Abstract
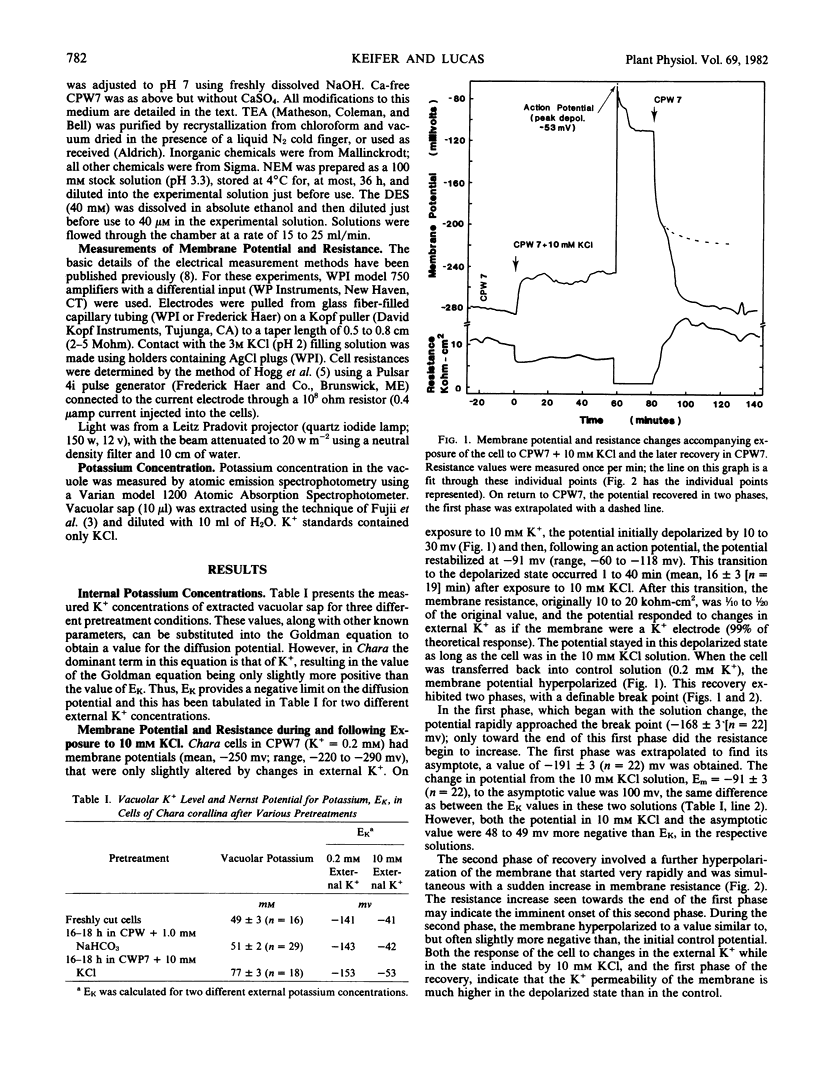
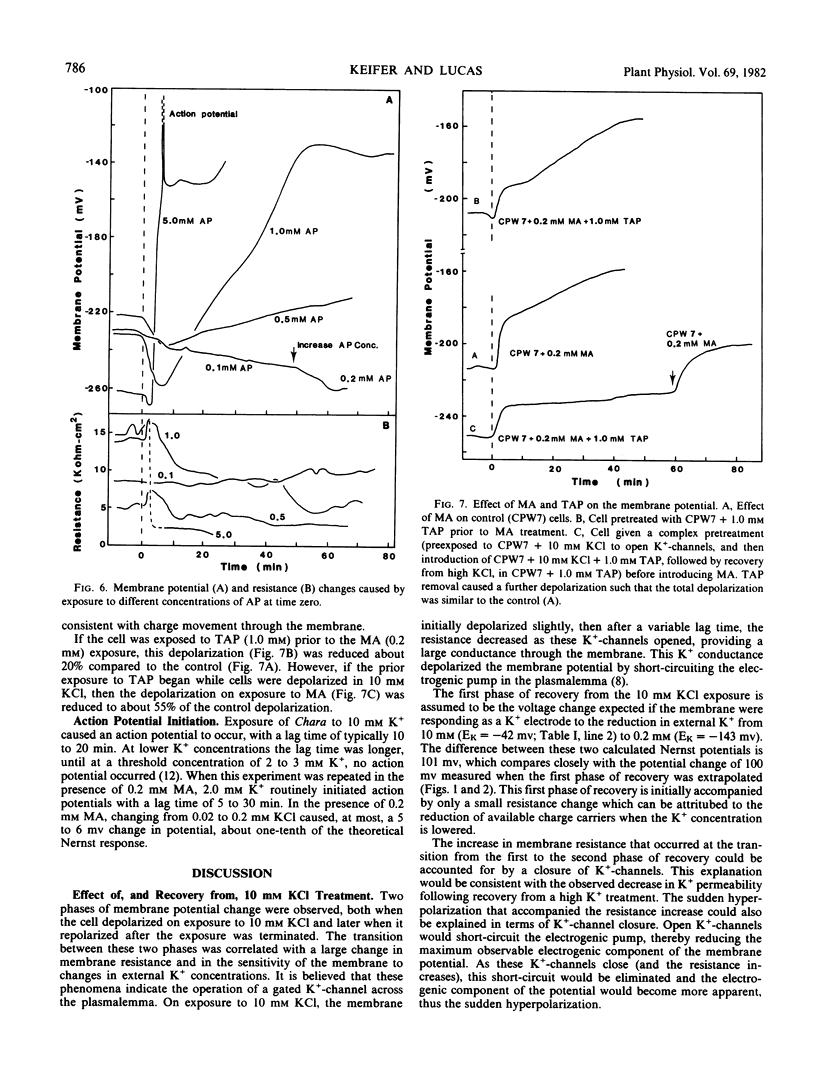
Plasmalemma electrical properties were used to investigate K+ transport and its control in internodal cells of Chara corallina Klein ex Willd., em R.D.W. Cell exposure to solutions containing 10 mm KCl caused the potential, normally −250 millivolts (average), to depolarize in two steps. The first step was a 21 millivolt depolarization that lasted from 1 to 40 minutes. The second step started with an action potential and left the membrane potential at −91 millivolts, with a 10-fold reduction in resistance. We suggest that the second step was caused by the opening of K+ -channels in the membrane. This lowered the resistance and provided a current pathway that partially short-circuited the electrogenic pump. Although largely short-circuited, the electrogenic pump was still operating as indicated by: (a) the depolarized potential of −91 millivolts was more negative than Ek (=−42 millivolts in 10 mm K+); (b) a large net K+ uptake occurred while the cell was depolarized; (c) both the electrogenic pump inhibitor, diethylstilbestrol, and the sulfhydryl-reagent N-ethylmaleimide (which increased the passive membrane permeability) further depolarized the potential in 10 mm KCl.
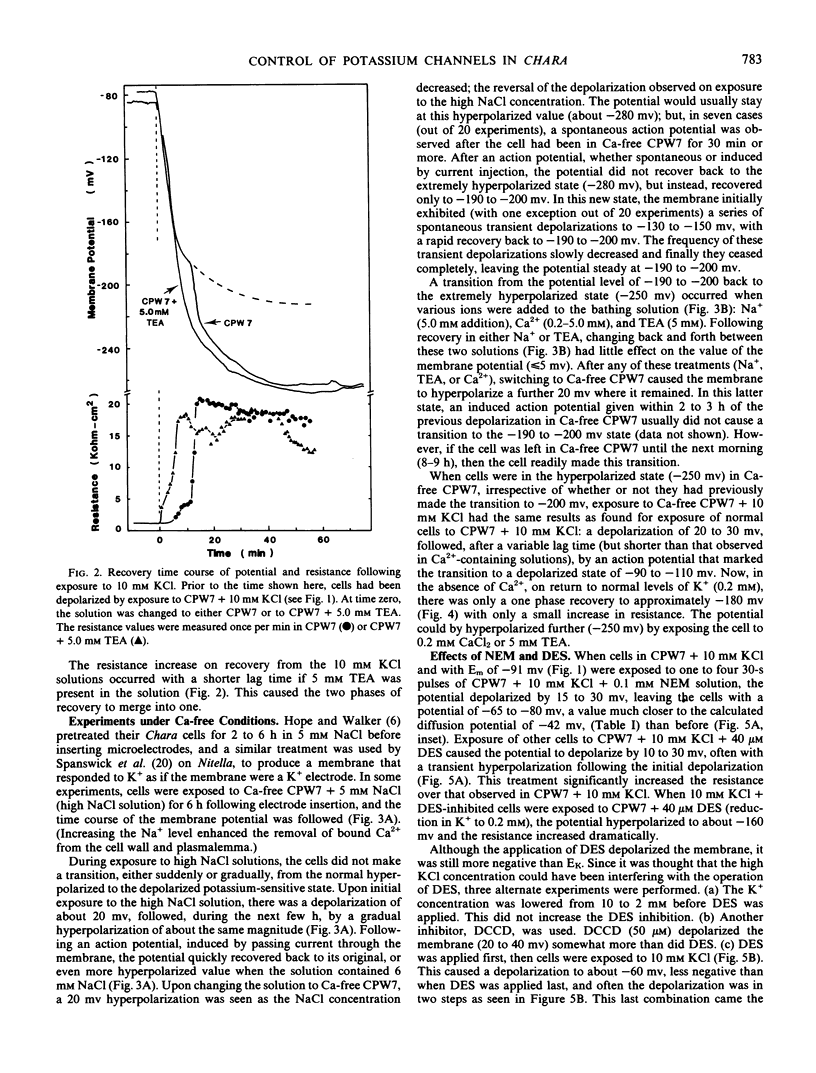
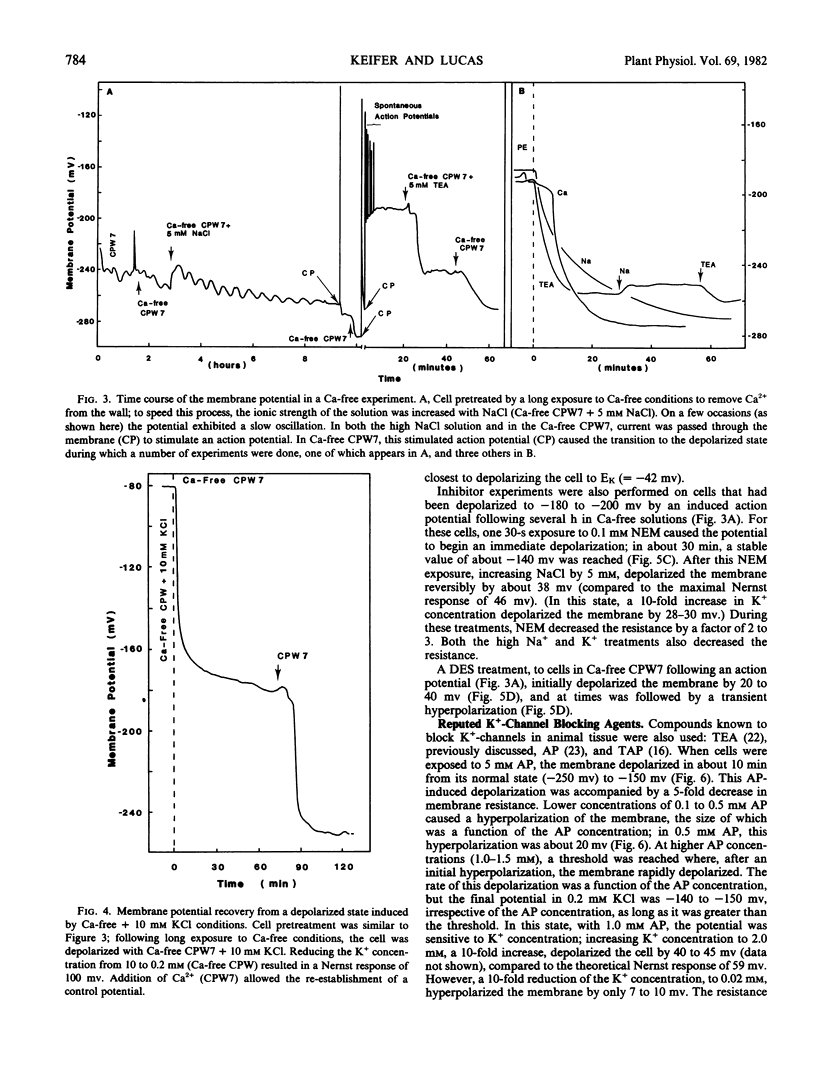
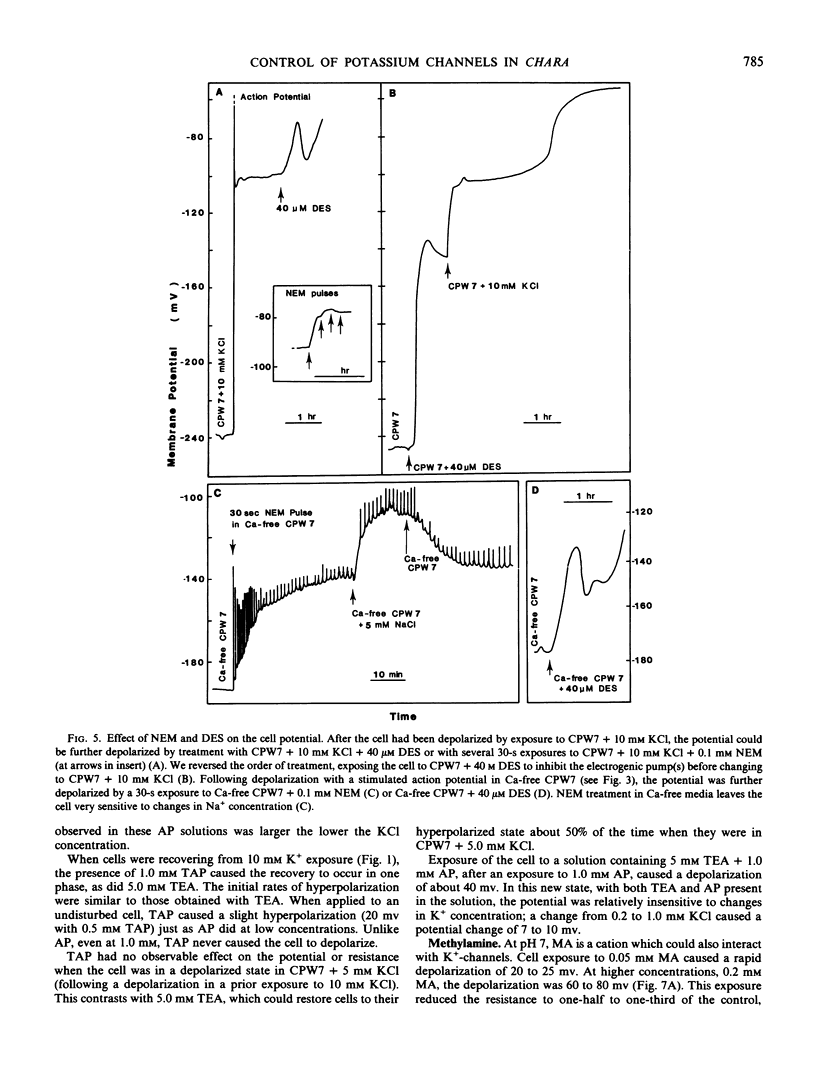
A two-phase recovery back to normal cell potentials occurred upon lowering the K+ concentration from 10 to 0.2 mm. The first phase was an apparent Nernst potential response to the change in external K+ concentration. The second phase was a sudden hyperpolarization accompanied by a large increase in membrane resistance. We attribute the second phase to the closing of K+ -channels and the removal of the associated short-circuiting effect on the electrogenic pump, thereby allowing the membrane to hyperpolarize. Further experiments indicated that the K+ -channel required Ca2+ for normal closure, but other ions could substitute, including: Na+, tetraethylammonium, and 2,4,6-triaminopyrimidine. Apparently, K+ -channel conductance is determined by competition between Ca2+ and K+ for a control (gating?) binding site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Findlay G. P., Hope A. B., Pitman M. G., Smith F. A., Walker N. A. Ionic fluxes in cells of Chara corallina. Biochim Biophys Acta. 1969;183(3):565–576. doi: 10.1016/0005-2736(69)90170-9. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg J., Williams E. J., Johnston R. J. A simplified method for measuring membrane resistances in Nitella translucens. Biochim Biophys Acta. 1968 Apr 29;150(3):518–520. doi: 10.1016/0005-2736(68)90152-1. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Activity of the Electrogenic Pump in Chara corallina as Inferred from Measurements of the Membrane Potential, Conductance, and Potassium Permeability. Plant Physiol. 1978 Oct;62(4):653–661. doi: 10.1104/pp.62.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Lucas W. J., Spanswick R. M. Effect of Sulfhydryl Reagents on the Biophysical Properties of the Plasmalemma of Chara corallina. Plant Physiol. 1981 Oct;68(4):899–904. doi: 10.1104/pp.68.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. HCO(3) Influx Across the Plasmalemma of Chara corallina: Divalent Cation Requirement. Plant Physiol. 1977 Dec;60(6):862–867. doi: 10.1104/pp.60.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J. HCO(3) Influx across the Plasmalemma of Chara corallina: Physiological and Biophysical Influence of 10 mm K. Plant Physiol. 1978 Apr;61(4):487–493. doi: 10.1104/pp.61.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Grady T. P. Triaminopyrimidinium (TAP+) blocks luminal membrane K conductance in Necturus gallbladder epithelium. J Membr Biol. 1979 Jul 31;48(3):285–298. doi: 10.1007/BF01872896. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBIAS J. M. A CHEMICALLY SPECIFIED MOLECULAR MECHANISM UNDERLYING EXCITATION IN NERVE: A HYPOTHESIS. Nature. 1964 Jul 4;203:13–17. doi: 10.1038/203013a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]



