Abstract
Aim
To examine the effects of a nurse-led case management programme for hospital-discharged older adults with co-morbidities.
Background
The most significant chronic conditions today involve diseases of the cardiovascular, respiratory, endocrine and renal systems. Previous studies have suggested that a nurse-led case management approach using either telephone follow-ups or home visits was able to improve clinical and patient outcomes for patients having a single, chronic disease, while the effects for older patients having at least two long-term conditions are unknown. A self-help programme using motivation and empowerment approaches is the framework of care in the study.
Design
Randomized controlled trial.
Method
The study was conducted from 2010–2012. Older patients having at least two chronic diseases were included for analysis. The participants were randomized into three arms: two study groups and one control group. Data were collected at baseline and at 4 and 12 weeks later.
Results
Two hundred and eighty-one patients completed the study. The interventions demonstrated significant differences in hospital readmission rates within 84 days post discharge. The two intervention groups had lower readmission rates than the control group. Patients in the two study arms had significantly better self-rated health and self-efficacy. There was significant difference between the groups in the physical composite score, but no significant difference in mental component score in SF-36 scale.
Conclusion
The postdischarge interventions led by the nurse case managers on self-management of disease using the empowerment approach were able to provide effective clinical and patient outcomes for older patients having co-morbidities.
Keywords: co-morbidities, hospital readmission, nurse-led case management, older adults, randomised control trial, self-efficacy
Why is this research or review needed?
Older patients suffering from multiple chronic diseases are more frequent users for medical and nursing care.
Self-management of long-term illness is particularly relevant for older people with multiple illnesses and overlapping symptoms in long-term conditions.
Most previous studies included patients with only one selected chronic diseases and there is a lack of data concerning discharged care for older patients with co-morbidities.
What are the key findings?
An integrative, multi-component intervention comprising pre- and postdischarge elements, intensive support and management during the high-risk period and ongoing monitoring of patients through self-care and education is able to reduce unplanned hospital readmission.
Older patients of age >65, having at least two medical diagnoses related to chronic respiratory disease, cardiovascular disease, type 2 diabetes and renal disease, benefitted from complex interventions delivered by the nurse-led case management programme.
The interventions provided by the nurse case manager, supported by nursing students, were able to demonstrate a statistically significant effect on the outcomes.
How should the findings be used to influence policy/practice/research/education?
Patient-centred care with mutual goal setting and empowerment strategies were able to bring about changes, with an increasing sense of strength and control for patients with co-morbidities.
The activities led by an Advanced Practice Nurse, rather than a general nurse, conducting individualized education with a cognitive behavioural approach, were able to provide positive clinical and patient outcomes.
Introduction
During the past two decades, there have been improvements in living conditions and healthcare delivery; people tend to live longer, which has led to an increase in the prevalence of chronic diseases (Garcia-Olmos et al. 2012). Ageing populations have increased the prevalence of multiple morbidities and the likelihood of older people having two or more chronic diseases is increasing (Beverly et al. 2011, Bardach & Schoenberg 2012). The definition of co-morbidities is the co-existence of two or more diseases or disorders. This may refer to acute-acute co-morbidity, acute-chronic co-morbidity and chronic-chronic co-morbidity (Wun et al. 1998). According to a global study, cardiovascular disease, cancer, chronic lung diseases and diabetes mellitus are the largest cause of death in the world (Yach et al. 2004). In the USA, approximately 62% of citizens above 65 years old have multiple chronic conditions such as cardiovascular diseases, chronic respiratory diseases and type 2 diabetes (Vogeli et al. 2007). The Hong Kong Census and Statistics Department (2009) revealed that approximately 800,000 older adults in Hong Kong had one or more chronic diseases and around 10% suffer from four or more chronic diseases. The management of chronic disease in the older population is therefore a vital area of research; the results will lead to improvements in healthcare management and the development of new, effective healthcare policies.
Background
Dealing with co-morbidities has become an enormous challenge for both the healthcare system and the patients in it. Older people, in particular those having chronic co-morbidities, experience substantial impacts and frustrations, which impair health-related quality of life (Ose et al. 2011). Senior citizens with low or inadequate health literacy could have reduced self-care abilities, leading to an excessive personal and public burden on our healthcare system (Cutilli 2007). The culture of the healthcare professional groups has contributed to the fragmentation of healthcare services for older patients. This has resulted in poor continuity of care and the inevitability of chronic conditions persisting after discharge (Glendinning 2003, Williams 2004). Recognizing the association between continuity of care and co-morbidities, it is necessary to determine how interventions during and after hospitalization affect older adults with multiple chronic diseases (Norris et al. 2008).
In 2002, the World Health Organization proposed a policy framework on active ageing to minimize chronic diseases and functional decline to better enable older people to continue their contribution to society (Yamamoto 2005). Self-management of long-term illness is particularly relevant for older people with multiple illnesses and overlapping symptoms in long-term conditions (Gallagher et al. 2008). Empowerment is defined as giving everyone the opportunity to achieve their full potential. The focus of patient empowerment initiatives is the process to encourage patient self-management of chronic conditions (McAllister et al. 2012). A systematic review by Chen and Li (2009) showed that empowerment interventions were able to produce significant improvements in HbA1c for people with diabetes and to increase patients' knowledge of their diseases and their ability to manage them. However, a review of published work on patient empowerment revealed a research gap in the area of older people suffering co-morbid, chronic diseases.
A structured review conducted by Sutherland and Hayter (2009) concluded that the use of nurse-led case management was able to impact on treatment adherence, self-care, patient satisfaction, service use and quality of life in three major chronic disease areas: diabetes, cardiovascular diseases and chronic pulmonary disease. The studies included patients with only one of the selected chronic diseases. A systematic review by Radhakrishnan (2011) showed that using multiple, tailored interventions for self-management was modestly successful in individuals with heart disease, hypertension or type 2 diabetes. Another contemporary review (Lupari et al. 2011) on nurse-led, home-based care case management services targeting people more than 65 years old with multi-morbid conditions focused its outcome measures primarily on health service utilization and satisfaction of patients, carers and physicians. There has been no study using validated tools to determine the impact of the intervention on quality of life or functionality of the patients. The studies conducted by Chow and Wong (2010) and Wong et al. (2010), using nurse case managers (NCMs) to conduct telephone follow-ups, demonstrated the effectiveness on quality of life and reducing postdischarge problems for patients undergoing peritoneal dialysis. Previous local and overseas studies were inconsistent in determining whether the different interventions, including combined home visits and telephone assistance, or only one of the two help methods (home visits or telephone calls) alone gave consistent effects across studies or with different patient groups. The different models of care for co-morbidities have not been compared and studied widely. This research gap needs further investigation before healthcare institutions adopt the new nursing intervention as a widespread model.
The study
Aim
The aim of this study was to examine the effects of a nurse-led case management programme for hospital-discharged, older adults with co-morbidities in Hong Kong. The primary outcome was whether the programme was able to reduce hospital readmission.
Other objectives explored:
whether the study groups experienced better quality of life than the control group,
whether the study groups demonstrated better self-efficacy in disease management than the control group,
whether the study groups experienced better self-rated health than the control group.
Design
This study was conducted from August 2010–June 2012 for a total of 23 months. A large, randomized controlled trial addressing different patient groups has been reported in the study by Wong et al. (2013). This study is part of a larger study examining the effects of a programme for patients with co-morbidities who required complex interventions to bring about changes in their health. The term, ‘co-morbidities’ refers to patients having primary and secondary diagnoses of specified chronic diseases as stated in the medical record on admission. Patients were recruited from the medical department of a 1700-bedded acute, general regional hospital in Hong Kong.
Sample size calculation
No similar study has been conducted using three arms comparisons on older patients having chronic diseases. The study by Naylor et al. (2004) on transitional care of older, hospitalized adults with heart failure was used as the primary outcome in computing the sample size. The proportions of rehospitalization due to co-morbidities for study group and control group were 0·19 and 0·41 respectively. To adjust for three groups comparisons, with alpha of 0·05/3 = 0·0167 and power = 80%, a sample size of 74 participants for each group was required.
Patient recruitment and randomization
Eligible patients were recruited during their hospital admission in the medical ward. The inclusion criteria were as follows: older patients of age >65, admitted with a medical diagnosis related to chronic respiratory, cardiac, type 2 diabetes and renal diseases; able to speak Cantonese and to communicate; resident in the hospital service area and able to be contacted by telephone after discharge. The exclusion criteria were as follows: those identified as having cognitive problems, Mini Mental State Examination <20; discharged for institutional care; being followed by a designated disease management programme after discharge; unable to communicate and patients who were terminally ill. The above chronic conditions were chosen as they are most prevalent in the study population and records show a high number of inpatient discharges from public hospitals in the past 6 years (Hospital Authority, Hong Kong n.d.). Also, these patients with long-term chronic conditions have postdischarge care needs as suggested by previous studies (Wong et al. 2002, Mattke et al. 2007).
Randomization – sequence generation
Patients who had agreed to take part were randomized into three arms by the researcher: two study groups and one control group, using randomly computer-generated numbers; the ratio was 1:1:1 ensuring a balanced allocation of groups.
Randomization – allocation concealment mechanism
All consenting patients were required to complete all the baseline assessments. The allocation sequence was concealed until baseline data collection was completed.
Randomization – implementation
Another researcher accessed the computer-generated assignment of each patient. When the group assignments were confirmed, she informed the case managers of the assignments to implement interventions to the patients in the two study arms.
Blinding (masking)
The participants and data collectors were blinded to the group assignments. The study group had two arms: one group received home visits and telephone calls and the other received telephone calls only. The control group received standard, inpatient care and placebo calls. All groups received standard, inpatient care, which included inpatient nursing care, basic health advice, information on medication and adherence, and arrangements for outpatient follow-up. In this study, we report patients having two or more chronic diseases.
The nurse case management intervention
The nurse case management in this study adopted an integrated, multi-component intervention comprising pre- and postdischarge elements. The self-help programme using motivation and empowerment approaches is the framework of care in the study.
Pre-discharge assessment
Intervention began with the NCM carrying out a pre-discharge assessment using the Omaha System on the two intervention arms patients. The Omaha System is comprehensive and comprises the Problem Classification Scheme, Intervention Scheme and Problem Rating Scale for Outcomes (Martin 2005). The system originates from the USA and has been validated and tested by the authors for reliability in various patient groups in the local Hong Kong community (Wong et al. 2008). The NCM used the system to assess and implement the interventions based on the constructs for the home and call groups. The patients in the two intervention arms received the interventions weekly for 4 weeks with different delivery approaches. No assessments and related interventions were conducted to the control group.
The programme design was based on the theoretical perspective of self-management of chronic disease. Older adults coping with multiple co-morbidities are particularly vulnerable when trying to cope with the complexity of medication and treatment regimens during the transition from hospital to home (Naylor et al. 2004). Patients must be knowledgeable to manage their own health, perform daily activities and apply the skills necessary for maintaining adequate psychosocial functioning (Clark et al. 1991). The unique features of the programme were that patients made decisions and took action to monitor their conditions and that tailor-made interventions are considered the major goals for chronic disease management. During the interventions, patients were asked: ‘What are the issues that you consider affect your ability to manage your medical conditions and health?’ Based on Bandura's self-efficacy theory and behavioural change (Bandura 1977), the NCM analysed the barriers and developed mutual goals with the patients to ensure that they were able to perform health maintenance activities, such as nutrition, monitoring of symptoms and medication adherence. As a consequence of performance accomplishments, improvement in self-efficacy would transfer to other daily activities that may enhance self-management for chronic diseases. The NCM coordinated all the interventions including the home visits and some telephone calls conducted by senior year nursing students.
For the home visit group, the first visit was undertaken by the NCM accompanied by the nursing students within 72 hours of discharge. The NCM made a telephone call in the second week to evaluate the interventions and advice given during the home visit. During the third week, the nursing students conducted a home visit. The purpose of the second home visit was to examine whether patients were able to continue managing their health needs after hospital discharge. To ensure continuity of care for effectiveness of intervention, the nursing students received instructions on each patient's particular issues that needed to be followed up from the NCM before making their home visit. The NCM made the final telephone call in the fourth week post discharge to remind patients about adherence behaviours and to motivate and support them before concluding the interventions.
For the call group, the NCM made the first telephone call based on the patients' needs, which had been identified using the Omaha system prior to discharge. The NCM passed these cases over to the nursing students, who were required to call the patients in the second and third week post discharge. The students referred to the mutual goals developed by the NCM and the patients to continue with the interventions. If the students encountered any problems or difficulties, they were instructed to call the NCM immediately for assistance. Similar to the home visit group, the NCM made the closing call to motivate and support the patients in maintaining self-management behaviours. A recent study conducted by the author showed that a 4-week intervention is considered sufficient to affect the clinical and health outcomes (Wong et al. 2011).
In this study, nursing students were recruited as affiliated members of the service providers. Social capital is important to the efficient functioning of modern society; it promotes social cohesion and co-operation between two or more individuals (Fukuyama 2001). The local and overseas studies conducted by Wong et al. (2011) and Kennedy et al. (2007) using lay persons as volunteers to assist participants in developing self-efficacy and management behaviours to manage their illnesses were successful. During the process, students were not only instilled with the professional values of caring for patients with greater healthcare needs in the community but also the notions of interpersonal trust, mutual aid and bonds were further developed throughout the interventions.
For the control group, the research assistants called the patients twice within 4 weeks. The calls were social calls that involved topics such as the weather, television programmes or leisure activities without self-management content. The duration of the call would be approximately 5 minutes. If there were questions raised by the patients related to their health, the research assistants would advice the patients to seek help from the healthcare professionals with the necessary direct contact numbers.
Preparation of the case managers and nursing students
The two case managers were advanced nursing practitioners, having over 15 years of medical nursing and discharge planning experiences in the study hospital. The case managers went through a 12-hour structured training programme on transitional care, the Omaha system, home visits and telephone nursing, patient empowerment and chronic disease management of older patients.
The senior year nursing students were recruited to assist the NCMs in part of the interventions. They received 6 hours of training on transitional care, communication with older patients, patient education and chronic disease management, which was provided by the researchers. The nursing students were assessed for competency before they participated in patient intervention.
Fidelity in delivery of tailored intervention
There are mechanisms to ensure that tailored interventions are appropriate for patients and that fidelity is ensured. This was especially important for this study, as interventions were delivered by both the NCM and nursing students. A pilot study using 10 cases to test the intervention protocols was first carried out. The research team and the case managers met to discuss the feasibility of the interventions and the problems encountered. During the course of the study, the case managers and the research team met periodically to review and discuss the recruited patients. For process evaluation, the documentation and audio records of the telephone interviews by both the nursing students and the NCMs were reviewed by the clinical partners and the researchers. The regular meetings and review of case records were used to monitor the quality of the case management approach.
Data collection
Data were collected at three time points. Time 1 (T1) was the baseline at the time of discharge, Time 2 (T2) was at 4 weeks after discharge when the interventions were completed and Time 3 (T3) was at 12 weeks to examine the sustained effects of interventions.
Questionnaires
Different questionnaires were used to evaluate the outcome measures. The patients' demographic and clinical data were collected at T1. Unplanned hospital readmission rate was the primary dependent variable of this study. The data for 28- and 84-day readmission were retrieved from the hospital's administrative record system. Other outcomes included self-efficacy, self-rated health and quality of life. For self-efficacy, the six-item Chinese version of the Short-form Chronic Disease Self-efficacy scales was used. The local version of the scale has gone through concurrent and convergent validity test and has high stability on the reliability test (Chow & Wong 2013). For quality of life, we used the MOS 36-item Short-Form Health Survey (SF-36) to examine the quality of life related to eight domains of physical and mental health (Ware n.d.). An overall physical and mental health component score summarized the overall quality of life for physical and mental well-being of the patients. The Hong Kong Chinese version was tested and results showed that the translated scale satisfied almost all the test on conceptual validity when it was used on the general Chinese adult population in Hong Kong (Lam & Gandek 2001). The self-rated health status scale was a single-item scale and patients responded using a 5-point Likert scale, which ranged from excellent to poor. The scale has been commonly used in overseas and local studies (DeSalvo et al. 2005, Chow & Chan 2010). All participants answered the above questionnaires three times, at baseline and at 4 and 12 weeks.
Reliability and validity
The intervention outcomes on self-efficacy, quality of life and self-rated health were assessed by validity and reliability tools to be used for the local population. The CONSORT statement 2010 (Schulz et al. 2010) was used to guide the design and conduct of the RCT. The study was conducted on the ward and in outpatient units in genuine clinical situations. The researchers, who are experienced with clinical trials, monitored the study design, study protocols, patient recruitment, blinding, interventions provided by the case managers, subject drop outs and confidentiality of the patients' information. The scrupulous study design ensured quality management and high external validity.
Ethical considerations
The study was approved by the Research Ethics Committees of the university and the study hospital to which the research team members were affiliated. Patients were informed that their participation was voluntary and that they could withdraw from the study at any time. They were reassured that their withdrawal would not prevent them from receiving the care that they would normally receive. The participants were identified by research codes and research information remained confidential.
Data analysis
The Statistical Package for Social Sciences (version 19: SPSS Inc., Chicago, IL, USA) was used for data analysis. Chi-square test and Kruskal–Wallis test were used to compare baseline data among groups for categorical and continuous variables. The readmissions among groups were compared using Fisher's exact test. Repeated measures analysis of variance (anova) was used to investigate differences in hospital readmission, quality of life, self-rated health and self-efficacy for the three time points among the three groups. The analyses were conducted on intention-to-treat basis and the significance level was set at P < 0·05, two-tailed test. Paired t-tests with Bonferroni adjustment were conducted for multiple comparisons among the three arms and changes within groups among the three points. The missing data were replaced by group means.
Results
Participant characteristics
There were 610 participants recruited in the main study with 312 patients having co-morbid, chronic conditions as confirmed by the physician in the medical records. There were 281 patients included in the final data analysis. The demographic characteristics included age, gender, education attainment and occupation. The mean age was 76·5, range 60–95. There were more female than male patients, at 52·5% and 47·5% respectively. Approximately 76% of the participants had received no formal education or had received primary education or below. More than 80% of the study population had two chronic conditions. Two patients suffered more than three chronic diseases. The average length of stay in hospital was 4 days, range 1–18 days. Table 1 shows the demographic and clinical variables for the three arms at baseline. The Consort statement (Figure 1) shows how the trail was designed, analysed and interpreted.
Table 1.
Comparison between groups for demographic and clinical data
| Control (n = 98) | Home visit (n = 87) | Call (n = 96) | Total (n = 281) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Gender | ||||||||
| Male | 49 | 50·0 | 41 | 47·1 | 44 | 45·8 | 134 | 47·7 |
| Female | 49 | 50·0 | 46 | 52·9 | 52 | 54·2 | 147 | 52·3 |
| Marital status | ||||||||
| Single | 4 | 4·1 | 0 | 0·0 | 0 | 0·0 | 4 | 1·4 |
| Married | 59 | 60·2 | 49 | 56·3 | 60 | 62·5 | 168 | 59·8 |
| Divorced | 2 | 2·0 | 5 | 5·7 | 1 | 1·0 | 8 | 2·8 |
| Widow | 33 | 33·7 | 33 | 37·9 | 35 | 36·4 | 101 | 36·0 |
| Education level | ||||||||
| No formal education | 28 | 28·6 | 26 | 29·9 | 31 | 32·3 | 85 | 30·2 |
| Primary education or below | 40 | 40·8 | 47 | 54·0 | 41 | 42·7 | 128 | 45·6 |
| Secondary education | 17 | 17·3 | 10 | 11·5 | 19 | 19·8 | 46 | 16·4 |
| Tertiary | 13 | 13·3 | 4 | 4·5 | 5 | 5·2 | 22 | 7·8 |
| Occupation | ||||||||
| Full time | 0 | 0·0 | 1 | 1·1 | 0 | 0·0 | 1 | 0·4 |
| Part-time | 6 | 6·1 | 0 | 0·0 | 4 | 4·2 | 10 | 3·6 |
| Not working | 92 | 93·9 | 86 | 98·9 | 92 | 95·9 | 270 | 96·1 |
| Age (years) | ||||||||
| Median (range) | 77·00 | (60–89) | 75·00 | (60–92) | 75·50 | (60–89) | 76·00 | (60–92) |
| Number of co-morbid disease | ||||||||
| Two diseases | 81 | 82·7 | 71 | 81·6 | 74 | 77·1 | 226 | 80·4 |
| Three diseases | 17 | 17·3 | 15 | 17·2 | 22 | 22·9 | 54 | 19·2 |
| More than three diseases | 0 | 0·0 | 1 | 1·1 | 0 | 0·0 | 1 | 0·4 |
| Length of stay | ||||||||
| Median (range) | 3·00 | (1–16) | 5·00 | (1–17) | 3·00 | (1–18) | 4·00 | (1–18) |
| Days between first readmission and index discharge | ||||||||
| Median (range) | 10 | (1–28) | 8·5 | (1–28) | 3 | (1–23) | 5 | (1–28) |
Figure 1.
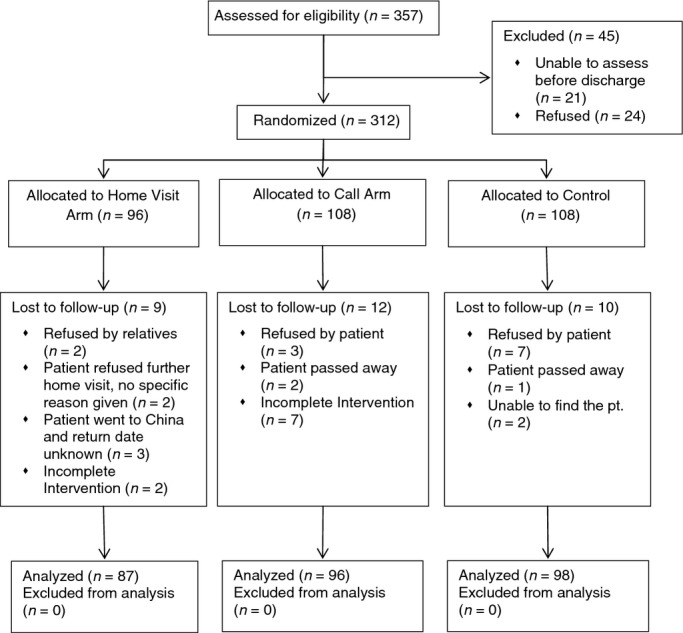
CONSORT diagram of the trial flow.
Readmission rates
For the readmission rate among groups within 28 days post discharge, the interventions demonstrated differences in the home group and the call group. The home visit group (15·4%) and the call group (16·0%) had lower readmission rates than the control group (22·9%). The result was not statistically significant (χ2 = 2·34, P = 0·311) as data collection was within the intervention period. The interventions demonstrated significant differences within 84 days post discharge (χ2 = 8·03, P = 0·018); the readmission rate for control group was 45·4%, whereas for home visit and call group were 33·0% and 28·3% respectively (see Table 2). Further analysis revealed the significant difference when the call group was compared with control group (χ2 = 7·25, P = 0·007) and results approached significant when home visit group was compared with control group (χ2 = 3·55, P = 0·059).
Table 2.
Comparisons of readmission rate
| Total | Control | Home visit | Call | χ2 | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Within 28 days post discharge | ||||||||||
| Readmitted | 54 | 18·2 | 24 | 22·9 | 14 | 15·4 | 16 | 16·0 | 2·34 | 0·311 |
| No readmission | 242 | 81·8 | 81 | 77·1 | 77 | 84·6 | 84 | 84·0 | ||
| Within 84 days post discharge | ||||||||||
| Readmitted | 121 | 36·3 | 59 | 45·4 | 32 | 33·0 | 30 | 28·3 | 8·03 | 0·018* |
| No readmission | 212 | 63·7 | 71 | 54·6 | 65 | 67·0 | 76 | 71·7 | ||
Significant at *P < 0·05.
Quality of life
Responses on the SF-36 were scored and aggregated into physical component score (PCS) and mental component score (MCS). Repeated measures ancova showed a significant difference between groups across the three time points in PCS [F(2, 277) = 4·31, P = 0·014] and approached significant in MCS [F(2, 277) = 2·77, P = 0·064]. There were significant differences in majority of the domains in the SF36 scale. Five of the eight domains showed significant differences among the three arms over time. They included physical functioning [F(2, 277) = 12·1, P < 0·001], role physical [F(2, 277) = 11·1, P < 0·001], vitality [F(2, 277) = 11·6, P < 0·001], social function [F(2,277) = 4·63, P = 0·01] and mental health [F(2, 277) = 8·0, P < 0·001]. Post hoc analysis using the Bonferroni multiple comparison procedures indicated that the improvements came from the study groups, either from the home visit or telephone arm. There was significant difference among the three arms in the physical composite score [F(2, 277) = 4·31, P = 0·014], but no significant difference in the MCS.
Self-efficacy and self-rated health
For self-efficacy, results showed significant difference between groups across the three time points [F(2, 277) =7·72, P < 0·001]. Post hoc analysis using the Bonferroni multiple comparison procedures indicated that the home visit arm (P = 0·005) and the call arm (P = 0·001) had significant higher self-efficacy than the control arm. For within-group effects, both the home visit arm [F(2, 172) =6·06, P = 0·002] and the call arm [F(1·8, 171) = 8·43, P < 0·001] demonstrated significant improvements across time. For self-rated health, the three arms in the three different time points showed overall significance [F(2, 277) = 19·7, P < 0·001]. Post hoc analysis showed significant difference between the baseline and different stages of assessment, as well as the effectiveness of intervention in T2 and T3. Results are summarized in Table 3.
Table 3.
Comparisons of SF-36, self-efficacy and self-rated health
| T1 | T2 | T3 | Between groups | Within groups | Interaction effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | sd | n | Mean | sd | n | Mean | sd | F (P value) [X:Y:Z] | F (P value) [A:B:C] | F (P value)§ | |
| Physical functioning | ||||||||||||
| Control | 98 | 43·0 | 8·3 | 98 | 43·7 | 6·4 | 98 | 42·7 | 6·3 | 12·1 (<0·001**) [0·956, <0·001**, 0·001**] | 2·52 (0·112) | 2·76 (0·064) |
| Home visit arm | 87 | 44·3 | 8·5 | 87 | 43·8 | 6·9 | 87 | 45·0 | 7·2 | 1·08 (0·335) [0·999, 0·999, 0·242] | ||
| Call arm | 96 | 43·3 | 8·1 | 96 | 46·3 | 5·5 | 96 | 46·4 | 6·0 | 1·15 (0·318) [0·999, 0·958, 0·486] | ||
| F (P value) [X:Y:Z]†‡ | 0·60 (0·547) [0·871, 0·999, 0·999] | 7·35 (<0·001**) [0·999, 0·006**, <0·001**] | 10·8 (<0·001**) [0·088, <0·001**, 0·060*] | 15·2 (<0·001**) [<0·001**, <0·001**, 0·999] | ||||||||
| Role physical | ||||||||||||
| Control | 98 | 42·5 | 12·2 | 98 | 44·3 | 10·8 | 98 | 42·4 | 10·1 | 11·1 (<0·001**) [<0·001**, <0·001**, 0·999] | 2·67 (0·102) | 1·00 (0·366) |
| Home visit arm | 87 | 42·8 | 12·5 | 87 | 47·5 | 9·0 | 87 | 47·3 | 8·1 | 1·48 (0·229) [0·435, 0·999, 0·266] | ||
| Call arm | 96 | 40·1 | 12·7 | 96 | 47·3 | 8·4 | 96 | 47·4 | 8·5 | 10·9 (<0·001**) [<0·001**, <0·001**, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 1·27 (0·280) [0·999, 0·550, 0·470] | 4·70 (0·009**) [0·050, 0·015*, 0·999] | 11·2 (<0·001**) [<0·001**, <0·001**, 0·999] | 19·4 (<0·001**) [<0·001**, <0·001**, 0·999] | ||||||||
| Bodily pain | ||||||||||||
| Control | 98 | 46·7 | 12·7 | 98 | 49·3 | 10·8 | 98 | 47·8 | 10·5 | 2·01 (0·134) [0·136, 0·948, 0·915] | 0·20 (0·653) | 1·26 (0·284) |
| Home visit arm | 87 | 48·7 | 13·4 | 87 | 51·6 | 8·3 | 87 | 51·4 | 9·6 | 2·46 (0·091) [0·121, 0·999, 0·393] | ||
| Call arm | 96 | 47·2 | 13·6 | 96 | 49·4 | 10·9 | 96 | 50·2 | 11·1 | 3·21 (0·048*) [0·071, 0·200, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 0·60 (0·547) [0·866, 0·999, 0·999] | 0·90 (0·406) [0·785, 0·999, 0·670] | 2·53 (0·081) [0·105, 0·276, 0·999] | 2·82 (0·061) [0·334, 0·084, 0·999] | ||||||||
| General health | ||||||||||||
| Control | 98 | 31·1 | 9·2 | 98 | 33·2 | 7·6 | 98 | 32·5 | 7·9 | 2·68 (0·069) [0·405, 0·999, 0·067] | 0·84 (0·359) | 0·16 (0·850) |
| Home visit arm | 87 | 33·7 | 9·8 | 87 | 35·0 | 8·5 | 87 | 34·7 | 7·7 | 2·13 (0·127) [0·186, 0·677, 0·999] | ||
| Call arm | 96 | 30·3 | 8·6 | 96 | 31·9 | 6·8 | 96 | 31·9 | 7·4 | 0·90 (0·406) [0·599, 0·999, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 3·38 (0·035*) [0·159, 0·999, 0·039*] | 1·99 (0·138) [0·907, 0·934, 0·140] | 1·86 (0·156) [0·429, 0·999, 0·197] | 2·25 (0·112) [0·207, 0·330, 0·999] | ||||||||
| Vitality | ||||||||||||
| Control | 98 | 52·1 | 9·2 | 98 | 52·4 | 6·9 | 98 | 50·8 | 7·3 | 11·6 (<0·001**) [0·120, <0·001**, 0·028*] | 3·35 (0·068) | 0·67 (0·510) |
| Home visit arm | 87 | 53·7 | 9·5 | 87 | 53·8 | 6·0 | 87 | 53·8 | 8·5 | 1·52 (0·221) [0·999, 0·567, 0·203] | ||
| Call arm | 96 | 51·4 | 11·4 | 96 | 55·6 | 8·5 | 96 | 54·8 | 8·5 | 0·01 (0·989) [0·999, 0·999, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 1·19 (0·304) [0·822, 0·999, 0·401] | 6·62 (0·001**) [0·999, 0·001**, 0·040*] | 8·05 (<0·001**) [0·077, <0·001**, 0·308] | 9·85 (<0·001**)[<0·001**,0·004**,0·999] | ||||||||
| Social functioning | ||||||||||||
| Control | 98 | 53·9 | 6·2 | 98 | 50·7 | 9·0 | 98 | 52·3 | 5·6 | 4·63 (0·010**) [0·886, 0·141, 0·009**] | 0·28 (0·594) | 0·39 (0·675) |
| Home visit arm | 87 | 52·8 | 8·0 | 87 | 50·0 | 6·2 | 87 | 51·1 | 8·4 | 6·47 (0·003**) [0·009**, 0·084, 0·201] | ||
| Call arm | 96 | 53·2 | 6·9 | 96 | 52·8 | 6·6 | 96 | 53·3 | 7·1 | 3·11 (0·052) [0·021*, 0·654, 0·770] | ||
| F (P value) [X:Y:Z]†‡ | 0·64 (0·526) [0·790, 0·999, 0·999] | 3·70 (0·025*) [0·999, 0·123, 0·032*] | 2·01 (0·135) [0·887, 0·964, 0·136] | 0·14 (0·866) [0·999, 0·999, 0·999] | ||||||||
| Role emotional | ||||||||||||
| Control | 98 | 50·4 | 10·4 | 98 | 51·0 | 7·2 | 98 | 47·4 | 10·8 | 2·51 (0·083) [0·081, 0·999, 0·470] | 3·45 (0·064) | 2·57 (0·077) |
| Home visit arm | 87 | 49·8 | 10·9 | 87 | 51·4 | 7·1 | 87 | 51·2 | 7·0 | 4·67 (0·010*) [0·999, 0·077, 0·007**] | ||
| Call arm | 96 | 47·7 | 11·7 | 96 | 50·5 | 8·7 | 96 | 48·6 | 10·3 | 1·15 (0·315) [0·632, 0·720, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 1·52 (0·218) [0·999, 0·289, 0·612] | 0·17 (0·836) [0·999, 0·999, 0·999] | 4·08 (0·017*) [0·013*, 0·620, 0·330] | 2·55 (0·088) [0·085, 0·999, 0·198] | ||||||||
| Mental health | ||||||||||||
| Control | 98 | 53·1 | 10·1 | 98 | 52·3 | 7·1 | 98 | 51·7 | 8·2 | 8·00 (<0·001**) [0·011*, <0·001**, 0·999] | 0·39 (0·532) | 0·59 (0·554) |
| Home visit arm | 87 | 52·9 | 10·8 | 87 | 54·3 | 6·1 | 87 | 54·6 | 6·4 | 1·08 (0·338) [0·999, 0·558, 0·999] | ||
| Call arm | 96 | 51·5 | 11·6 | 96 | 55·4 | 7·8 | 96 | 54·2 | 10·2 | 1·61 (0·205) [0·558, 0·435, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 0·59 (0·550) [0·999, 0·949, 0·999] | 7·08 (<0·001**) [0·085, <0·001**, 0·454] | 4·29 (0·014*) [0·040*, 0·033**, 0·999] | 5·77 (0·004**) [0·005**, 0·117, 0·700] | ||||||||
| Physical component SF36 | ||||||||||||
| Control | 98 | 37·9 | 9·6 | 98 | 40·3 | 8·2 | 98 | 39·3 | 7·3 | 4·31 (0·014*) [0·104, 0·016*, 0·999] | 0·65 (0·419) | 1·93 (0·146) |
| Home visit arm | 87 | 40·0 | 9·6 | 87 | 42·1 | 7·0 | 87 | 42·4 | 7·4 | 3·34 (0·041*) [0·052, 0·526, 0·567] | ||
| Call arm | 96 | 38·0 | 8·9 | 96 | 41·6 | 6·4 | 96 | 42·6 | 7·6 | 4·41 (0·013*) [0·060, 0·039*, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 1·50 (0·223) [0·363, 0·999, 0·424] | 1·04 (0·353) [0·792, 0·544, 0·999] | 5·91 (0·003**) [0·050, 0·003*, 0·999] | 16·0 (<0·001**) [<0·001**, <0·001**, 0·478] | ||||||||
| Mental component SF36 | ||||||||||||
| Control | 98 | 56·4 | 8·9 | 98 | 54·7 | 6·0 | 98 | 53·6 | 7·9 | 2·77 (0·064) [0·269, 0·076, 0·999] | 2·73 (0·099) | 0·86 (0·424) |
| Home visit arm | 87 | 55·5 | 9·6 | 87 | 55·4 | 5·5 | 87 | 55·5 | 6·5 | 4·17 (0·016*) [0·310, 0·024*, 0·570] | ||
| Call arm | 96 | 54·3 | 10·6 | 96 | 56·4 | 7·9 | 96 | 54·8 | 11·0 | 0·01 (0·982) [0·999, 0·999, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 1·08 (0·338) [0·999, 0·429, 0·999] | 2·61 (0·074) [0·999, 0·071, 0·548] | 1·76 (0·173) [0·247, 0·439, 0·999] | 1·81 (0·166) [0·171, 0·999, 0·425] | ||||||||
| Self-efficacy | ||||||||||||
| Control | 98 | 30·6 | 9·4 | 98 | 32·2 | 8·2 | 98 | 32·0 | 8·7 | 7·72 (<0·001**) [0·005*, 0·001**, 0·999] | 0·42 (0·514) | 0·99 (0·371) |
| Home visit arm | 87 | 32·3 | 10·7 | 87 | 34·8 | 7·1 | 87 | 36·3 | 9·0 | 1·50 (0·224) [0·363, 0·464, 0·999] | ||
| Call arm | 96 | 32·4 | 10·2 | 96 | 36·1 | 9·0 | 96 | 35·7 | 8·9 | 6·06 (0·002**) [0·070, 0·004**, 0·628] | ||
| F (P value) [X:Y:Z]†‡ | 0·90 (0·407)[0·780,0·714,0·999] | 5·10 (0·006**)[0·161,0·005**,0·761] | 5·39 (0·005**)[0·007**,0·031*,0·999] | 8·43 (<0·001**) [0·003**, 0·005**, 0·999] | ||||||||
| Self-rated health | ||||||||||||
| Control | 98 | 2·2 | 0·9 | 98 | 2·2 | 0·6 | 98 | 2·2 | 0·7 | 19·7 (<0·001**) [0·008**, <0·001**, 0·006**] | <0·01 (0·944) | 1·30 (0·272) |
| Home visit arm | 87 | 2·3 | 0·9 | 87 | 2·5 | 0·7 | 87 | 2·4 | 0·8 | 0·06 (0·913) [0·999, 0·999, 0·999] | ||
| Call arm | 96 | 2·1 | 0·8 | 96 | 2·8 | 0·8 | 96 | 2·6 | 0·8 | 3·25 (0·045*) [0·025*, 0·353, 0·999] | ||
| F (P value) [X:Y:Z]†‡ | 0·51 (0·597) [0·999, 0·999, 0·964] | 19·3 (<0·001**) [0·083, <0·001**, 0·083] | 8·67 (<0·001**) [0·077, <0·001**, 0·077] | 28·4 (<0·001**) [<0·001**, <0·001**, 0·063] | ||||||||
Significant at P < 0·05;
P < 0·01.
At T1 one-way anova.
At T2 and T3 ancova adjusted by T1.
Repeated measured adjusted by T1.
[A:B:C] multiple comparisons (Bonferroni), A: T1 vs. T2; B: T1 vs. T3; C: T2 vs. T3'. [X:Y:Z] multiple comparisons (Bonferroni), X: home visit vs. control; Y: call vs. control; Z: home visit vs. call.
Discussion
The results demonstrate that implementation of tailored interventions on empowerment by NCMs significantly contributes to enhance self-efficacy of older patients with co-morbid, chronic diseases. In this study, improvement in self-efficacy for chronic disease management subsequently reduced hospital readmission and increased quality of life.
Model of care delivery for patients having chronic co-morbidities
For successful management of older patients with multiple chronic diseases, the new model of care uses multiple component strategies targeting at comprehensive assessment of discharge needs, support for patient-centred processes and shared decision-making with co-ordination of the NCM. These form the key ingredients for a successful programme.
Two studies by Kwok et al. (2004, 2008) concluded that an intensive home visit by general community nurse was not effective in reducing hospital readmission in older patients with chronic lung disease and chronic heart failure. In a Cochrane review on initiated telephone follow-up by a hospital-based health professional, as a means to reduce postdischarge problems for different disease group patients, the results were inconclusive (Mistiaen & Poot 2008). Patient empowerment with self-management support using the collaborative process could be potentially valuable for patients having chronic conditions (McAllister et al. 2012). The majority of patients in our sample were poorly educated. We may, therefore, presume that home visits by the NCM would be most beneficial to manage patients' health status and functional performance due to direct personal contact and interventions in the patient's home environment. Our results showed that the bundle of care, consisting of home visits and telephone follow-ups or telephone calls alone produced similar significant effects. Patients were receiving one-stop service without the need to consult multiple professionals for problems after hospital discharge. It is not the platform of communication, but the nurse–patient relationship and empowerment that make the greater contribution to the improvements. Lattimer (2011) concluded that the key elements to successful care management for co-morbidities are the empowerment of patients and their caregivers with mutual understanding. The essential interventions in our study that included problem identification, decision support and mutual goal setting with professional support are the crucial components compared with simple home visits and telephone follow-ups conducted by general nurses. While acquiring a holistic perspective, the effects also depend on the duration and number of follow-up visits and the personality of the nurse (Frich 2003). Nurses caring for patients with co-morbidities should not only focus on the diseases but also to manage the complexities of the recovery processes and prioritize the problems with patients, their families and related healthcare professionals. In the study, the NCM ensured the flexibility of interventions and allow professional judgment for each individual decision.
Effectiveness of the interventions on hospital readmission and quality of life
The significant reduction in hospital readmission within 84 days post discharge confirmed the effectiveness of the intervention arms. The call arm showed better outcomes than the home visit arm when compared with the control group. Previous studies by Mistiaen and Poot (2008) and Clark et al. (2007) showed that telephone calls alone with remote monitoring were more effective at shortening hospital stay but not potent enough to control readmission at a significant level, while nurse telephone follow-ups without a strong transitional care component was insufficient to reduce healthcare expenditure (Peikes et al. 2009). Patients suffering co-morbidities required complex interventions. Wong et al.'s (2013) study on patients having single and multiple chronic diseases showed no significant difference between home/call and the control group. When examining the absolute percentage on readmission rates, there were 21·4% for home visit group, 20·6% for call group and 25·7% for control group. The readmission rates for combined group analysis were lower than patients having multiple chronic diseases due to the complexity in disease management and higher symptom burden with single disease alone.
Although not all studies reported an improvement in quality of life measures, our interventions were able to demonstrate improvements in some components in the physical and mental health of the patients indicated in SF36 scale. The call arm group showed significant improvement in physical functioning across time, but the home visit arm did not. For physical role, both intervention groups demonstrated improvements in T2 and T3. Patients in the interventions groups received individualized education regarding promotion of optimal medication regimens and to increase physical activities if needed and they reported less work- or activities-related anxiety as a result of their physical health. Through continuous motivation, support and identification of barriers to physical activities, the physical functions of the patients in both intervention arms were improved over time. Consequently, patients showed rigour and consistency in energy levels as indicated in the subscale for vitality. As older people prefer to perform exercise at home (Gill et al. 2003), it is imperative to encourage patients to increase physical activities at their own convenience.
Effectiveness of the intervention on self-rated health and self-efficacy on multiple chronic disease management
Lupari et al. (2011) delineated the research gap on self-efficacy in their summary review on service evaluation of nurse-led case management services for older patients with multiple chronic conditions. Our study was able to demonstrate a psychological construct, whereby self-efficacy was associated with the intervention. Wen et al. (2006) showed that population who were less educated or living in poor neighbourhoods showed low self-efficacy. As 76% of our study sample were educated to primary school level or below and were retired, it was encouraging to note that the self-efficacy scores improved dramatically after intervention and that they were able to sustain, particularly the call arm group. At the same time, the self-rated health of both intervention arms improved as a result of the interventions. These significant differences imply that empowerment and self-efficacy enhancing interventions can be considered as successful pathways to increase self-management behaviours. Despite the low socioeconomic status and educational attainment of the study participants, patient-centred care with mutual goal setting and empowerment strategies were able to bring about changes with an increasing sense of strength and control for patients with co-morbidities.
There has been a notable increase in intervention research targeting the health promoting effects in relation to social bonds and health-related behaviours (Kawachi et al. 2008). The programme used in our study, conducted on theory-based implementation by an Advanced Practice Nurse with support from nursing students, has provided high-quality evidence in the context of enhancing self-efficacy among patients with multiple chronic diseases. The key findings from this research have proven that a well-structured, sophisticated transitional care programme was able to improve self-management behaviours for older patients with co-morbidities.
Limitations
In patient recruitment, patients who consented to participate in the study might be more health conscious and may already be independent and wanting to perform self-care activities after discharge. Although the sample size was sufficient, the study participants were only recruited from a single hospital setting. Further studies in diverse study settings or across countries are necessary for us to generalize the study findings in relation to the contrasting findings from overseas studies. The programme should be further explored using different disease groups with a larger sample size.
Conclusion
Collaboration between patients and nurses on mutual decision-making to increase self-care abilities among older patients with co-morbidities were able to achieve the best outcomes. Despite the different intervention platforms, the effects depended on whether the assessment and interventions were holistic and multi-dimensional, the frequencies and duration of intervention, the personality of the nurses, expertise in case management and having a substantial amount of time to be with each patient. The activities led by an Advanced Practice Nurse instead of a general nurse conducting individualized education with a cognitive behavioural approach were able to provide positive clinical and patient outcomes.
The findings also confirmed the relationships between chronic disease management and self-efficacy, hospital readmission and quality of life. However, the causal relationships among these variables remain to be empirically tested in future studies.
Acknowledgments
The authors thank the patients who participated in the study.
Funding
The work described in this paper was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (RGC Ref. No. 547909).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
All authors have agreed on the final version and meet at least one of the following criteria [recommended by the ICMJE (http://www.icmje.org/ethical_1author.html)]:
substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
drafting the article or revising it critically for important intellectual content.
The Journal of Advanced Nursing (JAN) is an international, peer-reviewed, scientific journal. JAN contributes to the advancement of evidence-based nursing, midwifery and health care by disseminating high quality research and scholarship of contemporary relevance and with potential to advance knowledge for practice, education, management or policy. JAN publishes research reviews, original research reports and methodological and theoretical papers.
For further information, please visit JAN on the Wiley Online Library website: www.wileyonlinelibrary.com/journal/jan
Reasons to publish your work in JAN:
High-impact forum: the world's most cited nursing journal, with an Impact Factor of 1·527 – ranked 14/101 in the 2012 ISI Journal Citation Reports © (Nursing (Social Science)).
Most read nursing journal in the world: over 3 million articles downloaded online per year and accessible in over 10,000 libraries worldwide (including over 3,500 in developing countries with free or low cost access).
Fast and easy online submission: online submission at http://mc.manuscriptcentral.com/jan.
Positive publishing experience: rapid double-blind peer review with constructive feedback.
Rapid online publication in five weeks: average time from final manuscript arriving in production to online publication.
Online Open: the option to pay to make your article freely and openly accessible to non-subscribers upon publication on Wiley Online Library, as well as the option to deposit the article in your own or your funding agency's preferred archive (e.g. PubMed).
References
- Bandura A. Self-efficacy: towards a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bardach SH. Schoenberg NE. Primary care physician counseling with patients with multiple morbidity. Qualitative Health Research. 2012;22:1599–1611. doi: 10.1177/1049732312458183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Wray LA, Chiu CJ. Weinger K. Perceived challenges and priorities in co-morbidity management of older patients with Type 2 diabetes. Diabetic Medicine. 2011;28:781–784. doi: 10.1111/j.1464-5491.2011.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census and Statistics Department. Thematic Household Survey Report No. 40. Hong Kong: Census and Statistics Department; 2009. [Google Scholar]
- Chen Y. Li IC. Effectiveness of interventions using empowerment concept for patients with chronic disease: a systematic review; Effectiveness of Nurse Practitioners in nursing homes: a systematic review; Effectiveness of strategies to promote safe transition of older people across care settings; Management of disruptive behaviour within nursing work environments: a comprehensive systematic review of the evidence. Journal of Advanced Nursing. 2009;66:1446–1451. [Google Scholar]
- Chow SKY. Chan WC. Depression: problem-solving appraisal and self-rated health among Hong Kong Chinese migrant women. Nursing and Health Sciences. 2010;12:352–359. doi: 10.1111/j.1442-2018.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- Chow SKY. Wong FKY. Health-related quality of life in patients undergoing peritoneal dialysis: effects of a nurse-led case management programmed. Journal of Advanced Nursing. 2010;66:1780–1792. doi: 10.1111/j.1365-2648.2010.05324.x. [DOI] [PubMed] [Google Scholar]
- Chow SKY. Wong FKY. The reliability and validity of the Chinese version of the short-form chronic disease self-efficacy scales for older adults. Journal of Clinical Nursing. 2013 doi: 10.1111/jocn.12298. doi: 10.1111/jocn.12298. [DOI] [PubMed] [Google Scholar]
- Clark NM, Becker MH, Janz NK, Lorig K, Rakowski W. Anderson L. Self-management of chronic disease by older adults: a review and questions for research. Journal of Aging and Health. 1991;3:3–27. [Google Scholar]
- Clark R, Inglis SC, McAlister FA, Cleland J. Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. British Medical Journal. 2007;334:942–945. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutilli CC. Health literacy in geriatric patients: an integrative review of the literature. Orthopedic Nursing. 2007;26:43–48. doi: 10.1097/00006416-200701000-00014. [DOI] [PubMed] [Google Scholar]
- DeSalvo KB, Boser N, Reynolds K, He J. Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. Journal of General Internal Medicine. 2005;20:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frich LMH. Nursing interventions for patients with chronic conditions. Journal of Advanced Nursing. 2003;44:137–153. doi: 10.1046/j.1365-2648.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Fukuyama F. Social capital, civil society and development. Third World Quarterly. 2001;22:7–20. [Google Scholar]
- Gallagher R, Donoghue J. Stein-Parbury J. Self-management in older patients with chronic illness. International Journal of Nursing Studies. 2008;14:373–382. doi: 10.1111/j.1440-172X.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Olmos L, Salvador CH, Alberquilla A, Lora D, Carmona M, Garcia-Sagredo P, Pascual M, Munoz A, Monteagudo JL. Garcia-Lopez F. Comorbidity patterns in patients with chronic diseases in general practice. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Baker DI, Gottschalk M, Gahbauer EA, Charpentier PA, de Regt PT. Wallace SJ. A prehabilitation program for physically frail community-living older persons. Archives of Physical Medicine and Rehabilitation. 2003;84:394–404. doi: 10.1053/apmr.2003.50020. [DOI] [PubMed] [Google Scholar]
- Glendinning C. Breaking down barriers: integrating health and care services for older people in England. Health Policy. 2003;65:139–151. doi: 10.1016/s0168-8510(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Hospital Authority, Hong Kong (n.d.) Hospital Authority Statistical Report (2010–2011) Retrieved from http://www.ha.org.hk/upload/publication_15/411.pdf on 15 March 2013.
- Kawachi I, Subramanian SV. Kim D. Social Capital and Health. New York: Springer; 2008. pp. 1–26. [Google Scholar]
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, Gardner C, Gately C. Rogers A. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. Journal of Epidemiology and Community Health. 2007;61:254–261. doi: 10.1136/jech.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T, Lum CM, Chan HS, Ma HM, Lee D. Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmission of older patients with chronic lung disease. The American Geriatrics Society. 2004;52:1240–1246. doi: 10.1111/j.1532-5415.2004.52351.x. [DOI] [PubMed] [Google Scholar]
- Kwok T, Lee J, Woo J, Lee DTF. Griffith S. A randomized controlled trial of a community nurse-supported hospital discharge programme in older patients with chronic heart failure. Journal of Clinical Nursing. 2008;17:109–117. doi: 10.1111/j.1365-2702.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- Lam CLK. Gandek B. Cross-cultural adaptation of the SF-36 and SF-12. Quality of Life Research. 2001;10:283. [Google Scholar]
- Lattimer C. When it comes to transitions in patient care, effective communication can make all the difference. Journal of the American Society on Aging. 2011;35:69–72. [Google Scholar]
- Lupari M, Coatges V, Adamson G. Crealey GE. ‘We're just not getting it right’ – how should we provide care to the older person with multi-morbid chronic conditions. Journal of Clinical Nursing. 2011;20:1225–1235. doi: 10.1111/j.1365-2702.2010.03620.x. [DOI] [PubMed] [Google Scholar]
- Martin K. The Omaha System: A Key to Practice, Documentation and Information Management. 2. St, Louis, MO: Elsevier Saunders; 2005. [Google Scholar]
- Mattke S, Seid M. Ma S. Evidence for the effect of disease management: is $1 billion a year a good investment? American Journal of Managed Care. 2007;13:670–676. [PubMed] [Google Scholar]
- McAllister M, Dunn G, Payne K. Todd C. Patient empowerment: the need to consider it as a measurable patient-reported outcome for chronic conditions. BMC Health Services Research. 2012;12:157–164. doi: 10.1186/1472-6963-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistiaen P. Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2008:4. doi: 10.1002/14651858.CD004510.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M, Brooten D, Campbell R, Maislin G, McCauley KM. Schwartz S. Transitional care of older adults hospitalized with heart failure: a randomized controlled trial. Journal of the American Geriatrics Society. 2004;52:675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- Norris SL, High K, Gill TM, Hennessy S, Kutner JS, Reuben DB, Unutzer J. Landefeld CS. Health care for older Americans with multiple chronic conditions: a research agenda. Journal of the American Geriatrics Society. 2008;56:149–159. doi: 10.1111/j.1532-5415.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- Ose D, Miksch A, Urban E, Natanzon I, Szecsenyi J, Kunz CU. Freund T. Health related quality of life and comorbidity. A descriptive analysis comparing EQ-5D dimensions of patients in the German disease management program for type 2 diabetes and patients in routine care. BMC Health Services Research. 2011;11:179. doi: 10.1186/1472-6963-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikes D, Chen A, Schore J. Brown R. Effects of care coordination on hospitalization, quality of care and health care expenditures among medicare beneficiaries. JAMA. 2009;301:603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K. The efficacy of tailored interventions for self-management outcomes of type 2 diabetes, hypertension or heart disease: a systematic review. Journal of Advanced Nursing. 2011;68:496–510. doi: 10.1111/j.1365-2648.2011.05860.x. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG. Moher D for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152:1–8. [Google Scholar]
- Sutherland D. Hayter M. Structured review: evaluating the effectiveness of nurse case managers in improving health outcomes in three major chronic diseases. Journal of Clinical Nursing. 2009;18:2978–2992. doi: 10.1111/j.1365-2702.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB. Blumenthal D. Multiple chronic conditions: prevalence, health consequences and implications for quality, care management and costs. Journal of General Internal Medicine. 2007;22(Suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE. SF-36 Literature Construction of the SF-36 Version 2.0 Psychometric Considerations Translations Discussion. Retrieved from http://www.sf-36.org/tools/sf36.shtml#MODEL. retrieved on 29 May 2013.
- Wen M, Hawkley LC. Cacioppo JT. Objective and perceived neighbourhood environment, individual SES and psychosocial factors and self-rated health: an analysis of old adults in Cook County, Illinois. Social Science & Medicine. 2006;63:2575–2590. doi: 10.1016/j.socscimed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Williams A. Patients with comorbidities: perceptions of acute care services. Journal of Advanced Nursing. 2004;46:13–22. doi: 10.1111/j.1365-2648.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- Wong FKY, Ho ML, Chiu I, Lui WK, Chan C. Lee KM. Factors contributing to hospital readmission in a Hong Kong regional hospital: a case-controlled study. Nursing Research. 2002;51:40–49. doi: 10.1097/00006199-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Wong FKY, Chow SKY, Chung L, Chang K, Chan T, Lee WM. Lee R. Can home visits help reduce hospital readmissions? Randomized controlled trial. Journal of Advanced Nursing. 2008;62:585–596. doi: 10.1111/j.1365-2648.2008.04631.x. [DOI] [PubMed] [Google Scholar]
- Wong FKY, Chow SKY. Chan T. Evaluation of a nurse led disease management programme for chronic kidney disease management: a randomized controlled trial. International Journal of Nursing Studies. 2010;47:268–278. doi: 10.1016/j.ijnurstu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Wong FKY, Ho ML, Yeung SY, Tam SK. Chow SKY. Effects of a health-social partnership transitional program on hospital readmission: a randomized controlled trial. Social Science & Medicine. 2011;73:960–969. doi: 10.1016/j.socscimed.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Wong FKY, Chow SKY, Chan TMF. Tam SKY. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age and Ageing. 2013 doi: 10.1093/ageing/aft123. doi: 10.1093/ageing/aft123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun YT, Chan K. Lee A. Co-morbidity in general practice. Family Practice. 1998;15:266–268. doi: 10.1093/fampra/15.3.266. [DOI] [PubMed] [Google Scholar]
- Yach D, Hawkes C, Gould L. Hofman KJ. The global burden of chronic diseases overcoming impediments to prevention and control. The Journal of the American Medical Association. 2004;291:2616–2622. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- Yamamoto PPE. Improving the oral health of older people: the approach of the WHO global oral health programme. Community Dentistry and Oral Epidemiology. 2005;33:81–92. doi: 10.1111/j.1600-0528.2004.00219.x. [DOI] [PubMed] [Google Scholar]


