Abstract
2,4-Dichlorophenoxyacetic acid (2,4-D) stimulated the formation of scopoletin and scopolin in tobacco (Nicotiana tabacum L. `Bright Yellow') cell culture. It especially stimulated the uptake of scopoletin from culture medium into the cells and the glucosylation of scopoletin to its monoglucoside, scopolin. This phenomenon is peculiar to 2,4-D, in contrast to other plant hormones. 2,4-D (1 μg/ml) stimulated the glucosylation of scopoletin to scopolin by enhancing UDP-glucose:scopoletin glucosyltransferase (SGTase) activity. The enhancement of SGTase activity caused by treatment with 2,4-D was observed when the syntheses of RNA and protein were inhibited by either actinomycin-D and/or cycloheximide. However, the stimulatory effect of 2,4-D was inhibited by treatment with dinitrophenol. Furthermore, SGTase with or without treatment by 2,4-D in vivo for 24 hours, was isolated from cultured tobacco cells. The enzymes were purified about 200-fold by precipitation with (NH4)2SO4 and chromatography with Sephadex G-100, DEAE-cellulose, and hydroxyapatite. The specific activity of 2,4-D-treated SGTase was 10 times higher than that of untreated SGTase even in the purified fraction, which showed one protein band under electrophoresis. These results suggest that the enhancement of SGTase activity by 2,4-D is due to the energy-dependent activation of the enzyme already present, but not due to the de novo synthesis of the enzyme.
Full text
PDF
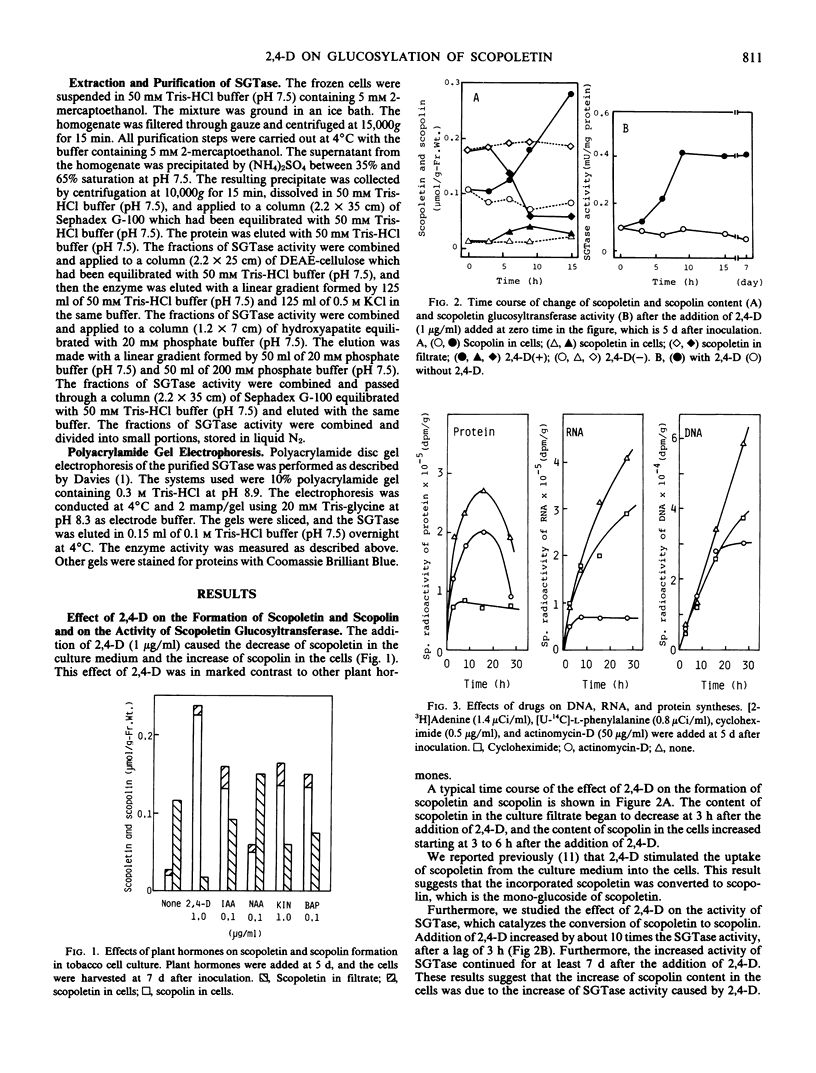
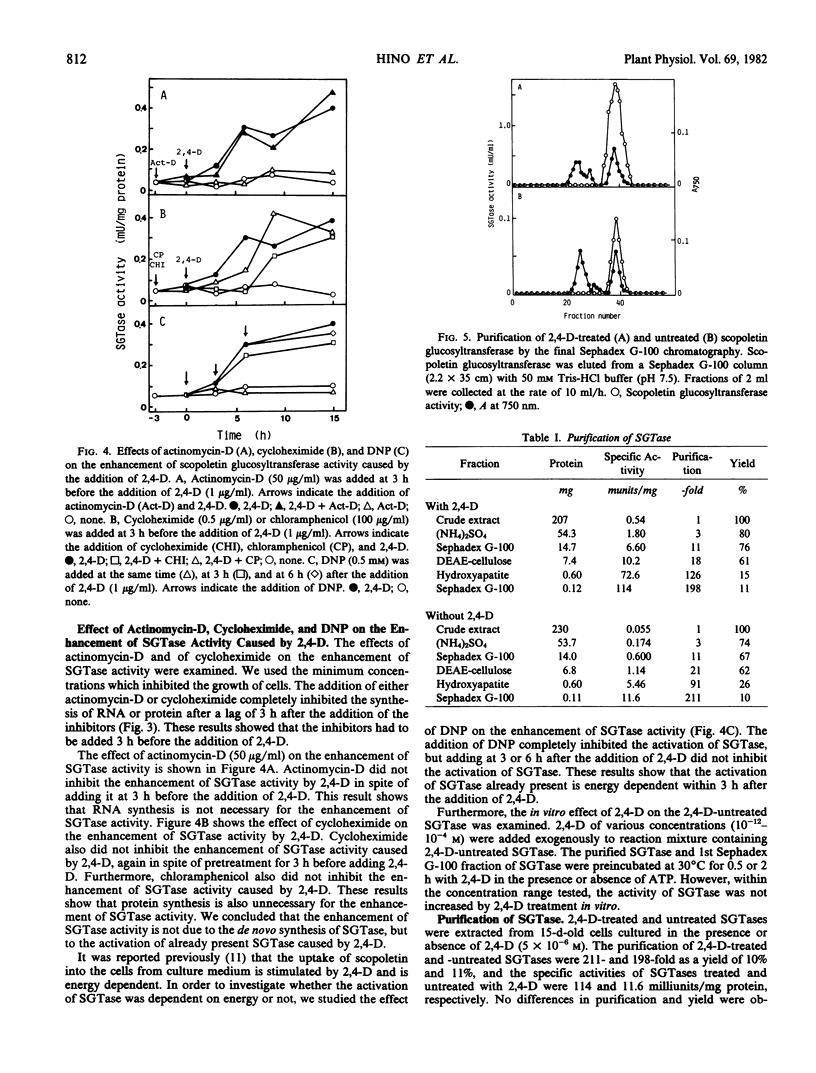

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthysse A. G., Phillips C. A protein intermediary in the interaction of a hormone with the genome. Proc Natl Acad Sci U S A. 1969 Jul;63(3):897–903. doi: 10.1073/pnas.63.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkissian I. V. Hormonal modification of plant citrate synthase in vitro. Biochem Biophys Res Commun. 1970 Sep;40(6):1385–1390. doi: 10.1016/0006-291x(70)90020-3. [DOI] [PubMed] [Google Scholar]
- Teissere M., Penon P., van Huystee R. B., Azou Y., Ricard J. Hormonal control of transcription in higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):391–402. doi: 10.1016/0005-2787(75)90274-9. [DOI] [PubMed] [Google Scholar]




