Abstract
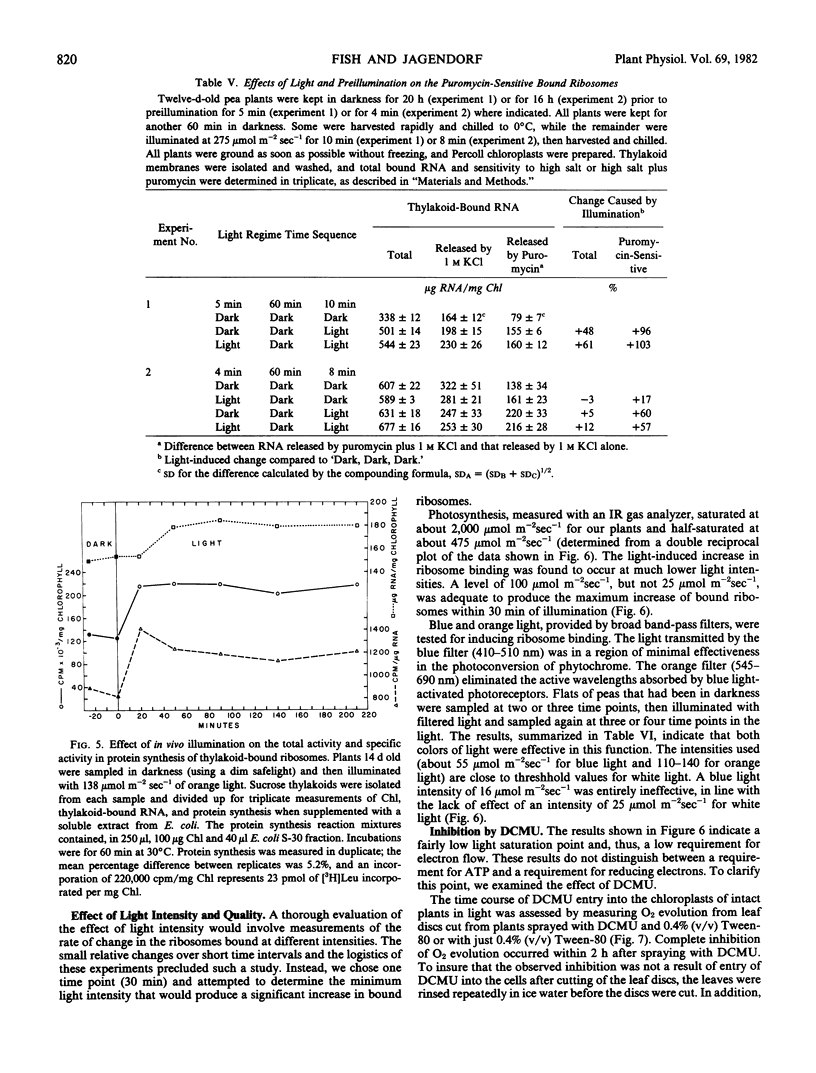
Within 8 to 10 minutes of illumination, chloroplast thylakoids of pea (Pisum sativum) became enriched 30 to 100% in ribosomes bound by nascent chains. Following (or, in some experiments, coincident with) this apprarent redistribution was a 25 to 65% increase in the total bound ribosome population, which was then maintained at this higher level during the normal light period. On transfer of plants to darkness, the bound ribosome population decreased to the lower dark level. White, blue (400 to 520 nanometers), and orange (545 to 690 nanometers) light were all effective in producing an increase in the bound ribosome population. The level of bound ribosomes in the oldest leaves of 16-day-old plants was 15-fold less than in the still-maturing leaf but was still increased by illumination.
In vivo experiments with chloramphenicol and lincomycin indicated a requirement for protein synthesis by the 70S ribosomes both for the light-induced shift to the population bound by nascent chains and for the increase in the total thylakoid-bound population. When thylakoids from plants in darkness or exposed to light for increasing periods were incubated in an Eschericia coli cell-free protein synthesizing system, 15 minutes of prior illumination in vivo produced a 60% increase in [3H]leucine incorporation. This stimulation preceded the increase in total bound ribosomes but corresponded in time to observed increases in the ribosomes bound by nascent chains.
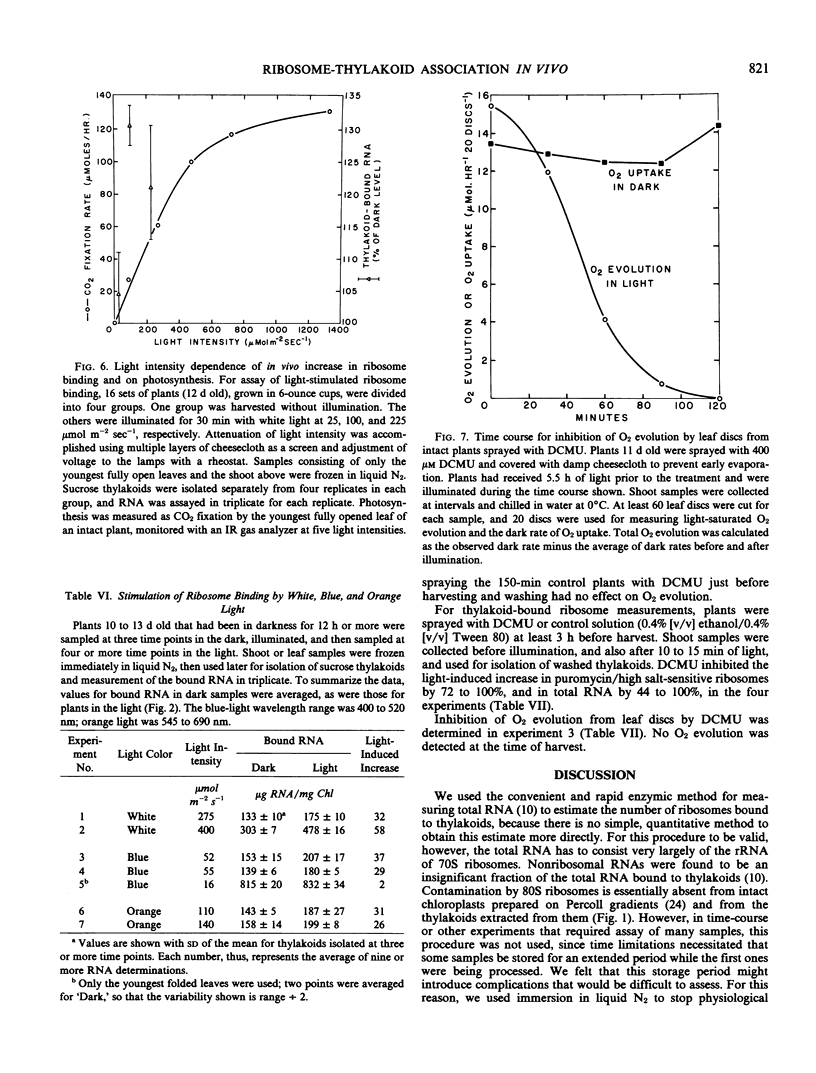
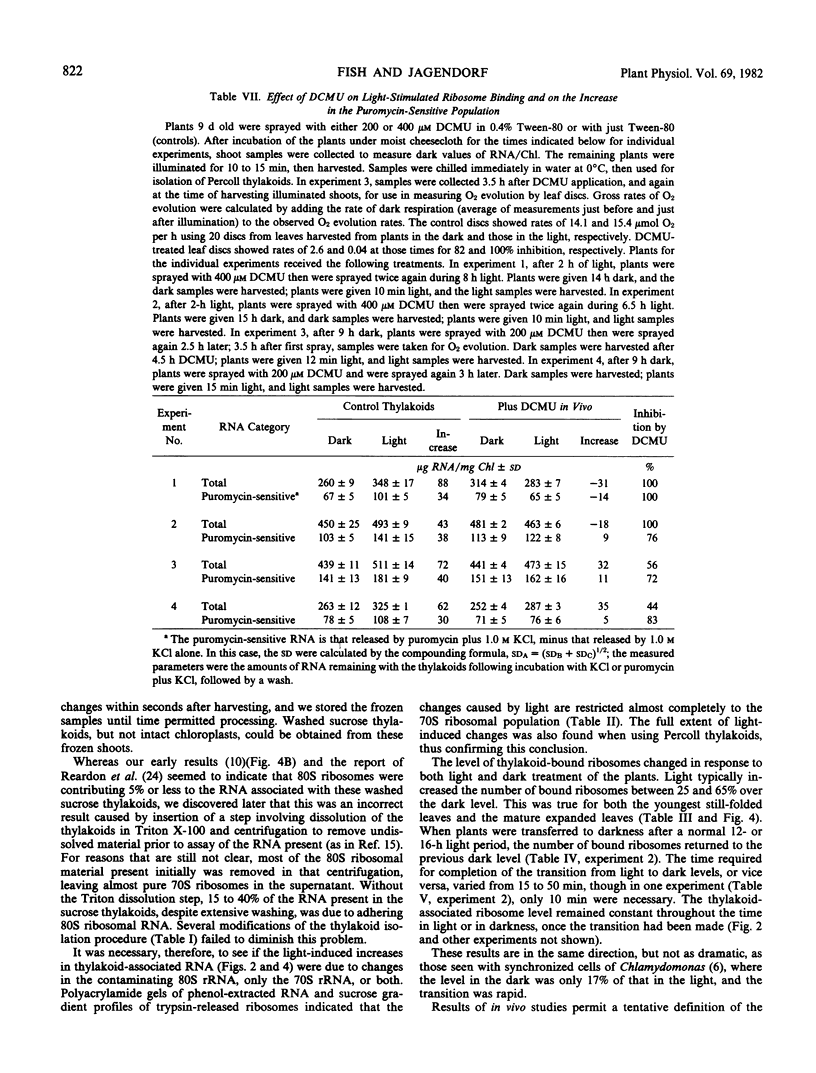
A light intensity of 100 micromoles per meter2 per second, but not 25 micromoles per meter2 per second, caused a significant increase in bound ribosomes over a 30-minute period. Strong inhibition in vivo by 3′,4′-dichlorophenyl-1, 1-dimethylurea suggests that noncyclic electron flow is essential for light-induced ribosome redistribution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alscher-Herman R., Jagendorf A. T., Grumet R. Ribosome-thylakoid association in peas: influence of anoxia. Plant Physiol. 1979 Aug;64(2):232–235. doi: 10.1104/pp.64.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher R., Patterson R., Jagendorf A. T. Activity of Thylakoid-bound Ribosomes in Pea Chloroplasts. Plant Physiol. 1978 Jul;62(1):88–93. doi: 10.1104/pp.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Attachment of chloroplast polysomes to thylakoid membranes in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1973 May;70(5):1554–1558. doi: 10.1073/pnas.70.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Periodic variations in the ratio of free to thylakoid-bound chloroplast ribosomes during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1976 Nov;71(2):497–514. doi: 10.1083/jcb.71.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk H. Rough thylakoids: polysomes attached to chloroplast membranes. J Cell Biol. 1969 Aug;42(2):582–587. doi: 10.1083/jcb.42.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L., Jagendorf A. T. A method for enzymic extraction and the measurement of chloroplast RNA. Plant Physiol. 1980 Apr;65(4):746–750. doi: 10.1104/pp.65.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E. Inhibitors of the Hill reaction. Plant Physiol. 1961 Nov;36(6):788–803. doi: 10.1104/pp.36.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Steinback K. E., Bogorad L. Comparison of the Molecular Weights of Proteins Synthesized by Isolated Chloroplasts with Those Which Appear during Greening in Zea mays. Plant Physiol. 1979 Mar;63(3):436–439. doi: 10.1104/pp.63.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoigt G., Louvel C. Etude de la regulation du développement plastidial chez Euglena gracilis. II. Localisation fonctionnelle et synthèse des particules ribosomiques chloroplastiques. Biochim Biophys Acta. 1979 Jul 26;563(2):432–444. doi: 10.1016/0005-2787(79)90062-5. [DOI] [PubMed] [Google Scholar]
- Lockshin A., Falk R. H., Bogorad L., Woodcock C. L. A coupling factor for photosynthetic phosphorylation from plastids of light- and dark-grown maize. Biochim Biophys Acta. 1971 Mar 2;226(2):366–382. doi: 10.1016/0005-2728(71)90104-6. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Margulies M. M., Michaels A. Ribosomes bound to chloroplast membranes in Chlamydomonas reinhardtii. J Cell Biol. 1974 Jan;60(1):65–77. doi: 10.1083/jcb.60.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Release of ribosomes from thylakoids and endoplasmic reticulum with trypsin. Biochim Biophys Acta. 1980;606(1):13–19. doi: 10.1016/0005-2787(80)90093-3. [DOI] [PubMed] [Google Scholar]
- Michaels A., Margulies M. M. Amino acid incorporation into protein by ribosomes bound to chloroplast thylakoid membranes: formation of discrete products. Biochim Biophys Acta. 1975 May 16;390(3):352–362. doi: 10.1016/0005-2787(75)90356-1. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J. M., Campo F. F., Arnon D. I. Photosynthetic phosphorylation as energy source for protein synthesis and carbon dioxide assimilation by chloroplasts. Proc Natl Acad Sci U S A. 1968 Feb;59(2):606–612. doi: 10.1073/pnas.59.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Stone A. B. A simplified method for preparing sucrose gradients. Biochem J. 1974 Jan;137(1):117–118. doi: 10.1042/bj1370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Price L. Filters for the Isolation of Narrow Regions in the Visible and Near-Visible Spectrum. Plant Physiol. 1953 Jan;28(1):105–114. doi: 10.1104/pp.28.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Burke J., Autz G., Jagendorf A. T. Bound Ribosomes of Pea Chloroplast Thylakoid Membranes: Location and Release in Vitro by High Salt, Puromycin, and RNase. Plant Physiol. 1981 May;67(5):940–949. doi: 10.1104/pp.67.5.940. [DOI] [PMC free article] [PubMed] [Google Scholar]



