Abstract
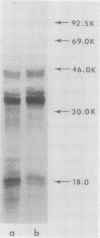
A total RNA extract was prepared from developing wheat seeds using guanidine-HCl to eliminate endogenous RNase activity. The RNA preparation, substantially free of protein, carbohydrate and DNA, was chromatographed on either a poly uridylic acid-agarose or poly guanylic acid-agarose column to yield a gliadin-enriched mRNA fraction. Only slight differences were observed for the products synthesized in a wheat germ cell-free translation system when either poly adenylic acid-enriched or cytosine-rich RNA was used as a template. These results are consistent with the high proline content of the gliadins and indicate that a large proportion of the mRNA activity in these RNA preparations is directed toward gliadin synthesis. After a second affinity chromatography step, the gliadin-enriched mRNA fraction was fractionated by two cycles on sucrose-density gradient centrifugation under denaturing conditions. The RNA sedimented as a broad band with a peak at 14S and a shoulder at the 11S region of the sucrose gradient. RNA from the peak 14S fraction translated predominantly the two major gliadin polypeptides which had molecular weights of 34,000 and 36,000. Analysis of the 14S RNA by methylmercury hydroxide-agarose gel electrophoresis revealed the presence of a predominant RNA species with a molecular size of 415,000 (1,200 nucleotides).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Greene F. C. In Vitro Synthesis of Wheat (Triticum aestivum L.) Storage Proteins. Plant Physiol. 1981 Sep;68(3):778–783. doi: 10.1104/pp.68.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane L. C., Kaesberg P. Multiple genetic components in bromegrass mosaic virus. Nat New Biol. 1971 Jul 14;232(28):40–43. doi: 10.1038/newbio232040a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Decaleya R., Rappaport L. Synthesis of a possible precursor of alpha-amylase in wheat aleurone cells. Plant Physiol. 1979 Jan;63(1):195–200. doi: 10.1104/pp.63.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Wrigley C. W., Shepherd K. W. Electrofocusing of grain proteins from wheat genotypes. Ann N Y Acad Sci. 1973 Jun 15;209:154–162. doi: 10.1111/j.1749-6632.1973.tb47526.x. [DOI] [PubMed] [Google Scholar]