Abstract
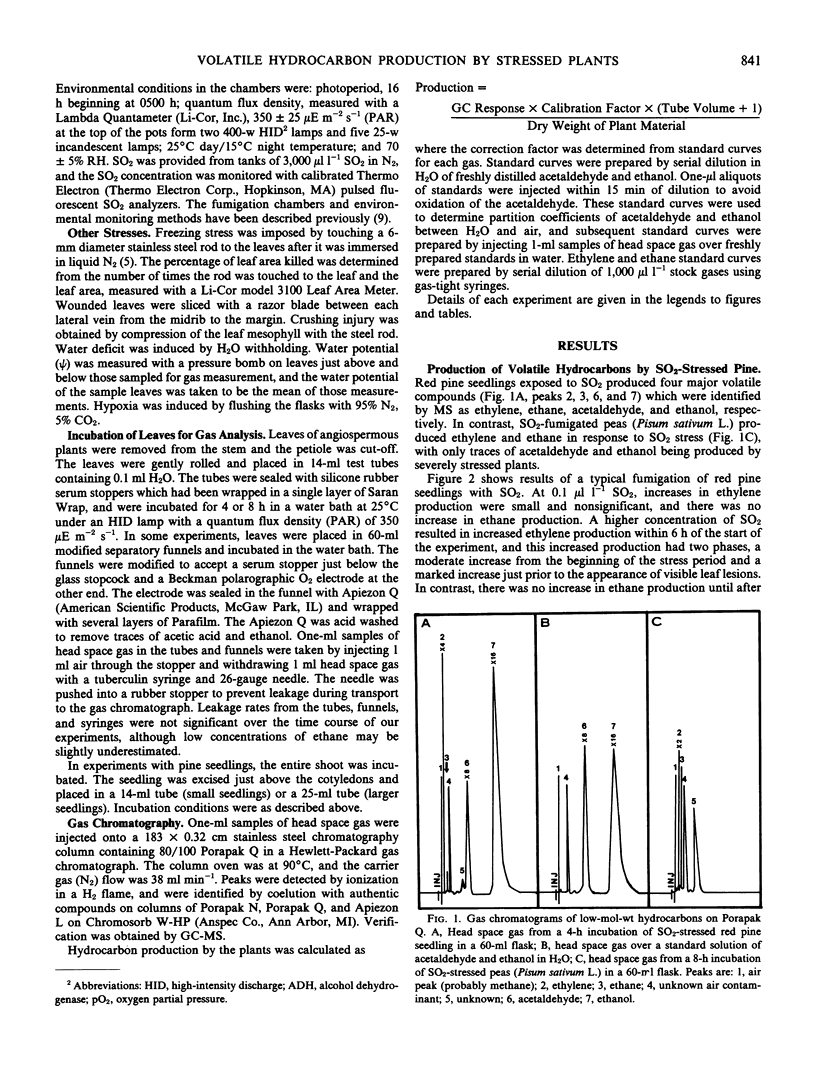
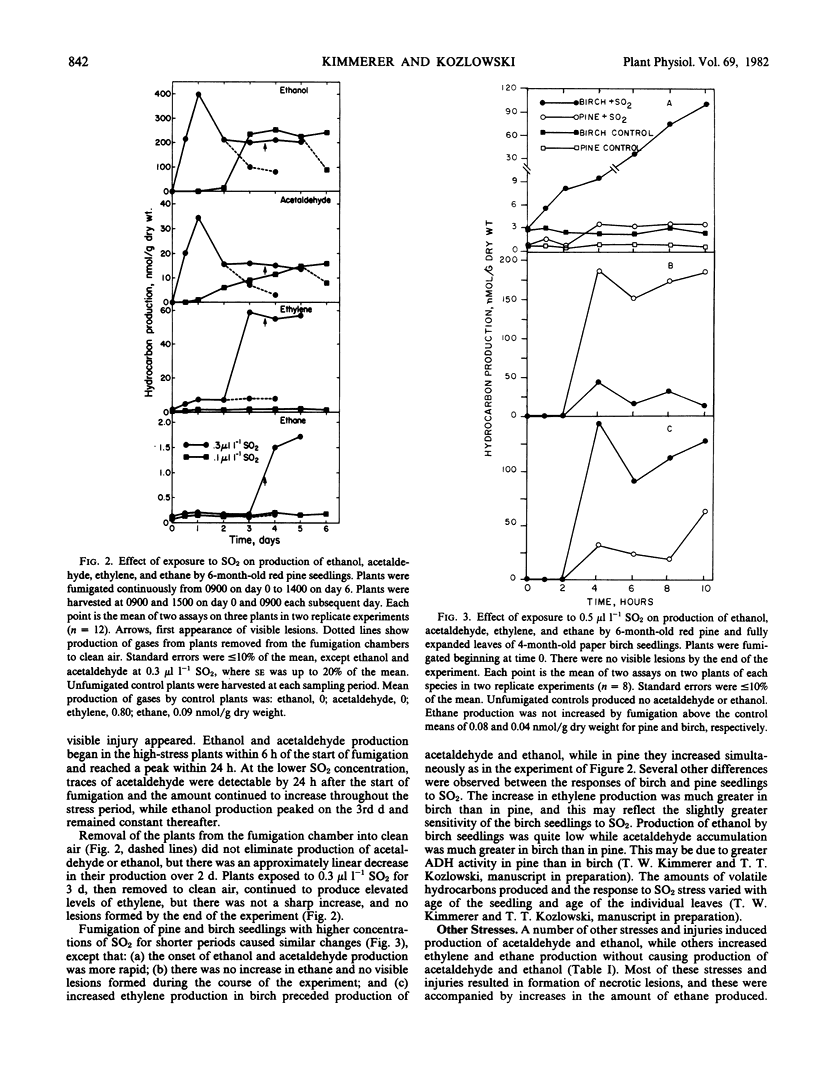
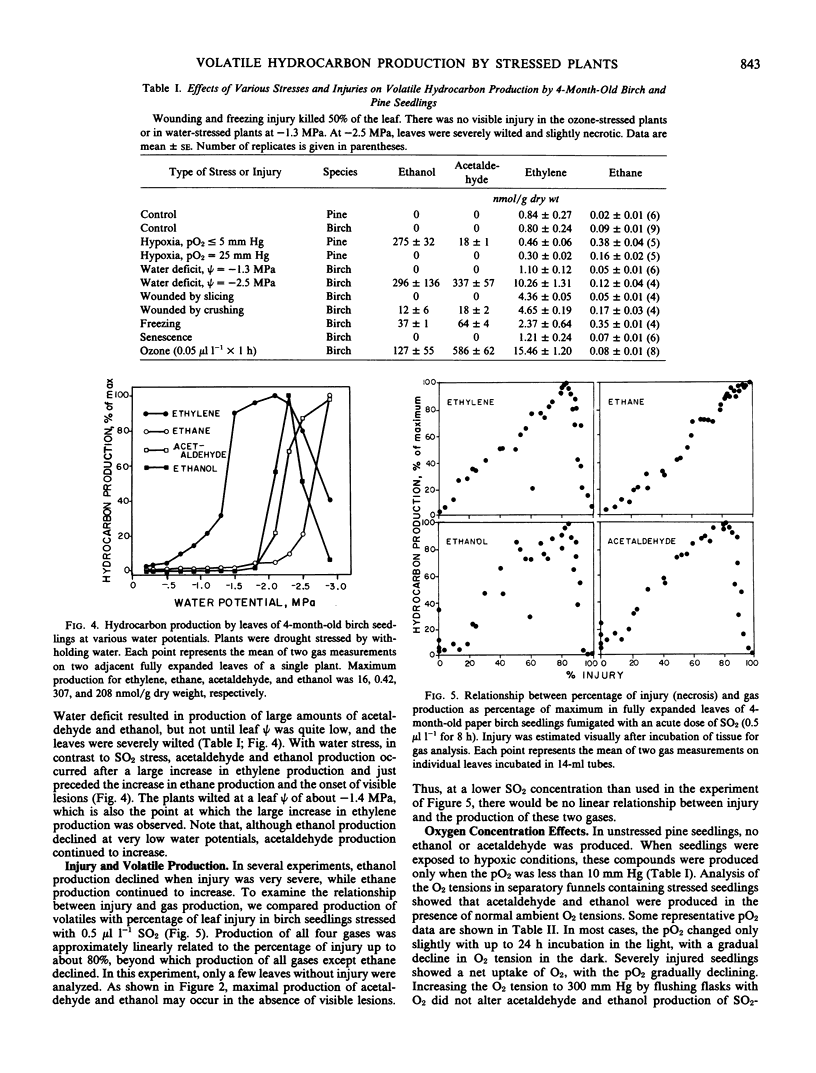
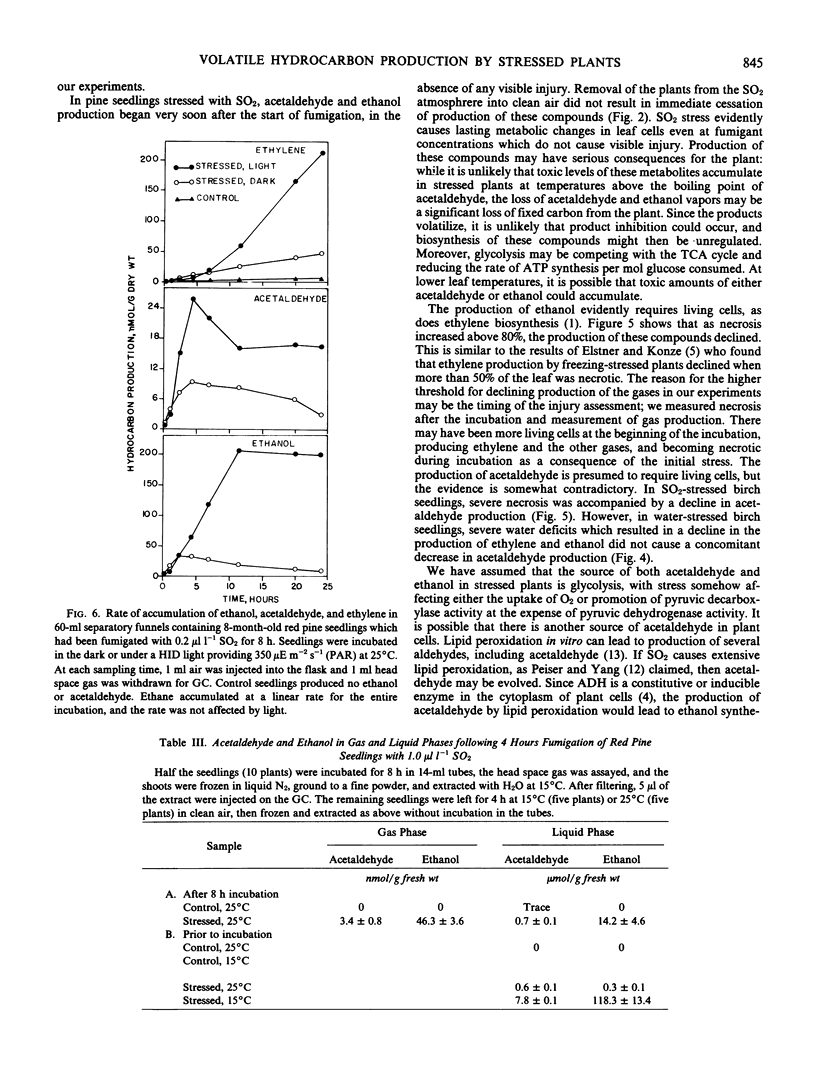
Red pine (Pinus resinosa Ait.) and paper birch (Betula papyrifera Marsh.) seedlings exposed to sulfur dioxide produced acetaldehyde and ethanol, and exhibited increased production of ethylene and ethane. Gas chromatographic measurement of head space gas from incubation tubes containing leaves or seedlings was a simple method of simultaneously measuring all four compounds. Increased ethylene production had two phases, a moderate increase from the beginning of the stress period and a large increase just prior to appearance of leaf lesions. Ethane production in SO2-stressed plants did not increase until lesions appeared. Acetaldehyde and ethanol production began within 6 hours at 0.3 microliter per liter SO2 and 24 hours at 0.1 microliter per liter SO2 and continued throughout a 6-day fumigation. Production of acetaldehyde and ethanol continued when plants were removed to clean air for up to 2 days. A higher concentration of SO2 (0.5 microliter per liter) induced acetaldehyde and ethanol production within 2 hours of the start of fumigation of birch and pine seedlings. A number of other stresses, including water deficit, freezing, and ozone exposure induced production of acetaldehyde and ethanol. Production of these compounds was not due to hypoxia, as the O2 partial pressure in the incubation vessels did not decline. Increasing the O2 partial pressure to 300 millimeters Hg did not affect production of these compounds. Production of ethylene, acetaldehyde, and ethanol declined when more than 80% of the leaf area became necrotic, while ethane production was linearly related to the percentage of necrosis. A number of woody and herbaceous plant species produced acetaldehyde and ethanol in response to freezing stress, while others did not. Measurement of these four compounds simultaneously in the gas phase may be a valuable method for monitoring plant stress, particularly air pollution stress.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressan R. A., Lecureux L., Wilson L. G., Filner P. Emission of ethylene and ethane by leaf tissue exposed to injurious concentrations of sulfur dioxide or bisulfite ion. Plant Physiol. 1979 May;63(5):924–930. doi: 10.1104/pp.63.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John W. W., Curtis R. W. Isolation and Identification of the Precursor of Ethane in Phaseolus vulgaris L. Plant Physiol. 1977 Mar;59(3):521–522. doi: 10.1104/pp.59.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Kozlowski T. T. Stomatal Conductance and Sulfur Uptake of Five Clones of Populus tremuloides Exposed to Sulfur Dioxide. Plant Physiol. 1981 May;67(5):990–995. doi: 10.1104/pp.67.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze J. R., Elstner E. F. Ethane and ethylene formation by mitochondria as indication of aerobic lipid degradation in response to wounding of plant tissue. Biochim Biophys Acta. 1978 Feb 27;528(2):213–221. doi: 10.1016/0005-2760(78)90195-9. [DOI] [PubMed] [Google Scholar]
- Nursten H. E., Williams A. A. Fruit aromas: a survey of components identified. Chem Ind. 1967 Mar 25;12:486–497. [PubMed] [Google Scholar]
- Peiser G. D., Yang S. F. Ethylene and Ethane Production from Sulfur Dioxide-injured Plants. Plant Physiol. 1979 Jan;63(1):142–145. doi: 10.1104/pp.63.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey D. T., Standley C., Field R. W. Stress ethylene evolution: a measure of ozone effects on plants. Atmos Environ. 1976;10(11):969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]
- Woodstock L. W., Taylorson R. B. Ethanol and acetaldehyde in imbibing soybean seeds in relation to deterioration. Plant Physiol. 1981 Mar;67(3):424–428. doi: 10.1104/pp.67.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]


