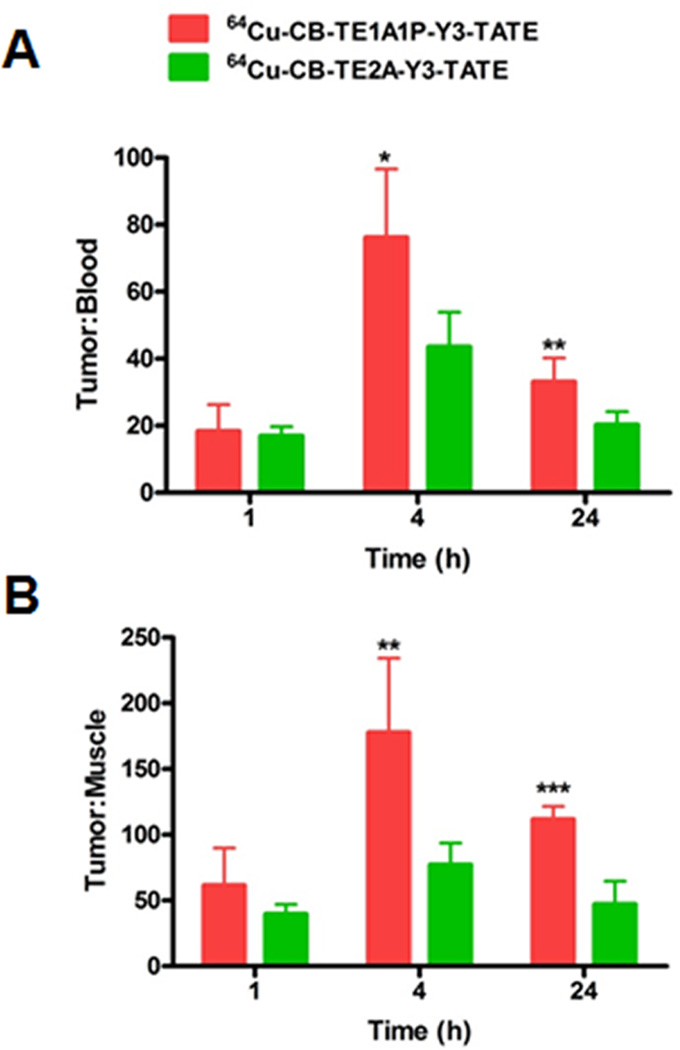
The amount of activity associated with SSTr2 positive tumor and pancreas was also quantified. Initially at 1 h, 64Cu uptake in pancreas was high for both 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE (CB-TE1A1P = 10.2 ± 2.7% ID/g, CB-TE2A = 5.7 ± 0.4 ID/g); however, clearance appeared to be quite efficient as 79% and 85% of the 1 h activity had been cleared by 24 h, respectively. In addition, more 64Cu accumulation in the pancreas was observed for 64Cu-CB-TE1A1P-Y3-TATE than for 64Cu-CB-TE2A-Y3-TATE at both 1 and 4 h after administration of radiopharmaceuticals (p ≤ 0.01). Both 64Cu-labeled conjugates were retained in the tumor for at least 4 h, as no significant change of tumor uptake was observed from 1 to 4 h. The two radiopharmaceuticals cleared from the tumor at a similar rate over time (72% of 64Cu-CB-TE1A1P-Y3-TATE and 69% of 64Cu-CB-TE2A-Y3-TATE from 1 to 24 h). Tumor to blood ratios for 64Cu-CB-TE1A1P-Y3-TATE were 76 ± 20 and 33 ± 7 at 4 and 24 h, respectively, and 44 ± 10 and 20 ± 4, respectively, for 64Cu-CB-TE2A-Y3-TATE (p ≤ 0.01) (Figure 4). Similarly, the tumor to muscle ratio for 64Cu-CB-TE1A1P-Y3-TATE was significantly higher than for 64Cu-CB-TE2A-Y3- TATE at both 4 h (CB-TE1A1P = 178 ± 56, CB-TE2A = 77 ± 16; p < 0.01) and 24 h (CB-TE1A1P = 112 ± 10, CB-TE2A = 47 ± 18, p < 0.0001).
Figure 4.
Comparison of tumor/blood (A) and tumor/muscle (B) ratios of 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CBTE2A-Y3-TATE11 at 1, 4, and 24 h postinjection in AR42J pancreatic tumor-bearing male lewis rats (*p < 0.05, **p < 0.01, ***p < 0.001).
A separate blocking study was performed at 4 h after injection for 64Cu-CB-TE1A1P-Y3-TATE by coinjecting cold peptide Y3-TATE (Figure 3A). Injection of Y3-TATE increased the kidney uptake from 1.9 ± 0.03%ID/g to 2.4 ± 0.2%ID/g. This observation is in agreement with previous studies of 64Cu-labeled somatostatin analogues and may be due to the increase in bioavailability of radioactive compounds when excess cold peptide blocks receptor-mediated uptake. All SSTr2 expressing tissues showed at least 90% reduction in 64Cu uptake when blocked with Y3-TATE (pituitary, 7.5 ± 1.1%ID/g; pituitary blocked, 0.3 ± 0.07%ID/g; adrenals, 8.6 ± 0.2%ID/g; adrenals blocked, 0.2 ± 0.02%ID/g; pancreas 6.0 ± 0.1%ID/g; pancreas blocked, 0.6 ± 0.04%ID/g). Tumor uptake of 64Cu-CB-TE1A1P-Y3-TATE was reduced from 3.3 ± 0.3%ID/g to 0.7 ± 0.09%ID/g (p < 0.0001), demonstrating that this novel radiopharmaceutical specifically targets SSTr2.
Small Animal PET/CT Imaging
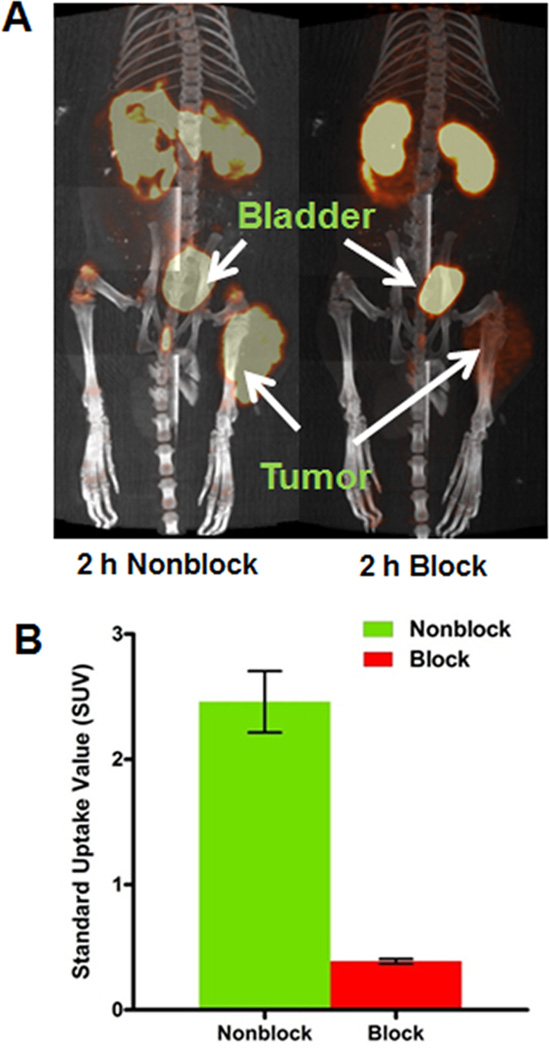
Figure 5 shows the small animal PET/CT projection images of AR42J tumor-bearing male Lewis rats. As early as 2 h after administration of 64Cu-CB-TE1A1P-Y3-TATE, the tumor was clearly visible in PET image with an average SUV of 2.5 ± 0.2. The coinjection of cold Y3-TATE resulted in a significant reduction in the tumor signal, and the average tumor SUV fell to 0.4 ± 0.02. On the basis of SUV analysis, 85% of the tumor uptake was effectively blocked by coinjecting excess cold peptide (p = 0.007). This further confirms the specificity of 64Cu-CB-TE1A1P-Y3-TATE to SSTr2 and reveals a low level of nonspecific binding. Prominent uptake was observed in the bladder and kidneys of all animals, which was primarily due to the fact that renal clearance was the major excretion route for small peptide radiopharmaceuticals. Excess Y3-TATE also blocked uptake in the bone. This finding correlates with the results from previously performed biodistribution experiments (data not shown).
Figure 5.
Small animal PET/CT projection images of AR42J tumor bearing rats at 2 h postinjection of 64Cu-CBTE1A1P-Y3-TATE, with or without 24 h preinjection of excess amount of Y3-TATE (A). PET/CT image at 2 h after administration of 64Cu-CB-TE1A1P-Y3-TATE (B). Standard uptake values (SUV) were calculated by measuring the activity in regions of interest from PET images of AR42J pancreatic tumor bearing Lewis rats (n = 2; bars ± SE; p = 0.007).
DISCUSSION
CB-TE2A has been reported as a promising chelator for 64Cu radiopharmaceuticals owing to its remarkable kinetic inertness.2,6,17 However, the requirement of harsh labeling conditions has precluded its applications in heat-sensitive targeting vectors. Methanephosphonic acid (−CH2−PO(OH)2) groups were introduced into macrocyclic chelators to improve thermodynamic and kinetic stability.7,18,19 Unlike previously reported cross-bridged cyclam chelators, CB-TE1A1P readily forms 64Cu complexes at room temperature under no-carrier-added conditions. This advantage of CB-TE1A1P over CB-TE2A is mostly preserved after conjugation to Y3-TATE. Radiolabeling of CB-TE1A1P-Y3-TATE with 64Cu reached 98% at 40 °C after 60 min without the need of further purification, while more rigorous conditions (95 °C, 1 h) are necessary for labeling CB-TE2A-Y3-TATE. CB-TE1A1P-Y3-TATE is the first cross-bridged chelator–peptide conjugate that can be labeled with 64Cu at 40 °C. We anticipate that expanding the length of the covalent linkage between the CB-TE1A1P and the Y3-TATE peptide will enhance facile radiolabeling, which is particularly important for radiolabeling proteins and monoclonal antibodies.
64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE have similar binding affinities as for SSTr2, but the total binding sites on AR42J membranes is greater for the CB-TE1A1P conjugate than for the CB-TE2A conjugate. Reubi et al. have demonstrated that relatively small changes in a radiolabeled peptide, such as changing the chelator, can markedly change the SSTr binding profile of the somatostatin analogues.20 In fact, the difference in the Bmax value between 64Cu-labeled CB-TE1A1P-and CB-TE2A-Y3-TATE conjugates (3410 ± 220 vs 1090–2100 fmol/mg) is much less dramatic than that between CB-TE2A and TETA (Bmax = 192 fmol/mg).6 The structural change from Cu(II)-TETA to Cu(II)-CB-TE2A is significant, as the two oxygen donors from carboxylate groups adapt axial positions in the pseudo-octahedral geometry of Cu(II)-TETA, but the two oxygens are in the plane of the Cu(II)-CB-TE2A complex.21−23 The X-ray structure of Cu(II)-CB-TE1A1P reveals a full six-coordinate distorted octahedral N4O2 envelopment of the cation with Jahn–Teller elongation along the O(1)–Cu(1)–N(2) axis, which is very similar to the structure of Cu(II)-CB-TE2A.19 Thus, the relatively small yet significant increase in Bmax may be attributed to the minor structural alteration and stabilization of the Cu(II)-CB-TE1A1P chelate in the lipid-rich environment of the receptor.
Assays were performed to determine the extent of radiopharmaceutical internalization of 64Cu-CB-TE1A1P-Y3-TATE in AR42J rat pancreatic carcinoma cells. Rapid internalization was observed for the initial 30 min, followed by a static period from 30 to 120 min. Another active internalizing period occurred after 120 min and was sustained through 240 min. These observations are consistent with a previous report that somatostatin agonists and the SSTr2 receptor are predominantly recycled after receptor-mediated endocytosis rather than being routed to lysosomes for degradation.24 After the initial 30 min of rapid internalization, most receptors that are bound to the radiopharmaceutical have been internalized and few receptors are available on the cell surface to internalize more radioligands. The recycling of SSTr2 receptors from the cell cytosol to the surface takes place between 30 and 120 min after the administration of radiopharmaceutical, which explains the static period and a subsequent round of active endocytosis after 120 min. Internalization of 64Cu-CB-TE1A1P-Y3-TATE was blocked by excess cold Y3-TATE, validating that this is a receptormediated process.
Comparison of in vivo behaviors between 64Cu-CB-TE1A1P-Y3-TATE and 64Cu-CB-TE2A-Y3-TATE12 was made in AR42J tumor-bearing male Lewis rats. To facilitate comparison, the same amounts of radiopharmaceuticals, both mass- and activity-wise, were administrated in both biodistribution studies. The two radiopharmaceuticals demonstrated substantial uptake in SSTr2 positive tissues and AR42J tumors. The uptake was significantly reduced by coinjection of cold Y3-TATE as a blocking agent, demonstrating that the interaction between the radiotracers and the organs is a specific receptor-mediated process in vivo. Comparable tumor uptake values were observed at every time point. In addition, they were cleared from the tumors at similar rates, which is correlated with the fact that 64Cu-CB-TE1A1PY3-TATE has similar binding affinity to 64Cu-CB-TE2A-Y3-TATE for SSTr2 in AR42J tumor membranes.
There was greater accumulation of 64Cu-CB-TE2A-Y3-TATE in the liver than 64Cu-CB-TE1A1P-Y3-TATE at 1 h postinjection, which may be attributed to charge differences of the two conjugates. Cu(II)-CB-TE2A-Y3-TATE has a net charge of +2 on the entire conjugate, while Cu(II)-CB-TE1A1P-Y3-TATE is +1 due to the one extra negative charge of the methanephosphonate arm. Jones-Wilson et al. reported that positively charged cyclam and Et-cyclam had higher accumulation in the liver of normal rats.25 On the basis of several studies that had been conducted to investigate the effect of charge on kidney uptake of radiolabeled peptides,3,25,26 renal retention of radioactivity was typically greatest for positively charged peptide conjugates and lowest for negatively charged ones. We observed increased renal retention for 64Cu-CB-TE1A1P-Y3-TATE compared to 64Cu-CB-TE2A-Y3-TATE, suggesting that more factors than charge should be considered when one is predicting the in vivo performance of a radiopharmaceutical. Sun et al. examined the in vivo behaviors of a series of 64Cu-labeled methanephosphonate tetraaza macrocyclic ligands (DO2P, DO3P, and DOTP).8 Their results suggested that the renal retention of the Cu(II) complexes increased as the number of methanephosphonate groups increased, regardless of the extra negative charges from phosphonic acid moieties.
The results from animal studies stand in contrast to those from in vitro stability tests. An acid inertness study of Cu(II)-CBTE1A1P in 5 M HCl at 90 °C gave a half-life of 6.8 h,9 which indicates significantly less stability under these conditions than Cu(II)-CB-TE2A (half-life = 154 h).22 This discrepancy can be explained by the possibility that conjugation of CB-TE1A1P to Y3-TATE improves the stability of Cu-CB-TE1A1P complex, or that the acid stability studies are not highly accurate for predicting in vivo behavior.
Recently, Fani et al. reported the evaluation of two novel 64Cu-labeled somatostatin antagonists, 64Cu-CB-TE2A-LM3 and 64Cu-NODAGA-LM3,27 and demonstrated a strong dependence of the affinity and pharmacokinetics of the radiolabeled somatostatin antagonists on the chelators. Similar to our observations with CB-TE2A and CB-TE1A1P, they observed a significant improvement of tumor-to-normal-tissue ratios with a NOTA-based chelator, NODAGA, compared to CB-TE2A, which was attributed to the neutral charge and higher hydrophilicity of 64Cu-NODAGA-LM3. In addition, they indicated that the antagonist LM3 was more sensitive to Nterminal modification than somatostatin agonists. At 4 and 24 h postinjection in human embryonic kidney (HEK)-sst2 tumor bearing nude mice, the liver uptake of 64Cu-CB-TE2A-LM3 was 3–4-fold higher than 64Cu-NODAGA-LM3, and the kidney uptake of 64Cu-CB-TE2A-LM3 was over 5-fold higher than 64Cu-NODAGA-LM3. Using the agonist Y3-TATE as our targeting peptide for SSTr2 in AR42J tumor-bearing rats, we also observed decreased liver uptake of 64Cu-CB-TE1A1P-Y3-TATE compared to 64Cu-CB-TE2A-Y3-TATE at 1 h postinjection, although this was significantly less than Fani et al.’s observations with LM3. Moreover, we observed unexpectedly higher kidney uptake of 64Cu-CB-TE1A1P-Y3-TATE than 64Cu-CB-TE2A-Y3-TATE at all time points postinjection. The advantages of CB-TE1A1P to CB-TE2A may be amplified by conjugation of CB-TE1A1P to somatostatin antagonists. Interestingly, CB-TE2A performed better than NODAGA when compared as RGD conjugates,28 confirming that the target tissue and nontarget organ uptake is dependent on both the chelator and the targeting peptide.
CONCLUSIONS
In summary, a novel PET radiopharmaceutical, 64Cu-CB-TE1A1P-Y3-TATE, has been successfully synthesized and evaluated in vitro and for imaging SSTr2 positive tumors in an AR42J tumor-bearing rat model. The data suggest that CBTE1A1P is a promising bifunctional chelator for 64Cu, owing to the improved tumor-to-blood and tumor-to-muscle ratios, as well as excellent tumor detection by PET. Integration of a methanephosphonate group to the cross-bridged macrocyclic chelator results in much milder labeling conditions and higher specific activity. These results demonstrate the promise of CBTE1A1P as a chelator for stably complexing 64Cu for a wide variety of biological applications, including proteins such as monoclonal antibodies. Additionally, the 64Cu-labeled CB-TE1A1P-Y3-TATE conjugate shows improved biodistribution compared to 64Cu-CB-TE2A-Y3-TATE.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Christopher Sherman for performing the biodistribution studies and Dexing Zeng for assistance with compound characterization. This research was supported by NIH/NCI 5 R01 CA 093375 (CJA) and DOE DEFG02-08ER64671 (Integrated Research Training Program of Excellence in Radiochemistry awarded to Michael J. Welch supporting YG).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Eiblmaier M, Andrews R, Laforest R, Rogers BE, Anderson CJ. Nuclear uptake and dosimetry of 64Cu-labeled chelator somatostatin conjugates in an SSTr2-transfected human tumor cell line. J. Nucl. Med. 2007;48:1390–1396. doi: 10.2967/jnumed.107.039990. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem. 2002;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 3.Bass LA, Wang M, Welch MJ, Anderson CJ. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjugate Chem. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 4.Boswell CA, McQuade P, Weisman GR, Wong EH, Anderson CJ. Optimization of labeling and metabolite analysis of copper-64-labeled azamacrocyclic chelators by radio-LC-MS. Nucl. Med. Biol. 2005;32:29–38. doi: 10.1016/j.nucmedbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Rogers BE, Anderson CJ, Connett JM, Guo LW, Edwards WB, Sherman EL, Zinn KR, Welch MJ. Comparison of four bifunctional chelates for radiolabeling monoclonal antibodies with copper radioisotopes: biodistribution and metabolism. Bioconjugate Chem. 1996;7:511–522. doi: 10.1021/bc9600372. [DOI] [PubMed] [Google Scholar]
- 6.Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, Anderson CJ. Preparation and biological evaluation of copper-64-labeled tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin. Cancer Res. 2004;10:8674–8682. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 7.Stigers DJ, Ferdani R, Weisman GR, Wong EH, Anderson CJ, Golen JA, Moore C, Rheingold AL. A new phosphonate pendant-armed cross-bridged tetraamine chelator accelerates coppe (ii) binding for radiopharmaceutical applications. Dalton Trans. 2010;39:1699–1701. doi: 10.1039/b920871b. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Wuest M, Kovacs Z, Sherry AD, Motekaitis R, Wang Z, Martell AE, Welch MJ, Anderson CJ. In vivo behavior of copper-64-labeled methanephosphonate tetraaza macrocyclic ligands. J. Biol. Inorg. Chem. 2003;8:217–225. doi: 10.1007/s00775-002-0408-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferdani R, Stigers DJ, Fiamengo AL, Wei L, Li BT, Golen JA, Rheingold AL, Weisman GR, Wong EH, Anderson CJ. Synthesis, Cu(ii) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms. Dalton Trans. 2012;41:1938–1950. doi: 10.1039/c1dt11743b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 11.Wadas TJ, Anderson CJ. Radiolabeling of TETA- and CB-TE2A-conjugated peptides with copper-64. Nat. Protoc. 2006;1:3062–3068. doi: 10.1038/nprot.2006.431. [DOI] [PubMed] [Google Scholar]
- 12.Wadas TJ, Eiblmaier M, Zheleznyak A, Sherman CD, Ferdani R, Liang K, Achilefu S, Anderson CJ. Preparation and biological evaluation of 64Cu-CB-TE2A-sst2-ANT, a somatostatin antagonist for PET imaging of somatostatin receptor-positive tumors. J. Nucl. Med. 2008;49:1819–1827. doi: 10.2967/jnumed.108.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Caruano AL, Lewis MR, Meyer LA, Vander Waal RP, Anderson CJ. Subcellular localization of radiolabeled somatostatin analogues: implications for targeted radiotherapy of cancer. Cancer Res. 2003;63:6864–6869. [PubMed] [Google Scholar]
- 14.Lewis JS, Srinivasan A, Schmidt MA, Anderson CJ. In vitro and in vivo evaluation of 64Cu-TETA-Tyr3-octreotate. A new somatostatin analog with improved target tissue uptake. Nucl. Med. Biol. 1999;26:267–273. doi: 10.1016/s0969-8051(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 15.Mucha A, Grembecka J, Cierpicki T, Kafarski P. Hydrolysis of the phosphonamidate bond in phosphono dipeptide analogues - The influence of the nature of the N-terminal functional group. Eur. J. Org. Chem. 2003:4797–4803. [Google Scholar]
- 16.Mucha A, Kunert A, Grembecka J, Pawelczak M, Kafarski P. A phosphonamidate containing aromatic N-terminal amino group as inhibitor of leucine aminopeptidase - design, synthesis and stability. Eur. J. Med. Chem. 2006;41:768–772. doi: 10.1016/j.ejmech.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraaza-macrocyclic complexes. J. Med. Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 18.Lukes I, Kotek J, Vojtisek P, Hermann P. Complexes of tetraazacycles bearing methylphosphinic/phosphonic acid pendant arms with coppe(II), zinc(II) and lanthanides(III). A comparison with their acetic acid analogues. Coordin. Chem. Rev. 2001;216:287–312. [Google Scholar]
- 19.Svobodova I, Lubal P, Plutnar J, Havlickova J, Kotek J, Hermann P, Lukes I. Thermodynamic, kinetic and solid-state study of divalent metal complexes of 1,4,8,11-tetraazacyclote-tradecane (cyclam) bearing two trans (1,8-)methylphosphonic acid pendant arms. Dalton Trans. 2006;43:5184–5197. doi: 10.1039/b603251f. [DOI] [PubMed] [Google Scholar]
- 20.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Macke HR. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CJ, Jones LA, Bass LA, Sherman EL, McCarthy DW, Cutler PD, Lanahan MV, Cristel ME, Lewis JS, Schwarz SW. Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor-bearing rats. J. Nucl. Med. 1998;39:1944–1951. [PubMed] [Google Scholar]
- 22.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong EH, Weisman GR, Hill DC, Reed DP, Rogers ME, Condon JS, Fagan MA, Calabrese JC, Lam KC, Guzei IA, Rheingold AL. Synthesis and characterization of cross-bridged cyclams and pendant-armed derivatives and structural studies of their coppe(II) complexes. J. Am. Chem. Soc. 2000;122:10561–10572. [Google Scholar]
- 24.Koenig JA, Kaur R, Dodgeon I, Edwardson JM, Humphrey PP. Fates of endocytosed somatostatin sst2 receptors and associated agonists. Biochem. J. 1998;336(Pt 2):291–298. doi: 10.1042/bj3360291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones-Wilson TM, Deal KA, Anderson CJ, McCarthy DW, Kovacs Z, Motekaitis RJ, Sherry AD, Martell AE, Welch MJ. The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nucl. Med. Biol. 1999;25:523–530. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 26.Akizawa H, Arano Y, Mifune M, Iwado A, Saito Y, Mukai T, Uehara T, Ono M, Fujioka Y, Ogawa K, Kiso Y, Saji H. Effect of molecular charges on renal uptake of 111In-DTPA-conjugated peptides. Nucl. Med. Biol. 2001;28:761–768. doi: 10.1016/s0969-8051(01)00241-4. [DOI] [PubMed] [Google Scholar]
- 27.Fani M, Del Pozzo L, Abiraj K, Mansi R, Tamma ML, Cescato R, Waser B, Weber WA, Reubi JC, Maecke HR. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J. Nucl. Med. 2011;52:1110–1118. doi: 10.2967/jnumed.111.087999. [DOI] [PubMed] [Google Scholar]
- 28.Dumont RA, Deininger F, Haubner R, Maecke HR, Weber WA, Fani M. Novel 64Cu- and 68Ga-labeled RGD conjugates show improved PET imaging of alphaνbeta3 integrin expression and facile radiosynthesis. J. Nucl. Med. 2011;52:1276–1284. doi: 10.2967/jnumed.111.087700. [DOI] [PubMed] [Google Scholar]