Abstract
Sleep problems in children are associated with poor health, behavioural and cognitive problems, as are deficiencies of long-chain omega-3 fatty acids such as docosahexaenoic acid. Theory and some evidence support a role for these fatty acids in sleep regulation, but this issue has received little formal investigation. We examined associations between blood fatty acid concentrations (from fingerstick blood samples) and subjective sleep (using an age-standardized parent questionnaire) in a large epidemiological sample of healthy children aged 7–9 years (n = 395) from mainstream UK schools. In a randomized controlled trial, we then explored whether 16-week supplementation (600 mg day−1) with algal docosahexaenoic acid versus placebo might improve sleep in a subset of those children (n = 362) who were underperforming in reading. In a randomly selected subsample (n = 43), sleep was also assessed objectively via actigraphy. In 40% of the epidemiological sample, Child Sleep Habits Questionnaire scores indicated clinical-level sleep problems. Furthermore, poorer total sleep disturbance scores were associated weakly but significantly with lower blood docosahexaenoic acid (std coeff. −0.105*) and a lower docosahexaenoic acid : arachidonic acid ratio (std coeff. −0.119**). The treatment trial showed no significant effects on subjective sleep measures. However, in the small actigraphy subsample, docosahexaenoic acid supplementation led on average to seven fewer wake episodes and 58 min more sleep per night. Cautiously, we conclude that higher blood levels of docosahexaenoic acid may relate to better child sleep, as rated by parents. Exploratory pilot objective evidence from actigraphy suggests that docosahexaenoic acid supplementation may improve children's sleep, but further investigations are needed.
Keywords: actigraphy, children, docosahexaenoic acid, omega-3, randomized controlled trial, sleep
Introduction
Numerous studies have found that good sleep is essential for children's general health, cognitive functioning and emotional wellbeing (Ivanhoe et al., 2007; Liu et al., 2012). However, epidemiological studies indicated that lack of sleep in children is common in most western countries (Smaldone et al., 2007). Substantial evidence now links poor sleep with attention deficit/hyperactivity disorder (ADHD) (Cortese et al., 2009; Wiggs et al., 2005), as well as with other difficulties with behaviour and learning in children (Dewald et al., 2010; Kopasz et al., 2010).
Similarly, adequate dietary intakes of omega-3 and omega-6 fatty acids are fundamental to health, wellbeing and cognitive performance in both children and adults, as they and their derivatives are required for the proper structure and function of almost all cells and systems in the brain and body. However, most modern, western-type diets are seriously lacking in the long-chain omega-3 fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] that are most important for physical and mental wellbeing. Deficiencies in these omega-3s have been repeatedly implicated in ADHD and related child behaviour and learning difficulties (Richardson, 2006), and some evidence suggests benefits from supplementation with omega-3 fatty acids not only in these domains (Bloch and Qawasmi, 2011; Tan et al., 2012), but also for behaviour and cognition in children from the general school population (Richardson et al., 2012). Furthermore, very low blood levels of EPA and DHA were found in a recent study of healthy UK schoolchildren (Montgomery et al., 2013).
The importance of long-chain polyunsaturated fatty acids (LC-PUFA) for sleep initiation and maintenance has long been known (Yehuda et al., 1998). For example, arachidonic acid (AA) is required as a precursor for the sleep-promoting prostaglandin D2 (Urade and Hayaishi, 2011), and DHA also appears essential for sleep regulation (Lavialle et al., 2008). Evidence suggests that the balance of DHA and AA in the pineal gland regulates melatonin production (Zaouali-Ajina et al., 1999), with higher levels of DHA relating to increased levels of melatonin (Zhang et al., 1998). In the underlying processes DHA seems needed for one of the enzymes which transforms serotonin into melatonin (Catala, 2010; Peuhkuri et al., 2012).
In line with these mechanisms, epidemiological studies find higher levels of omega-3 fatty acids associated with fewer sleep problems in infants (Cheruku et al., 2002) and adults (Papandreou, 2013) and children with ADHD (Burgess et al., 2000). DHA was also associated with less severe sleep apnea (Ladesich et al., 2011).
Trials among clinical populations suggest that LC-PUFA supplementation might improve sleep. Treatments including omega-3 and omega-6 fatty acids with other nutrients improved sleep quality (Yehuda et al., 2011) and reduced sleep disorders (Huss et al., 2010) among children with behavioural problems.
From trials using fatty acids alone, it appears that omega-3 fatty acids might be more effective for sleep problems. A recent RCT with insomnia patients found little or no benefit from supplementation with the omega-6 linoleic acid (LA) (Cornu et al., 2010). In contrast, a trial among pregnant women found improved sleep patterns in infants born following omega-3 DHA supplementation (Judge et al., 2012).
To date, however, very little is known about the relationship of LC-PUFA and sleep in healthy children. In addition, given treatment formulations that combine fatty acids (FA) with other supplements, current evidence is insufficient to demonstrate benefits from DHA supplementation alone for sleep in children.
Objectives
The aims of this study were twofold: (1) to explore associations between child sleep and biochemical measures of blood fatty acid status; and (2) to assess the effect of DHA supplementation on children's sleep.
Hypotheses
On the basis of the existing literature we predicted that: (1) higher blood concentrations of long-chain omega-3 fatty acids would be associated with better child sleep; and (2) DHA supplementation would lead to improvements in child sleep.
Methods
Study design
The Docosahexaenoic Acid (DHA) Oxford Learning and Behaviour (DOLAB) Study was designed in two stages. Stage 1 was an epidemiological study of blood fatty acid status in relation to learning and behaviour. This formed the screening stage of the subsequent intervention trial. Stage 2 was a double-blind, fixed-dose, parallel-group randomized controlled trial (RCT) of supplementation with DHA versus placebo over 16 weeks.
Population
Epidemiological sample – stage 1
Healthy children were invited from Oxfordshire (UK) mainstream primary schools between January 2009 and November 2010 on the basis of Local Authority data of all children in the county (Montgomery et al., 2013).
Children from year groups 3, 4 and 5 (typically aged 7–9 years) were eligible if they had no significant learning difficulty [i.e. no statement of ‘special educational needs’ (Department of Education, 2001)], but were thought to have below-average literacy skills according to nationally standardized assessments at age 7 [Key Stage 1 categories: 2C, 1 and W (Standards and Testing Agency, 2009)] or teachers' current judgements. Children were excluded for specific medical disorders (e.g. visual or hearing impairment), first language other than English or if schools felt their social/family circumstances would make inclusion inappropriate.
Intervention sample – stage 2
From the epidemiological sample in stage 1362 children were included if they were underperforming in reading (≤33rd-centile) on age-standardized testing (British Ability Scales II) but were otherwise healthy, and not taking fatty acid supplements, nor eating fish more than twice a week (Richardson et al., 2012). Children were excluded if taking any medication thought likely to affect behaviour (e.g. Ritalin).
A random subsample was drawn from this population for the objective measurement of sleep using actigraphy.
The inclusion and exclusion criteria reflected clinical and research experience that this age and ability group was most likely to show significant improvements in literacy skills – the primary outcome – following omega-3 supplementation, without confounding effects due to medical or behavioural conditions (Richardson and Montgomery, 2005).
At neither stage were children selected on the basis of a sleep problem. Consent for every stage of the study was obtained in advance. Fig. 1 provides details of the sample.
Figure 1.
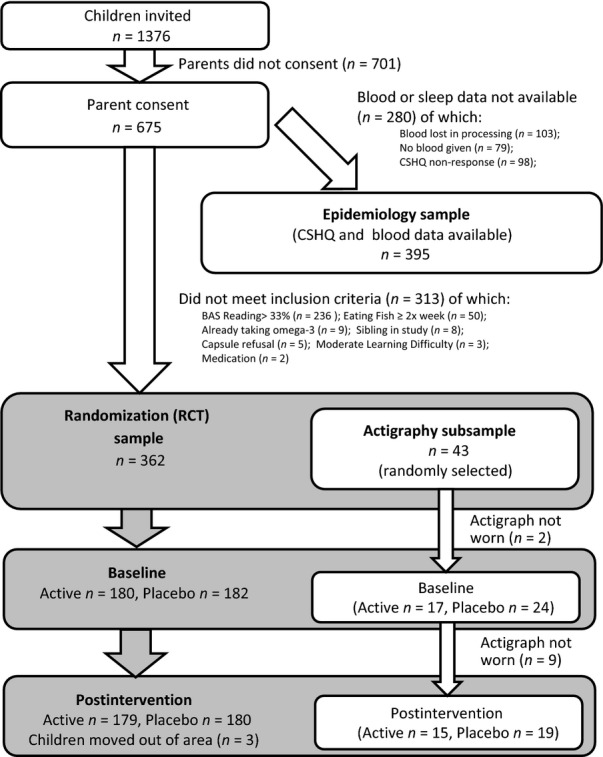
Flowchart.
Sample size
The RCT sample was powered based on the primary outcome ‘reading ability’, which indicated that 180 participants per group would provide 90% power with an α of 5%, for an effect size of r = −0.169 (Cohen's d = −0.343) (Richardson et al., 2012).
Randomization
Randomization was conducted by the University of Oxford's Centre for Statistics in Medicine, using minimization to ensure balanced treatment groups for sex and school, and including a random subsample of children whose sleep was assessed objectively using actigraphy.
Treatment
Active treatment in stage 2 was a fixed dose of 600 mg day−1 of algal DHA, delivered in three 500-mg capsules. Placebo treatment consisted of three 500-mg capsules per day containing corn/soybean oil, matched with the active treatment for taste and colour (both treatments were provided by DSM Nutritional Products, Columbia, MD, USA).
Treatments were dispensed in schools (during school days) and by parents (during weekends and school holidays), with instructions that three capsules should be taken daily with lunch. Parents and schools were given diaries to record capsule consumption, to encourage compliance and for monitoring purposes.
Outcomes: subjective and objective sleep
Sleep was measured subjectively via a parent-report questionnaire in all children at stage 1, and again postintervention for children who entered the RCT. In addition, objective sleep was measured via actigraphy at baseline and postintervention in a random subsample of the RCT children.
Subjective sleep – Child Sleep Habits Questionnaire (CSHQ)
The CSHQ is a well-validated parent-reported screening instrument for behaviourally and medically based sleep problems, designed and validated specifically for school children aged 4–10 years (Owens et al., 2000). Parents/carers are asked to rate their child's sleeping habits over a recent typical week on 45 items using a three-point scale (3 = usually/2 = sometimes/1 = rarely/never). Responses are scored to derive eight subscales (bedtime resistance; sleep duration; parasomnias; sleep disturbed breathing; night wakings; daytime sleepiness; sleep anxiety and sleep onset delay) and one total sleep disturbance scale.
Post-hoc, we considered children with total CSHQ scores >41 to be a subgroup with clinical-level sleep problems (Owens et al., 2000), i.e. meeting criteria for clinical ‘caseness’.
Objective Sleep – actigraphy and sleep diaries
Sleep duration, wake episodes and latency were recorded for 5 nights pre- and postintervention via actigraphy (MicroMini-Motionlogger™, Ambulatory Monitoring Inc., Ardsley, NY, USA), which has good properties in detecting sleep in children (Meltzer et al., 2012). To provide sufficient measurement reliability, the following variables are reported as average times/scores over five nights (Huang et al., 2011):
Sleep onset and offset times
Sleep duration in minutes (total sleep period between onset and offset)
Minutes awake between sleep onset and offset
Sleep efficiency (total sleep time divided by time in bed)
Sleep latency (minutes taken to fall asleep)
Number of night wakings after sleep onset
Actigraphy data were augmented by parents completing a sleep diary detailing their child's bedtime and rising time, any intervals when the actigraph was removed and their perception of the child's periods of sleep.
Blood fatty acid measures
Blood samples were taken by trained researchers via a finger-stick method (Bailey-Hall et al., 2008) [also see Montgomery et al. (2013)]. Blood fatty acid concentrations were assessed using gas-chromatography of total lipids in capillary whole blood (Ichihara et al., 2002), expressed as a weight percentage.
Other measures
Demographic variables
The children's age, sex, ethnicity and eligibility for free school meals (FSM) were obtained from Local Authority or school data, with FSM used as a proxy for socioeconomic status (SES).
Health status, medication and fish/supplement consumption
This information was collected by questionnaire from parents/guardians. In addition, weight was measured at baseline by researchers.
Statistical methods
Item-missing data on the CSHQ were imputed using the mean value of the respective subscale. If all observations in a subscale were missing, although ≥80% of the total CSHQ had been rated, the imputed value was the mean of that subscale for the whole sample. For calculating the total sleep disturbance score the mean of all non-duplicated items in the subscales were used, in keeping with existing literature.
The raw actigraphy data, zero-crossing-mode counts, were scored with the Sadeh algorithm (Sadeh et al., 1994) using the Action4 software (version 1.16; Ambulatory Monitoring Inc., Ardsley, NY, USA), which has well-validated benchmark properties in detecting sleep patterns compared with polysomnography (Tilmanne et al., 2009).
In the epidemiology sample, associations were examined with Spearman's rank correlations, accounting for distributional problems, and with ordinary least squares (OLS) regressions with bootstrapped standard errors controlling for demographic differences.
In the intervention sample, group differences in change-scores (postintervention minus baseline scores) were analysed using independent t-tests or Wilcoxon's rank sum tests, depending on the normality of the data (Shapiro–Wilks). These analyses were conducted on an intention-to-treat principle (ITT), i.e. including all children randomized, using mean imputation. Post-hoc subgroup analyses were also conducted on children for whom parent-ratings indicated clinical sleep problems at baseline.
All analyses were conducted in Stata version 11.2 (StataCorp., College Station, TX, USA). The syntax used is available on request.
Ethics
The study was approved by the Milton Keynes NHS Research Ethics Committee (08/H0603/49). Written informed consent was gained from parents/carers and verbal assent from the children, prior to initial screening. Trial protocol and the Consolidated Standards Of Reporting Trials (CONSORT) checklist are available at: Protocol: doi: 10.1371/journal.pone.0043909.s001; Checklist: doi: 10.1371/journal.pone.0043909.s002. The trial was registered with ClinicalTrials.gov (NCT01066182) and Controlled-Trials.com (ISRCTN99771026).
Results
Samples
Epidemiological sample – stage 1
Parents of 675 children attending 74 Oxfordshire schools consented to their child's participation (see Fig. 1). Blood samples were provided by 596 children, although 103 of these samples were either too small or were lost in the chemical preparation process. Overall, blood data from 493 children were available for analysis. Further, missing data on the subjective sleep measure meant that both blood and sleep data were available for 395 children.
Intervention sample – stage 2
A total of 362 of the children in stage 1 met inclusion criteria to take part in the randomized controlled trial assessing the effects of 16 weeks' supplementation with 600 mg day−1 of DHA or placebo.
Actigraphy subsample
It was only possible to justify this objective assessment on a random subset of children from the treatment trial, given resource constraints and the demands that actigraphy places on children and parents. Forty-three children took part in this exploratory pilot study.
Post-hoc subgroup with sleep problems
A total of 188 children were identified whose parent-rated sleep at baseline suggested sleep problems (CSHQ total sleep disturbance score >41). Additional post-hoc subgroup analyses on an ITT basis were carried out on these children for their potential clinical relevance.
Sample characteristics – demographics
Table 1 reports the demographic characteristics of the epidemiology sample (n = 395), the RCT sample (n = 362), the actigraphy sample (n = 43) and the RCT subgroup of children with sleep problems (CSHQ total sleep disturbance score >41, n = 188, includes imputation). The samples were compared for any differences in their demographic make-up.
Table 1.
Demographic characteristics of the samples analysed
| Epidemiology sample (CSHQ and blood data available, n = 395) | RCT sample (n = 362) | Actigraphy sample (n = 43) | CSHQ caseness* subgroup of RCT (n = 188) | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 177 (44.81) | 170 (46.96) | 21 (48.84) | 99 (52.38) |
| Male | 218 (55.19) | 192 (53.04) | 22 (51.16) | 89 (47.09) |
| Age in years, n (%) | ||||
| 6/7 years | 111 (28.1) | 80 (22.1) | 9 (20.93) | 39 (20.63) |
| 8 years | 143 (36.2) | 137 (37.85) | 18 (41.86) | 69 (36.51) |
| 9/10 years | 141 (35.7) | 145 (40.06) | 16 (37.21) | 80 (42.33) |
| Weight, kg | ||||
| Mean (SD) | 30.72 kg (7.68) | 30.86 kg (7.54) | 33.12 kg (8.24) | 30.72 kg (7.48) |
| Free school meals, n (%) | ||||
| Not eligible | 347 (87.85) | 289 (79.83) | 32 (74.42) | 138 (73.02) |
| Eligible | 48 (12.15) | 73 (20.17) | 11 (25.58) | 50 (26.46) |
| Ethnicity, n (%) | ||||
| White | 362 (91.65) | 330 (91.16) | 41 (95.35) | 171 (90.48) |
| Mixed | 12 (3.04) | 16 (4.42) | 1 (2.33) | 8 (4.23) |
| Asian | 6 (1.52) | 2 (0.55) | 0 (0) | 2 (1.06) |
| Black | 1 (0.25) | 1 (0.28) | 0 (0) | 1 (0.53) |
| Other | 5 (1.27) | 7 (1.93) | 1 (2.33) | 3 (1.59) |
| Missing information | 9 (2.28) | 6 (1.8) | 0 (0) | 3 (1.59) |
RCT, randomized clinical trial; SD, standard deviation; CSHQ, Child Sleep Habits Questionnaire.
‘Caseness’: clinical level sleep problems as indicated by total CSHQ score >41.
Some significant differences were found, largely reflecting the differing entry criteria for the RCT versus the epidemiological study (see Appendix 1, Table 7). For example, fewer parents of low-income families gave consent to their child taking part in the study at all. However, proportionally more such children were eligible for the RCT owing to their poor reading ability.
The significant differences relating to sleep problems appear when contrasting the subgroup of children with a CSHQ total of >41 with the overall RCT sample. This subgroup contains significantly more girls (χ2 = 5.10, P < 0.023) and more children with free school meal entitlement (χ2 = 11.42, P < 0.002). Furthermore, the actigraphy subsample had a higher average weight than the RCT sample (t = −2.08, P < 0.038).
Epidemiological results (n = 395)
Subjective sleep
Parent-rated sleep quality as measured by the CSHQ was found to be poor. The average score of the total sleep disturbance scale was 41.05 [standard deviation (SD = 6.98] and ranged up to 69 (Fig. 2). Almost 41% (n = 161) of the children had a total sleep disturbance score above the cut-off for clinical caseness (see Appendix 2, Table 8).
Figure 2.
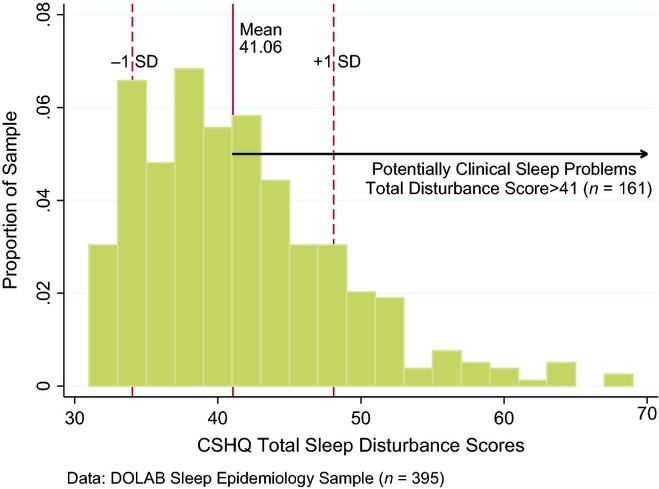
Child Sleep Habits Questionnaire (CSHQ) total sleep disturbance distribution in the epidemiology sample.
Fatty acid concentrations
The average DHA (22 : 6, n-3) concentration was 1.93% (SD = 0.54) and total omega-3 fatty acids were 4.11% (SD = 0.85) in the epidemiological sample (for full results see Appendix 3, Table 9). As discussed elsewhere, this is low from a health perspective (Montgomery et al., 2013).
Relationships between subjective sleep and fatty acids
The associations between blood fatty acid concentrations and subjective measures of sleep are reported in Table 2. DHA was related significantly and negatively to the total sleep disturbance score (ρ = −0.131, P < 0.009), bedtime resistance (ρ = −0.113, P < 0.025) and parasomnias (ρ = −0.106, P < 0.035). The ‘Omega-3 index’ (EPA + DHA) was correlated negatively with bedtime resistance (ρ = −0.109, P < 0.031). The DHA : AA ratio was associated significantly and negatively with the total sleep disturbance score (ρ = −0.133, P < 0.008). Concentrations of total omega-3 were correlated negatively with scores on the sleep duration subscale (ρ = −0.103, P < 0.04).
Table 2.
| Fatty acids | Bedtime resistance | Sleep onset delay | Sleep duration | Sleep anxiety | Night wakings | Parasomnia | Sleep disturbed breathing | Daytime sleepiness | Total sleep disturbance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | |
| DHA (22 : 6) | −0.113 | 0.025* | −0.033 | 0.515 | −0.068 | 0.181 | −0.061 | 0.224 | −0.023 | 0.644 | −0.106 | 0.035* | −0.052 | 0.304 | −0.086 | 0.086 | −0.131 | 0.009** |
| DPA (22 : 5) | −0.009 | 0.854 | 0.002 | 0.976 | −0.04 | 0.425 | 0.029 | 0.563 | 0.023 | 0.654 | 0.032 | 0.523 | 0.05 | 0.322 | 0.012 | 0.808 | 0.043 | 0.395 |
| EPA (20 : 5) | −0.018 | 0.723 | 0.02 | 0.699 | 0.002 | 0.972 | −0.019 | 0.708 | 0.067 | 0.184 | 0.092 | 0.068 | 0.014 | 0.782 | 0.017 | 0.735 | 0.063 | 0.214 |
| ALA (18 : 3) | −0.021 | 0.679 | 0.001 | 0.986 | −0.039 | 0.436 | 0.04 | 0.432 | 0.019 | 0.701 | 0.013 | 0.798 | 0.006 | 0.907 | −0.018 | 0.728 | 0.024 | 0.63 |
| Total omega-3 | −0.098 | 0.052 | −0.016 | 0.746 | −0.103 | 0.04* | −0.003 | 0.949 | 0.035 | 0.486 | −0.035 | 0.493 | 0.005 | 0.917 | −0.055 | 0.274 | −0.052 | 0.303 |
| DPA (n-6) (22 : 5) | −0.014 | 0.782 | −0.017 | 0.738 | 0.006 | 0.898 | −0.03 | 0.548 | 0.071 | 0.157 | 0.004 | 0.944 | −0.01 | 0.846 | −0.047 | 0.35 | −0.028 | 0.585 |
| Adrenic (22 : 4) | −0.009 | 0.86 | 0.013 | 0.798 | 0.049 | 0.327 | 0.002 | 0.971 | 0.044 | 0.385 | 0.002 | 0.966 | 0.071 | 0.16 | −0.048 | 0.346 | 0.005 | 0.92 |
| AA (20 : 4) | −0.045 | 0.373 | −0.022 | 0.657 | −0.01 | 0.848 | −0.009 | 0.865 | 0.036 | 0.47 | 0.005 | 0.921 | 0.018 | 0.726 | −0.044 | 0.387 | −0.027 | 0.595 |
| DGLA (20 : 3) | 0.016 | 0.749 | 0.032 | 0.526 | 0.04 | 0.428 | −0.011 | 0.83 | −0.007 | 0.887 | 0.034 | 0.497 | −0.035 | 0.488 | −0.016 | 0.746 | 0.007 | 0.891 |
| GLA (18 : 3) | −0.029 | 0.572 | 0.031 | 0.542 | 0.005 | 0.923 | 0.044 | 0.385 | 0.007 | 0.883 | 0.01 | 0.844 | −0.001 | 0.99 | −0.028 | 0.584 | 0.016 | 0.748 |
| LA (18 : 2) | 0.000 | 0.995 | −0.014 | 0.777 | −0.042 | 0.403 | 0.052 | 0.303 | 0.002 | 0.975 | −0.057 | 0.26 | −0.083 | 0.1 | −0.077 | 0.124 | −0.055 | 0.279 |
| Total omega-6 | −0.027 | 0.586 | −0.02 | 0.689 | −0.022 | 0.657 | 0.044 | 0.381 | 0.024 | 0.637 | −0.031 | 0.542 | −0.037 | 0.463 | −0.068 | 0.179 | −0.041 | 0.422 |
| Omega-3 index§ | −0.109 | 0.031* | −0.026 | 0.602 | −0.053 | 0.293 | −0.061 | 0.224 | 0.007 | 0.896 | −0.051 | 0.313 | −0.038 | 0.454 | −0.064 | 0.206 | −0.089 | 0.076 |
| Ratio DHA : AA | −0.093 | 0.064 | −0.037 | 0.466 | −0.085 | 0.093 | −0.058 | 0.25 | −0.053 | 0.293 | −0.094 | 0.062 | −0.078 | 0.123 | −0.08 | 0.111 | −0.133 | 0.008** |
| Ratio EPA : AA | 0.029 | 0.567 | 0.04 | 0.431 | −0.001 | 0.989 | −0.011 | 0.831 | 0.038 | 0.456 | 0.084 | 0.095 | 0.012 | 0.818 | 0.042 | 0.409 | 0.078 | 0.124 |
Fatty acid acronyms: ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid; CSHQ, Child Sleep Habits Questionnaire.
n = 395.
P < 0.01;
P < 0.05.
Spearman's rank correlations.
The strongest physiological rationale for this research exists for omega-3 and omega-6 [long-chain polyunsaturated fatty acids (LC-PUFA)], hence only results for these fatty acids are reported here.
This broadly corresponds to the ‘Omega-3 index’, a well-validated measure of cardiovascular risk in adults. However, values in this study were from whole blood, whereas the ‘Omega-3 index’ is typically calculated from EPA + DHA in red cell membranes (Harris and Klurfeld, 2011).
Subjective sleep and fatty acids – controlling for demographic differences
In Table 3 we report the associations between subjective sleep and blood fatty acids, while controlling for demographic differences between the children in terms of age, gender, weight and SES. Consistent with the uncontrolled correlation results we found that:
Table 3.
Epidemiology – standardized regression results† for subjective sleep (CSHQ) and blood fatty acids (LC-PUFA)‡ controlling for demographics§
| Fatty acids | Bedtime resistance | Sleep onset delay | Sleep duration | Sleep anxiety | Night wakings | Parasomnia | Sleep disturbed breathing | Daytime sleepiness | Total sleep disturbance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | Coeff. | P-value | |
| DHA (22 : 6) | −0.078 | 0.052 | −0.027 | 0.6 | −0.082 | 0.076 | −0.05 | 0.294 | −0.015 | 0.757 | −0.078 | 0.131 | −0.039 | 0.447 | −0.057 | 0.225 | −0.105 | 0.026* |
| DPA (22 : 5) | 0.076 | 0.375 | 0.009 | 0.87 | −0.042 | 0.401 | 0.087 | 0.063 | 0.008 | 0.877 | 0.107 | 0.043* | 0.096 | 0.056 | 0.051 | 0.463 | 0.093 | 0.057 |
| EPA (20 : 5) | −0.019 | 0.667 | 0.057 | 0.313 | −0.032 | 0.506 | 0.032 | 0.622 | 0.105 | 0.146 | 0.163 | 0.007** | 0 | 0.996 | 0.003 | 0.948 | 0.063 | 0.284 |
| ALA (18 : 3) | 0.012 | 0.839 | 0 | 0.996 | −0.077 | 0.094 | 0.114 | 0.047* | 0.077 | 0.279 | −0.003 | 0.964 | −0.037 | 0.347 | −0.014 | 0.789 | 0.003 | 0.96 |
| Total omega-3 | −0.023 | 0.646 | −0.001 | 0.979 | −0.097 | 0.068 | 0.048 | 0.377 | 0.056 | 0.368 | 0.033 | 0.565 | 0.002 | 0.973 | −0.017 | 0.741 | −0.011 | 0.848 |
| DPA (n-6) (22 : 5) | 0.044 | 0.367 | −0.053 | 0.303 | 0.024 | 0.638 | 0.017 | 0.722 | 0.072 | 0.173 | 0.043 | 0.377 | −0.024 | 0.719 | 0.014 | 0.781 | 0.037 | 0.48 |
| Adrenic (22 : 4) | 0.034 | 0.483 | −0.012 | 0.825 | 0.086 | 0.121 | 0.02 | 0.686 | 0.044 | 0.408 | −0.007 | 0.887 | 0.07 | 0.226 | −0.022 | 0.64 | 0.032 | 0.539 |
| AA (20 : 4) | 0.017 | 0.724 | −0.025 | 0.643 | 0.03 | 0.583 | 0.012 | 0.814 | 0.03 | 0.507 | 0.002 | 0.963 | 0.027 | 0.654 | 0 | 0.993 | 0.011 | 0.816 |
| DGLA (20 : 3) | 0.053 | 0.264 | 0.045 | 0.391 | 0.059 | 0.241 | 0.007 | 0.89 | −0.022 | 0.63 | 0.033 | 0.455 | −0.028 | 0.567 | 0.023 | 0.661 | 0.043 | 0.37 |
| GLA (18 : 3) | 0.011 | 0.848 | 0.028 | 0.612 | −0.001 | 0.99 | 0.092 | 0.077 | −0.001 | 0.978 | −0.002 | 0.974 | −0.037 | 0.353 | 0.005 | 0.914 | 0.017 | 0.748 |
| LA (18 : 2) | 0.067 | 0.169 | −0.04 | 0.455 | 0.018 | 0.718 | 0.031 | 0.538 | −0.004 | 0.929 | −0.052 | 0.326 | −0.04 | 0.588 | −0.024 | 0.658 | −0.012 | 0.827 |
| Total omega-6 | 0.062 | 0.2 | −0.032 | 0.551 | 0.042 | 0.426 | 0.039 | 0.434 | 0.016 | 0.747 | −0.024 | 0.653 | −0.014 | 0.835 | −0.015 | 0.779 | 0.01 | 0.852 |
| Omega-3 index | −0.068 | 0.089 | −0.003 | 0.949 | −0.076 | 0.108 | −0.03 | 0.58 | 0.021 | 0.713 | −0.012 | 0.839 | −0.031 | 0.534 | −0.045 | 0.351 | −0.065 | 0.215 |
| Ratio DHA : AA | −0.09 | 0.018* | −0.019 | 0.709 | −0.1 | 0.018* | −0.06 | 0.172 | −0.033 | 0.523 | −0.085 | 0.092 | −0.055 | 0.289 | −0.062 | 0.185 | −0.119 | 0.009** |
| Ratio EPA : AA | −0.039 | 0.359 | 0.051 | 0.38 | −0.046 | 0.319 | 0.018 | 0.767 | 0.087 | 0.23 | 0.162 | 0.007** | −0.004 | 0.944 | −0.009 | 0.864 | 0.047 | 0.43 |
Fatty acid acronyms: ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid; CSHQ, Child Sleep Habits Questionnaire.
n = 395.
P < 0.01;
P < 0.05.
OLS regressions with bootstrapped standard errors (seed 14 141, 500 repetitions) and standardized coefficients: a 1 standard deviation change in the fatty acid corresponds to the reported change in the sleep measure.
The strongest physiological rationale for this research exists for omega-3 and omega-6 [long-chain polyunsaturated fatty acids (LC-PUFA)], hence only results for these fatty acids are reported here.
The analyses control for gender, age in years, weight, socio-economic status (entitlement to free school meals); unstandardized results including coefficients for the demographic variables are listed in Appendix 5, Tables19.
DHA was related significantly and negatively with the total sleep disturbance score (std coeff. −0.105, P < 0.026); and
The DHA : AA ratio had a significant and negative association with the total sleep disturbance score (std coeff. −0.119, P < 0.009).
The other correlations were not robust in the light of demographic differences in the sample. However, additional significant relationships after controlling for these differences were found for the DHA : AA ratio with bedtime resistance (std coeff. −0.09, P < 0.018) and sleep duration (std coeff. −0.1, P < 0.018).
Contrary to expectations, positive relationships were found for DPA (n-3) and parasomnias (std coeff. 0.107, P < 0.043), EPA and parasomnias (std coeff. 0.163, P < 0.007) and the EPA : AA ratio and parasomnias (std coeff. 0.162, P < 0.007). Additionally, the short-chain omega-3 ALA was associated positively with sleep anxiety (0.114, P < 0.047; for full regression results see Appendix 5, Tables19).
RCT results (n = 362)
Tables4 and 5 report the baseline and postintervention means, change scores and statistical test results for group differences for both the subjective and objective sleep measures on an ITT basis.
Table 4.
CSHQ mean scores and group comparisons (ITT, n = 362)
| Group mean scores on CSHQ subscales (SD) | Change scores (SD) Baseline versus postintervention | Comparisons Active versus placebo 0–16-week Diff. | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (16 weeks) | |||||||
| Active (n = 180) Imputed n = 34 | Placebo (n = 182) Imputed n = 41 | Active (n = 180) Imputed n = 38 | Placebo (n = 182) Imputed n = 35 | Active (n = 180) | Placebo (n = 182) | Z-value | P-value | |
| Bedtime resistance | 7.12 (1.885) | 7.38 (1.895) | 6.99 (1.575) | 7.33 (2.02) | −0.13 (1.845) | −0.05 (1.856) | 0.998 | 0.318 |
| Sleep onset delay | 1.67 (0.72) | 1.66 (0.716) | 1.66 (0.676) | 1.62 (0.677) | −0.01 (0.695) | −0.04 (0.66) | 0.71 | 0.478 |
| Sleep duration | 3.94 (1.269) | 3.77 (1.126) | 3.94 (1.214) | 3.88 (1.202) | 0.00 (1.338) | 0.11 (1.198) | −0.724 | 0.469 |
| Sleep anxiety | 5.13 (1.22) | 5.34 (1.573) | 5.10 (1.303) | 5.09 (1.469) | −0.03 (1.396) | −0.26 (1.358) | 1.163 | 0.245 |
| Night wakings | 3.58 (0.952) | 3.69 (0.997) | 3.46 (0.721) | 3.63 (1.033) | −0.12 (0.869) | −0.06 (1.026) | 0.19 | 0.849 |
| Parasomnias | 8.84 (1.842) | 8.6 (1.578) | 8.58 (1.457) | 8.6 (1.432) | −0.26 (1.755) | 0.00 (1.565) | −1.294 | 0.196 |
| Sleep disturbed breathing | 3.57 (1.016) | 3.49 (0.754) | 3.51 (0.909) | 3.45 (0.768) | −0.06 (0.974) | −0.05 (0.791) | 0.232 | 0.816 |
| Daytime sleepiness | 9.74 (2.763) | 9.93 (2.81) | 9.56 (2.555) | 9.69 (2.766) | −0.18 (2.284) | −0.24 (2.753) | −0.128 | 0.898 |
| Total sleep disturbance | 41.3 (6.842) | 41.38 (6.177) | 40.48 (6.166) | 40.87 (6.084) | −0.82 (6.032) | −0.51 (5.364) | −0.682 | 0.495 |
ITT, mean imputation of missing observations; CSHQ, Child Sleep Habits Questionnaire; SD, standard deviation.
Table 5.
Actigraphy mean scores and group comparisons (ITT, n = 43)
| Group means of the actigraphy measures (SD) | Change scores (SD) Baseline versus postintervention | Comparisons† Active versus placebo 0–16-week Diff. | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (16 weeks) | |||||||
| Active (n = 19) Imputed n = 2 | Placebo (n = 24) Imputed n = 0 | Active (n = 19) Imputed n = 4 | Placebo (n = 24) Imputed n = 5 | Active (n = 19) | Placebo (n = 24) | Z/t-value | P-value | |
| Time child fell asleep | 20:27 (43 min) | 20:32 (51 min) | 21:3 (50 min) | 20:47 (61 min) | 36 min (45 min) | 15 min (69 min) | Z: 1.333 | 0.183 |
| Time child woke up | 7:31 (69 min) | 7:18 (72 min) | 7:31 (52 min) | 7:21 (76 min) | 0 min (91 min) | 3 min (66 min) | t: −0.12 | 0.909 |
| Minutes sleep duration | 654 min (59 min) | 640 min (49 min) | 639 min (52 min) | 611 min (66 min) | −16 min (87 min) | −29 min (53 min) | t: 0.6 | 0.551 |
| Minutes awake | 157 min (57 min) | 140 min (64 min) | 96 min (39 min) | 123 min (57 min) | −61 min (74 min) | −17 min (80 min) | t: −1.88 | 0.068+ |
| Minutes of sleep | 497 min (84 min) | 500 min (68 min) | 543 min (64 min) | 488 min (89 min) | 46 min (114 min) | −12 min (95 min) | Z: 2.177 | 0.029* |
| Sleep efficiency (ratio) | 0.76 (0.092) | 0.78 (0.098) | 0.85 (0.061) | 0.8 (0.098) | 0.09 (0.117) | 0.01 (0.132) | t: 2.000 | 0.052+ |
| Sleep latency (min) | 15 min (19 min) | 27 min (29 min) | 14 min (22 min) | 25 min (33 min) | −1 min (33 min) | −2 min (38 min) | Z: .379 | 0.704 |
| Wake episodes | 22.92 (6.622) | 19.29 (8.459) | 12.86 (3.93) | 15.78 (6.521) | −10.06 (8.39) | −3.52 (8.092) | t: −2.59 | 0.013* |
ITT, mean imputation of missing observations.
P < 0.05,
P < 0.1.
Some variables are normal, some non-normally distributed; if Shapiro – Wilk was P > 0.05, a t-test was used, otherwise Wilcoxon's rank sum.
DHA supplementation and changes in subjective sleep
During the 16-week intervention, slight but non-significant improvements were seen in both groups for all but one CSHQ subscale (sleep duration).
DHA supplementation and changes in objective sleep
Actigraphy results showed that total sleep duration increased by 58 min more in the active group than in controls (P < 0.029; Fig. 3). Correspondingly, the reduction in wake episodes was significantly greater in children on active treatment (seven fewer wake episodes per night, P < 0.013).
Figure 3.
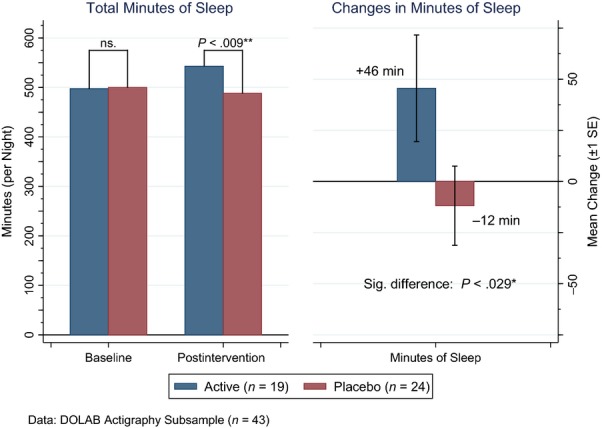
Total min sleep: baseline, postintervention and change by treatment group.
With the less conservative alpha-level applied for exploratory analyses, group differences were also evident for changes in min awake (active versus placebo: −44 min, P < 0.068) and overall sleep efficiency (+8%, P < 0.052).
Post-hoc RCT subgroup results for children with clinical sleep problems (n = 188)
DHA supplementation and changes in subjective sleep
Approximately 52% (188) of the RCT children were found to have clinical-level sleep problems at baseline, i.e. CSHQ total sleep disturbance score >41 (see Appendix 2, Table 8). Table 6 shows the effects of treatment on CSHQ scores in this post-hoc subgroup. Ratings on the parasomnias subscale fell significantly more in the active treatment group than controls (P < 0.039), as did total sleep disturbance (P < 0.049).
Table 6.
Post-hoc subgroup analysis – caseness: CSHQ mean scores and group comparisons (ITT, CSHQ total sleep disturbance >41, n = 188)
| Group mean scores on CSHQ subscales (SD) | Change scores (SD) Baseline versus postintervention | Comparisons† Active versus placebo 0–16-week Diff. | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (16 weeks) | |||||||
| Active (n = 94) Imputed n = 39 | Placebo (n = 94) Imputed n = 25 | Active (n = 94) Imputed n = 34 | Placebo (n = 94) Imputed n = 23 | Active (n = 94) | Placebo (n = 94) | Z-value | P-value | |
| Bedtime resistance | 7.94 (2.234) | 8.17 (2.206) | 7.43 (1.892) | 8.01 (2.366) | −0.51 (2.382) | −0.16 (2.286) | −0.634 | 0.526 |
| Sleep onset delay | 1.91 (0.694) | 1.91 (0.699) | 1.76 (0.675) | 1.83 (0.7) | −0.15 (0.764) | −0.08 (0.714) | −0.162 | 0.871 |
| Sleep duration | 4.53 (1.379) | 4.26 (1.265) | 4.22 (1.395) | 4.17 (1.331) | −0.32 (1.515) | −0.09 (1.359) | −1.519 | 0.129 |
| Sleep anxiety | 5.56 (1.276) | 5.95 (1.766) | 5.5 (1.503) | 5.52 (1.724) | −0.06 (1.683) | −0.43 (1.476) | 0.904 | 0.366 |
| Night wakings | 3.92 (1.113) | 4 (1.053) | 3.59 (0.783) | 3.88 (1.081) | −0.33 (0.948) | −0.12 (1.233) | −0.429 | 0.668 |
| Parasomnias | 9.48 (1.951) | 9.25 (1.734) | 8.85 (1.586) | 9.21 (1.56) | −0.63 (1.982) | −0.04 (2.021) | −2.059* | 0.039* |
| Sleep disturbed breathing | 3.86 (1.202) | 3.68 (0.858) | 3.68 (1.081) | 3.6 (0.946) | −0.18 (1.187) | −0.08 (0.97) | −0.231 | 0.817 |
| Daytime sleepiness | 11.24 (2.542) | 11.24 (2.866) | 10.49 (2.707) | 10.49 (2.75) | −0.75 (2.548) | −0.74 (3.035) | −0.314 | 0.753 |
| Total sleep disturbance | 45.98 (5.952) | 45.62 (5.347) | 43.04 (6.646) | 44.02 (5.992) | −2.94 (6.834) | −1.59 (6.404) | −1.963* | 0.049* |
ITT: mean imputation of missing observations. CSHQ, Child Sleep Habits Questionnaire; SD, standard deviation.
P < 0.05.
Wilcoxon's rank sum test.
DHA supplementation and changes in objective sleep
The sample size for the subgroup of children with clinical-level sleep problems for which actigraphy results are available is very small (active = 11, placebo = 9). The analyses for this subgroup did not detect any significant changes following the intervention (see Appendix 4, Table 10).
Discussion
To the authors’ knowledge, this is the first published study investigating associations between LC-PUFA status and sleep, and the effect of DHA supplementation on sleep, in healthy children. This sample was selected for below-average reading at age 7, but had no medical diagnoses or learning disabilities. Previous research has demonstrated clear links between children's sleep and their physiological, mental and emotional health as well as their cognition and behaviour (Dewald et al., 2010; Ivanhoe et al., 2007; Liu et al., 2012). Similarly, existing evidence points to associations between children's omega-3 LC-PUFA status and their cognition, as well as their behaviour.
In terms of the objectives of this study, our main findings are as follows:
- In this large epidemiological sample of healthy children:
- Forty per cent of these children appear to have a clinical-level sleep problem, as defined by the cut-off for clinical caseness on the CSHQ. This is consistent with other findings for the general child population in the United States and Europe (Smaldone et al., 2007).
- Higher blood DHA status was associated significantly with better sleep (std coeff.: −0.105, P < 0.026), as assessed by the total sleep disturbance scores from the CSHQ even when controlling for demographic variables. Correspondingly, associations between the DHA : AA ratio and total sleep disturbance score were found to be significant (std coeff.: −0.119, P < 0.009).
- No consistent associations were found between CSHQ scores and other fatty acids when controlling for demographic variables.
- In the subsequent RCT of supplementation with DHA:
- The pilot actigraphy subsample results indicated significant group differences in children's sleep following a 16-week treatment with 600 mg day−1 of DHA. Sleep duration improved by 58 min more in children receiving active treatment versus placebo, with fewer and shorter night-wakings and corresponding improvement in sleep efficiency.
- No significant effects of supplementation were evident on the subjective sleep measures for the whole sample. However, in a post-hoc subgroup with clinical-level sleep problems, DHA treatment did improve total sleep disturbance scores (Z = −1.963, P < 0.05).
Strengths and limitations
The findings presented in this paper should be interpreted cautiously, for a number of reasons. First, the epidemiological analysis finds relatively few statistically significant results in view of the number of relationships tested. While Bonferroni corrections would be inappropriate (owing to the strong intercorrelations always found between individual blood fatty acid values), further studies are warranted to confirm the inverse association observed here between blood DHA status and subjective sleep problems. In order to distinguish individual differences more clearly, these studies should also collect a wider range of demographic variables associated with sleep outcomes, such as waist circumference.
Secondly, the results from the intervention study must be regarded as very preliminary, as benefits of DHA supplementation were found only for objective measures of sleep, and these were available only for a small subset of the RCT sample.
Thirdly, it is important to emphasize that the children in the study were not selected for sleep problems, hence these results cannot be assumed to generalize to a clinically referred population. Conversely, the fact that this was a general school population sample, with 60% of children not reported to have clinical-level sleep problems at baseline, makes it all the more impressive that any treatment effects were detected.
Fourthly, there were inevitably some missing data, which always requires some caution in the interpretation of findings. In the RCT, around 20% of children had missing data for one or other of the sleep measures postintervention, which is broadly in line with other studies (see, for example, Price et al., 2012).
A major strength of this study is its large, non-clinical sample of children, broadly representative of the more general population of UK children aged 7–9 years. Another strength is the use of fingerstick blood samples, allowing objective measurement of blood fatty acid status. In addition, sleep was assessed both subjectively (via a well-validated and age-standardized parent rating scale) in the whole sample and objectively (via actigraphy) in a subset.
Implications
The improvements in sleep found in this study may be both clinically important and comparable to the improvements achieved by current front-line interventions for child sleep problems (Edinger et al., 2001; Weiss et al., 2006). These kinds of improvements could, if replicated, be expected to lead to significant benefits in physical health, mood, behaviour and cognition, including educational achievement. Indeed, improvements in both behaviour and learning were found in the DOLAB RCT (Richardson et al., 2012).
While the actigraphy can only provide simple measures of sleep duration and timing, it seems likely that the changes observed here would normally be accompanied by qualitative improvements in sleep architecture. Further studies using polysomnography would, of course, be needed to elucidate potential mechanisms.
These results are in line with the existing literature, as there are already some reports of associations between poor sleep outcomes and low blood concentrations of omega-3 LC-PUFA (Papandreou, 2013; Stevens et al., 1995), as well as some preliminary research indicating benefits for sleep from fatty acid supplementation in children with behavioural problems (Yehuda et al., 2011).
Importantly, these findings also fit well with theoretical mechanisms, as DHA is the main omega-3 found in the brain and fatty acids are involved in sleep regulation via melatonin metabolism (Lavialle et al., 2008), with increased DHA : AA ratios related directly to the increased secretion of melatonin (Peuhkuri et al., 2012). This provides a potential physiological explanation for our finding that DHA supplementation improves sleep continuity and duration.
As noted earlier, the epidemiology sample broadly matched the wider population of children in the United Kingdom, giving good reason to believe that the association found here between low blood DHA and sleep problems would be generalizable. By contrast, the RCT sample involved those children who were underperforming in reading (<33rd-centile), hence generalizability to the wider UK population cannot be assumed.
However, the treatment effect observed in the pilot actigraphy for these children was substantial, at almost 1 h of extra sleep per night on objective measurement. The physical, mental and social benefits that would be expected to follow from such an improvement are profound. In the post-hoc subgroup with sleep problems (CSHQ > 41), the effects on the subjective total sleep disturbance score appear to be positive and in line with the actigraphy results. This is suggestive that future work in a clinical sample of children with sleep problems would be justified.
Conclusion
DHA supplementation has long been shown to be safe and well-tolerated, as we have already confirmed in this sample (Richardson et al., 2012). Furthermore, we also found very low blood levels of this key fatty acid in a larger sample of healthy UK schoolchildren, indicating that omega-3 deficiencies are widespread in the general population (Montgomery et al., 2013). For these reasons, further investigations of the effects of this simple nutritional intervention on child sleep are needed, ideally in both clinical and general populations.
Acknowledgments
The authors owe thanks to: Oxfordshire County Council and the many participating children, parents, teachers and schools; Janet Walter, Annie Shrier, Stephanie Dobrowolski and Lindsay Shepard for their assistance with the data collection. Cenia Bahnsen for initial work on the actigraphy data; Tom Savage for administrative assistance; Eileen Bailey-Hall for her work on the blood analyses; Sean Grant for substantial comments on earlier drafts of this paper; two anonymous reviewers and the journal editor for their helpful comments. The authors thank Martek Biosciences Inc. now DSM Nutritional Lipids (http://www.lifesdha.com/) for funding this study. The authors also thank the Waterloo Foundation for its support for Dr Richardson's work in this area of research.
Appendices
Appendix 1 Sample comparison
Table 7.
Tests for demographic differences between samples
| Consent versus RCT sample | Consent versus epidemiology sample | RCT versus actigraphy sample | RCT versus epidemiology sample | RCT versus caseness RCT (CSHQ > 41) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | χ2† | P-value | Direction | χ 2 | P-value | χ 2 | P-value | Direction | χ 2 | P-value | Direction | χ 2 | P-value | |
| Sex, n (%) | 2.25 | 0.134 | 0.1 | 0.75 | 0.07 | 0.793 | 2.62 | 0.455 | RCT < caseness | 5.1 | 0.023* | |||
| Age in years, n (%) | Consent < RCT | 10.79 | 0.005** | 0.47 | 0.789 | 0.34 | 0.845 | 11.9 | 0.064 | 1.07 | 0.059 | |||
| Free school meals, n (%) | Consent < RCT | 4.37 | 0.037* | Consent > epid | 17.84 | 0.000*** | 0.89 | 0.346 | RCT > Epid | 22.33 | 0.000*** | RCT < caseness | 11.42 | 0.002** |
| Ethnicity, n (%) | Consent < RCT | 10.32 | 0.035* | 2.3 | 0.681 | 0.99 | 0.911 | 12.92 | 0.375 | 3.02 | 0.552 | |||
| t-value | P-value | t-value | P-value | t-value | P-value | t-value | P-value | t-value | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | −1.59 | 0.111 | −1.696 | 0.242 | RCT < acti −2.08 | 0.038* | −0.285 | 0.77 | 0.77 | 0.442 |
RCT, randomized controlled trial.
P < 0.001;
P < 0.01;
P < 0.05.
χ2 tests.
Appendix 2 CSHQ scores and ‘clinical caseness’ in the epidemiology sample
Table 8.
Descriptive values for the CSHQ subscales in the DOLAB study
| CSHQ epidemiology sample | CSHQ caseness subgroup† | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Bedtime resistance | 7.11 | 1.95 | 6–17 | 8.05 | 2.22 | 6–17 |
| Sleep onset delay | 1.66 | 0.79 | 1–3 | 1.91 | 0.69 | 1–3 |
| Sleep duration | 3.84 | 1.34 | 3–9 | 4.4 | 1.33 | 3–9 |
| Sleep anxiety | 5.18 | 1.54 | 4–12 | 5.75 | 1.55 | 4–11 |
| Night wakings | 3.66 | 1.13 | 3–9 | 3.96 | 1.08 | 3–9 |
| Parasomnias | 8.72 | 1.96 | 7–17 | 9.36 | 1.84 | 7–17 |
| Sleep disturbed breathing | 3.44 | 0.92 | 3–9 | 3.77 | 1.04 | 3–9 |
| Daytime sleepiness | 9.77 | 3.04 | 6–20 | 11.24 | 2.7 | 6–19 |
| Total sleep disturbance | 41.05 | 6.98 | 31–69 | 45.8 | 5.65 | 41.34–67 |
| †CSHQ total sleep disturbance >41 | n = 395 | n = 161 | ||||
CSHQ, Child Sleep Habits Questionnaire; SD, standard deviation; DOLAB, Docosahexaenoic Acid (DHA) Oxford Learning and Behaviour Study.
Appendix 3 Fatty acid levels in the CSHQ epidemiology sample
Table 9.
Blood fatty acid levels in the CSHQ epidemiology sample
| Mean | SD | Range | |
|---|---|---|---|
| DHA (22 : 6) | 1.93 | 0.54 | 0.9–3.8 |
| DPA (22 : 5) | 1.03 | 0.28 | 0.53–3.62 |
| EPA (20 : 5) | 0.56 | 0.21 | 0–2.03 |
| ALA (18 : 3) | 0.55 | 0.26 | 0–2.32 |
| Total omega-3 | 4.11 | 0.85 | 2.19–8.01 |
| DPA (n-6) (22 : 5) | 0.25 | 0.1 | 0–0.63 |
| Adrenic (22 : 4) | 1.10 | 0.22 | 0.42–1.81 |
| AA (20 : 4) | 8.18 | 1.31 | 4.3–11.73 |
| DGLA (20 : 3) | 1.55 | 0.34 | 0.81–2.9 |
| GLA (18 : 3) | 0.32 | 0.24 | 0–1.41 |
| LA (18 : 2) | 19.22 | 2.33 | 12.66–26.08 |
| Total omega-6 | 30.88 | 3.33 | 20.61–39.79 |
| Omega-3 index | 2.49 | 0.67 | 1.26–5.24 |
| Ratio DHA : AA | 0.24 | 0.06 | 0.14–0.53 |
| Ratio EPA : AA | 0.07 | 0.03 | 0–0.24 |
n = 395
CSHQ, Child Sleep Habits Questionnaire; SD, standard deviation; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
Appendix 4 Group comparisons actigraphy outcomes in CSHQ caseness subgroup
Table 10.
Actigraphy mean scores and group comparison (ITT, CSHQ > 41)
| Group means of the actigraphy measures (SD) | Change scores (SD) Baseline versus postintervention | Comparisons† Active versus placebo 0- to 16-week Diff. | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (16 weeks) | |||||||
| Active (n = 11) Imputed n = 2 | Placebo (n = 9) Imputed n = 0 | Active (n = 11) Imputed n = 4 | Placebo (n = 9) Imputed n = 3 | Active (n = 11) | Placebo (n = 9) | Z-Value/t-value | P-value | |
| Time child fell asleep | 20:26 (35 min) | 20:26 (63 min) | 20:52 (39 min) | 21:01 (38 min) | 26 min (55 min) | 35 min (65 min) | t: −0.34 | 0.74 |
| Time child woke up | 7:22 (51 min) | 7:25 (39 min) | 7:31 (62 min) | 7:09 (28 min) | 8 min (86 min) | −16 min (59 min) | t: 0.73 | 0.476 |
| Min sleep duration | 658 min (74 min) | 654 min (49 min) | 645 min (53 min) | 646 min (76 min) | −13 min (106 min) | −9 min (58 min) | t: −0.1 | 0.919 |
| Min awake | 156 min (54 min) | 148 min (59 min) | 101 min (42 min) | 115 min (31 min) | −54 min (73 min) | −33 min (64 min) | t: −0.70 | 0.494 |
| Min sleep | 502 min (101 min) | 507 min (70 min) | 543 min (70 min) | 531 min (86 min) | 41 min (131 min) | 24 min (94 min) | Z: 0.722 | 0.47 |
| Sleep efficiency (ratio) | 0.76 (0.092) | 0.77 (0.095) | 0.84 (0.066) | 0.82 (0.05) | 0.08 (0.118) | 0.04 (0.104) | t: 0.73 | 0.473 |
| Sleep latency (min) | 17.07 (21.463) | 39.11 (38.998) | 8.88 (9.649) | 31.19 (27.909) | −8.19 (28.036) | −7.92 (37.848) | t: −0.02 | 0.985 |
| Wake episodes | 23.11 (5.21) | 21.1 (9.731) | 12.65 (3.283) | 16.63 (5.333) | −10.46 (7.67) | −4.48 (10.427) | t: −01.48 | 0.157 |
CSHQ, Child Sleep Habits Questionnaire; SD, standard deviation. ITT, mean imputation of missing observations.
Some variables are normal, some non-normally distributed; if Shapiro–Wilk was P > 0.05 a t-test was used, otherwise Wilcoxon's rank sum.
Appendix 5 Blood fatty acid associations with the CSHQ–full regression results controlling for demographic differences
Table 11.
Full results–OLS regression model controlling for demographics: CSHQ subscale–bed resistance
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.28 | −0.08 | 0.052 | −0.07 | −0.07 | 0.746 | −0.32 | −0.13 | 0.017* | 0.03 | −1.10 | 0.047* | 1.10 | −0.05 | 0.009** |
| DPA (22 : 5) | 0.52 | 0.08 | 0.375 | −0.12 | 0.13 | 0.578 | −0.30 | 0.24 | 0.025* | 0.03 | 2.04 | 0.055* | 1.09 | 0.09 | 0.007** |
| EPA (20 : 5) | −0.18 | −0.02 | 0.667 | −0.09 | −0.05 | 0.671 | −0.32 | −0.08 | 0.017* | 0.03 | −0.71 | 0.043* | 1.14 | −0.03 | 0.007** |
| ALA (18 : 3) | 0.09 | 0.01 | 0.839 | −0.09 | 0.02 | 0.66 | −0.32 | 0.04 | 0.018* | 0.03 | 0.34 | 0.048* | 1.14 | 0.02 | 0.007** |
| Total omega-3 | −0.05 | −0.02 | 0.646 | −0.08 | −0.01 | 0.695 | −0.32 | −0.03 | 0.018* | 0.03 | −0.21 | 0.044* | 1.13 | −0.01 | 0.007** |
| DPA (n-6) (22 : 5) | 0.84 | 0.04 | 0.367 | −0.12 | 0.21 | 0.529 | −0.32 | 0.39 | 0.017* | 0.04 | 3.29 | 0.041* | 1.12 | 0.14 | 0.008** |
| Adrenic (22 : 4) | 0.30 | 0.03 | 0.483 | −0.12 | 0.08 | 0.555 | −0.32 | 0.14 | 0.019* | 0.03 | 1.17 | 0.047* | 1.13 | 0.05 | 0.007** |
| AA (20 : 4) | 0.03 | 0.02 | 0.724 | −0.10 | 0.01 | 0.617 | −0.31 | 0.01 | 0.018* | 0.03 | 0.10 | 0.046* | 1.14 | 0.00 | 0.007** |
| DGLA (20 : 3) | 0.30 | 0.05 | 0.264 | −0.13 | 0.08 | 0.528 | −0.31 | 0.14 | 0.018* | 0.03 | 1.17 | 0.048* | 1.15 | 0.05 | 0.006** |
| GLA (18 : 3) | 0.09 | 0.01 | 0.848 | −0.10 | 0.02 | 0.648 | −0.32 | 0.04 | 0.018* | 0.03 | 0.37 | 0.047* | 1.14 | 0.02 | 0.007** |
| LA (18 : 2) | 0.06 | 0.07 | 0.169 | −0.09 | 0.01 | 0.646 | −0.31 | 0.03 | 0.02* | 0.04 | 0.22 | 0.033* | 1.12 | 0.01 | 0.007** |
| Total omega-6 | 0.04 | 0.06 | 0.2 | −0.12 | 0.01 | 0.56 | −0.31 | 0.02 | 0.02* | 0.04 | 0.14 | 0.035* | 1.13 | 0.01 | 0.007** |
| Omega-3 index | −0.20 | −0.07 | 0.089 | −0.07 | −0.05 | 0.732 | −0.32 | −0.09 | 0.016* | 0.03 | −0.78 | 0.045* | 1.11 | −0.03 | 0.009** |
| Ratio DHA : AA | −2.90 | −0.09 | 0.018 | −0.10 | −0.73 | 0.636 | −0.31 | −1.34 | 0.021* | 0.03 | −11.30 | 0.042* | 1.09 | −0.49 | 0.009** |
| Ratio EPA : AA | −2.83 | −0.04 | 0.359 | −0.10 | −0.71 | 0.641 | −0.32 | −1.31 | 0.018* | 0.04 | −11.03 | 0.039* | 1.14 | −0.48 | 0.007** |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05,
P < 0.01.
Table 12.
Full results–OLS regression model controlling for demographics: CSHQ subscale–sleep onset delay
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value* | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.04 | −0.03 | 0.6 | −0.02 | −0.03 | 0.803 | 0.06 | −0.05 | 0.272 | −0.01 | −0.38 | 0.173 | 0.40 | −0.02 | 0.000*** |
| DPA (22 : 5) | 0.03 | 0.01 | 0.87 | −0.02 | 0.02 | 0.762 | 0.06 | 0.03 | 0.263 | −0.01 | 0.25 | 0.175 | 0.40 | 0.01 | 0.000*** |
| EPA (20 : 5) | 0.21 | 0.06 | 0.313 | −0.03 | 0.14 | 0.744 | 0.06 | 0.25 | 0.238 | −0.01 | 2.10 | 0.148 | 0.41 | 0.09 | 0.000*** |
| ALA (18 : 3) | 0.00 | 0.00 | 0.996 | −0.02 | 0.00 | 0.774 | 0.06 | 0.00 | 0.267 | −0.01 | −0.01 | 0.177 | 0.41 | 0.00 | 0.000*** |
| Total omega-3 | 0.00 | 0.00 | 0.979 | −0.02 | 0.00 | 0.774 | 0.06 | 0.00 | 0.268 | −0.01 | −0.01 | 0.177 | 0.41 | 0.00 | 0.000*** |
| DPA (n-6) (22 : 5) | −0.41 | −0.05 | 0.303 | −0.01 | −0.26 | 0.934 | 0.06 | −0.47 | 0.243 | −0.01 | −3.98 | 0.133 | 0.42 | −0.17 | 0.000*** |
| Adrenic (22 : 4) | −0.04 | −0.01 | 0.825 | −0.02 | −0.03 | 0.811 | 0.06 | −0.05 | 0.272 | −0.01 | −0.40 | 0.179 | 0.41 | −0.02 | 0.000*** |
| AA (20 : 4) | −0.02 | −0.03 | 0.643 | −0.02 | −0.01 | 0.834 | 0.06 | −0.02 | 0.29 | −0.01 | −0.15 | 0.166 | 0.41 | −0.01 | 0.000*** |
| DGLA (20 : 3) | 0.10 | 0.05 | 0.391 | −0.04 | 0.07 | 0.661 | 0.06 | 0.12 | 0.254 | −0.01 | 1.00 | 0.159 | 0.41 | 0.04 | 0.000*** |
| GLA (18 : 3) | 0.09 | 0.03 | 0.612 | −0.03 | 0.06 | 0.73 | 0.06 | 0.11 | 0.266 | −0.01 | 0.89 | 0.177 | 0.41 | 0.04 | 0.000*** |
| LA (18 : 2) | −0.01 | −0.04 | 0.455 | −0.02 | −0.01 | 0.781 | 0.06 | −0.02 | 0.28 | −0.01 | −0.13 | 0.152 | 0.41 | −0.01 | 0.000*** |
| Total omega-6 | −0.01 | −0.03 | 0.551 | −0.02 | −0.01 | 0.833 | 0.06 | −0.01 | 0.285 | −0.01 | −0.07 | 0.157 | 0.41 | 0.00 | 0.000*** |
| Omega-3 index | 0.00 | 0.00 | 0.949 | −0.02 | 0.00 | 0.776 | 0.06 | −0.01 | 0.268 | −0.01 | −0.04 | 0.177 | 0.41 | 0.00 | 0.000*** |
| Ratio DHA : AA | −0.25 | −0.02 | 0.709 | −0.02 | −0.16 | 0.77 | 0.06 | −0.28 | 0.261 | −0.01 | −2.40 | 0.179 | 0.40 | −0.10 | 0.000*** |
| Ratio EPA : AA | 1.49 | 0.05 | 0.38 | −0.02 | 0.94 | 0.802 | 0.06 | 1.73 | 0.261 | −0.01 | 14.57 | 0.141 | 0.41 | 0.63 | 0.000*** |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.001.
Table 13.
Full results–OLS regression model controlling for demographics: CSHQ subscale–sleep duration
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.20 | −0.08 | 0.076 | 0.04 | −0.08 | 0.798 | −0.06 | −0.14 | 0.49 | 0.00 | −1.16 | 0.967 | 0.11 | −0.05 | 0.682 |
| DPA (22 : 5) | −0.20 | −0.04 | 0.401 | 0.03 | −0.07 | 0.834 | −0.07 | −0.13 | 0.478 | 0.00 | −1.13 | 0.901 | 0.15 | −0.05 | 0.544 |
| EPA (20 : 5) | −0.20 | −0.03 | 0.506 | 0.02 | −0.08 | 0.877 | −0.06 | −0.14 | 0.493 | 0.00 | −1.16 | 0.909 | 0.13 | −0.05 | 0.593 |
| ALA (18 : 3) | −0.39 | −0.08 | 0.094 | 0.02 | −0.15 | 0.891 | −0.06 | −0.27 | 0.507 | 0.00 | −2.26 | 0.886 | 0.13 | −0.10 | 0.606 |
| Total omega-3 | −0.15 | −0.10 | 0.068 | 0.04 | −0.06 | 0.761 | −0.07 | −0.10 | 0.459 | 0.00 | −0.87 | 0.875 | 0.12 | −0.04 | 0.62 |
| DPA (n-6) (22 : 5) | 0.31 | 0.02 | 0.638 | 0.01 | 0.12 | 0.965 | −0.06 | 0.21 | 0.494 | 0.00 | 1.78 | 0.895 | 0.13 | 0.08 | 0.611 |
| Adrenic (22 : 4) | 0.52 | 0.09 | 0.121 | −0.03 | 0.19 | 0.849 | −0.05 | 0.35 | 0.541 | 0.00 | 2.98 | 0.946 | 0.13 | 0.13 | 0.603 |
| AA (20 : 4) | 0.03 | 0.03 | 0.583 | 0.01 | 0.01 | 0.971 | −0.05 | 0.02 | 0.546 | 0.00 | 0.18 | 0.916 | 0.14 | 0.01 | 0.589 |
| DGLA (20 : 3) | 0.23 | 0.06 | 0.241 | −0.01 | 0.09 | 0.934 | −0.06 | 0.16 | 0.533 | 0.00 | 1.32 | 0.994 | 0.15 | 0.06 | 0.568 |
| GLA (18 : 3) | 0.00 | 0.00 | 0.99 | 0.02 | 0.00 | 0.895 | −0.06 | 0.00 | 0.509 | 0.00 | −0.02 | 0.945 | 0.13 | 0.00 | 0.597 |
| LA (18 : 2) | 0.01 | 0.02 | 0.718 | 0.02 | 0.00 | 0.898 | −0.06 | 0.01 | 0.52 | 0.00 | 0.06 | 0.907 | 0.13 | 0.00 | 0.602 |
| Total omega-6 | 0.02 | 0.04 | 0.426 | 0.00 | 0.01 | 0.975 | −0.05 | 0.01 | 0.547 | 0.00 | 0.10 | 0.87 | 0.13 | 0.00 | 0.601 |
| Omega-3 index | −0.15 | −0.08 | 0.108 | 0.03 | −0.06 | 0.81 | −0.06 | −0.10 | 0.482 | 0.00 | −0.86 | 0.935 | 0.11 | −0.04 | 0.658 |
| Ratio DHA : AA | −2.16 | −0.10 | 0.018* | 0.01 | −0.81 | 0.921 | −0.05 | −1.48 | 0.57 | 0.00 | −12.47 | 0.917 | 0.10 | −0.54 | 0.697 |
| Ratio EPA : AA | −2.28 | −0.05 | 0.319 | 0.01 | −0.85 | 0.919 | −0.06 | −1.55 | 0.511 | 0.00 | −13.12 | 0.874 | 0.14 | −0.57 | 0.584 |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05.
Table 14.
Full results–OLS regression model controlling for demographics: CSHQ subscale–sleep anxiety
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22:6) | −0.14 | −0.05 | 0.294 | 0.03 | −0.05 | 0.837 | −0.20 | −0.08 | 0.048* | 0.02 | −0.71 | 0.126 | 0.44 | −0.03 | 0.09 |
| DPA (22:5) | 0.47 | 0.09 | 0.063 | 0.00 | 0.15 | 0.993 | −0.18 | 0.28 | 0.072 | 0.02 | 2.32 | 0.156 | 0.42 | 0.10 | 0.104 |
| EPA (20:5) | 0.24 | 0.03 | 0.622 | 0.02 | 0.08 | 0.909 | −0.19 | 0.14 | 0.048* | 0.02 | 1.18 | 0.133 | 0.46 | 0.05 | 0.076 |
| ALA (18:3) | 0.67 | 0.11 | 0.047* | 0.02 | 0.22 | 0.896 | −0.19 | 0.40 | 0.054 | 0.02 | 3.35 | 0.143 | 0.47 | 0.15 | 0.066 |
| Total omega-3 | 0.09 | 0.05 | 0.377 | 0.01 | 0.03 | 0.956 | −0.19 | 0.05 | 0.053 | 0.02 | 0.43 | 0.137 | 0.46 | 0.02 | 0.072 |
| DPA (n-6) (22:5) | 0.26 | 0.02 | 0.722 | 0.01 | 0.08 | 0.941 | −0.20 | 0.15 | 0.053 | 0.02 | 1.30 | 0.127 | 0.45 | 0.06 | 0.081 |
| Adrenic (22:4) | 0.14 | 0.02 | 0.686 | 0.01 | 0.04 | 0.948 | −0.19 | 0.08 | 0.053 | 0.02 | 0.68 | 0.129 | 0.46 | 0.03 | 0.077 |
| AA (20:4) | 0.01 | 0.01 | 0.814 | 0.02 | 0.01 | 0.922 | −0.19 | 0.01 | 0.051 | 0.02 | 0.07 | 0.123 | 0.46 | 0.00 | 0.076 |
| DGLA (20:3) | 0.03 | 0.01 | 0.89 | 0.02 | 0.01 | 0.912 | −0.19 | 0.02 | 0.052 | 0.02 | 0.16 | 0.124 | 0.46 | 0.01 | 0.077 |
| GLA (18:3) | 0.59 | 0.09 | 0.077 | −0.01 | 0.19 | 0.95 | −0.19 | 0.35 | 0.056 | 0.02 | 2.93 | 0.126 | 0.48 | 0.13 | 0.064 |
| LA (18:2) | 0.02 | 0.03 | 0.538 | 0.02 | 0.01 | 0.899 | −0.19 | 0.01 | 0.053 | 0.02 | 0.10 | 0.107 | 0.45 | 0.00 | 0.078 |
| Total omega-6 | 0.02 | 0.04 | 0.434 | 0.01 | 0.01 | 0.964 | −0.19 | 0.01 | 0.055 | 0.02 | 0.09 | 0.107 | 0.45 | 0.00 | 0.077 |
| Omega-3 index | −0.07 | −0.03 | 0.58 | 0.03 | −0.02 | 0.861 | −0.20 | −0.04 | 0.046* | 0.02 | −0.34 | 0.123 | 0.45 | −0.02 | 0.083 |
| Ratio DHA:AA | −1.51 | −0.06 | 0.172 | 0.02 | −0.49 | 0.908 | −0.19 | −0.89 | 0.061 | 0.02 | −7.55 | 0.118 | 0.43 | −0.33 | 0.092 |
| Ratio EPA:AA | 1.03 | 0.02 | 0.767 | 0.02 | 0.33 | 0.883 | −0.20 | 0.61 | 0.05* | 0.02 | 5.15 | 0.129 | 0.46 | 0.22 | 0.079 |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05.
Table 15.
Full results–OLS regression model controlling for demographics: CSHQ subscale–night waking
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value* | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.03 | −0.02 | 0.757 | 0.14 | −0.01 | 0.235 | −0.10 | −0.03 | 0.166 | 0.01 | −0.22 | 0.153 | 0.31 | −0.01 | 0.136 |
| DPA (22 : 5) | 0.03 | 0.01 | 0.877 | 0.14 | 0.01 | 0.246 | −0.10 | 0.03 | 0.173 | 0.01 | 0.22 | 0.159 | 0.32 | 0.01 | 0.14 |
| EPA (20 : 5) | 0.55 | 0.11 | 0.146 | 0.13 | 0.25 | 0.259 | −0.09 | 0.45 | 0.19 | 0.01 | 3.81 | 0.205 | 0.32 | 0.17 | 0.129 |
| ALA (18 : 3) | 0.33 | 0.08 | 0.279 | 0.14 | 0.15 | 0.234 | −0.10 | 0.27 | 0.162 | 0.01 | 2.26 | 0.175 | 0.32 | 0.10 | 0.124 |
| Total omega-3 | 0.07 | 0.06 | 0.368 | 0.13 | 0.03 | 0.278 | −0.10 | 0.06 | 0.183 | 0.01 | 0.50 | 0.171 | 0.32 | 0.02 | 0.125 |
| DPA (n-6) (22 : 5) | 0.77 | 0.07 | 0.173 | 0.11 | 0.35 | 0.338 | −0.11 | 0.63 | 0.152 | 0.01 | 5.35 | 0.107 | 0.30 | 0.23 | 0.152 |
| Adrenic (22 : 4) | 0.22 | 0.04 | 0.408 | 0.12 | 0.10 | 0.317 | −0.10 | 0.18 | 0.181 | 0.01 | 1.54 | 0.15 | 0.32 | 0.07 | 0.132 |
| AA (20 : 4) | 0.03 | 0.03 | 0.507 | 0.13 | 0.01 | 0.282 | −0.10 | 0.02 | 0.187 | 0.01 | 0.18 | 0.14 | 0.32 | 0.01 | 0.129 |
| DGLA (20 : 3) | −0.07 | −0.02 | 0.63 | 0.15 | −0.03 | 0.207 | −0.10 | −0.06 | 0.163 | 0.01 | −0.49 | 0.147 | 0.32 | −0.02 | 0.132 |
| GLA (18 : 3) | −0.01 | 0.00 | 0.978 | 0.14 | 0.00 | 0.236 | −0.10 | −0.01 | 0.169 | 0.01 | −0.04 | 0.152 | 0.32 | 0.00 | 0.132 |
| LA (18 : 2) | 0.00 | 0.00 | 0.929 | 0.14 | 0.00 | 0.237 | −0.10 | 0.00 | 0.166 | 0.01 | −0.01 | 0.155 | 0.32 | 0.00 | 0.131 |
| Total omega-6 | 0.01 | 0.02 | 0.747 | 0.14 | 0.00 | 0.262 | −0.10 | 0.00 | 0.175 | 0.01 | 0.04 | 0.141 | 0.32 | 0.00 | 0.131 |
| Omega-3 Index | 0.03 | 0.02 | 0.713 | 0.14 | 0.02 | 0.252 | −0.10 | 0.03 | 0.17 | 0.01 | 0.24 | 0.153 | 0.32 | 0.01 | 0.123 |
| Ratio DHA : AA | −0.60 | −0.03 | 0.523 | 0.14 | −0.27 | 0.237 | −0.10 | −0.49 | 0.179 | 0.01 | −4.16 | 0.147 | 0.31 | −0.18 | 0.141 |
| Ratio EPA : AA | 3.56 | 0.09 | 0.23 | 0.15 | 1.60 | 0.21 | −0.10 | 2.93 | 0.162 | 0.01 | 24.71 | 0.214 | 0.31 | 1.07 | 0.137 |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
Table 16.
Full results–OLS regression model controlling for demographics: CSHQ subscale–parasomnia
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.28 | −0.08 | 0.131 | 0.30 | −0.07 | 0.111 | −0.25 | −0.13 | 0.03* | 0.01 | −1.11 | 0.476 | 0.75 | −0.05 | 0.039* |
| DPA (22 : 5) | 0.71 | 0.11 | 0.043* | 0.24 | 0.19 | 0.192 | −0.23 | 0.34 | 0.054* | 0.01 | 2.85 | 0.58 | 0.73 | 0.12 | 0.041* |
| EPA (20 : 5) | 1.48 | 0.16 | 0.007** | 0.26 | 0.38 | 0.168 | −0.23 | 0.70 | 0.043* | 0.01 | 5.93 | 0.645 | 0.79 | 0.26 | 0.024* |
| ALA (18 : 3) | −0.02 | 0.00 | 0.964 | 0.28 | −0.01 | 0.137 | −0.25 | −0.01 | 0.034* | 0.01 | −0.08 | 0.458 | 0.79 | 0.00 | 0.029* |
| Total omega-3 | 0.07 | 0.03 | 0.565 | 0.27 | 0.02 | 0.159 | −0.25 | 0.04 | 0.035* | 0.01 | 0.29 | 0.483 | 0.80 | 0.01 | 0.027* |
| DPA (n-6) (22 : 5) | 0.81 | 0.04 | 0.377 | 0.25 | 0.21 | 0.198 | −0.25 | 0.38 | 0.03* | 0.01 | 3.23 | 0.381 | 0.77 | 0.14 | 0.033* |
| Adrenic (22 : 4) | −0.06 | −0.01 | 0.887 | 0.29 | −0.02 | 0.141 | −0.25 | −0.03 | 0.034* | 0.01 | −0.24 | 0.46 | 0.79 | −0.01 | 0.029* |
| AA (20 : 4) | 0.00 | 0.00 | 0.963 | 0.28 | 0.00 | 0.153 | −0.25 | 0.00 | 0.035* | 0.01 | 0.01 | 0.461 | 0.79 | 0.00 | 0.029* |
| DGLA (20 : 3) | 0.19 | 0.03 | 0.455 | 0.26 | 0.05 | 0.182 | −0.25 | 0.09 | 0.035* | 0.01 | 0.74 | 0.49 | 0.80 | 0.03 | 0.027* |
| GLA (18 : 3) | −0.01 | 0.00 | 0.974 | 0.28 | 0.00 | 0.15 | −0.25 | −0.01 | 0.032* | 0.01 | −0.05 | 0.46 | 0.79 | 0.00 | 0.028* |
| LA (18 : 2) | −0.04 | −0.05 | 0.326 | 0.28 | −0.01 | 0.139 | −0.25 | −0.02 | 0.029* | 0.01 | −0.17 | 0.581 | 0.81 | −0.01 | 0.026* |
| Total omega-6 | −0.01 | −0.02 | 0.653 | 0.29 | 0.00 | 0.137 | −0.25 | −0.01 | 0.031* | 0.01 | −0.05 | 0.514 | 0.79 | 0.00 | 0.028* |
| Omega-3 index | −0.03 | −0.01 | 0.839 | 0.28 | −0.01 | 0.137 | −0.25 | −0.02 | 0.032* | 0.01 | −0.13 | 0.457 | 0.79 | −0.01 | 0.031* |
| Ratio DHA : AA | −2.66 | −0.09 | 0.092 | 0.28 | −0.69 | 0.149 | −0.24 | −1.26 | 0.042* | 0.01 | −10.64 | 0.43 | 0.75 | −0.46 | 0.038* |
| Ratio EPA : AA | 11.46 | 0.16 | 0.007** | 0.30 | 2.97 | 0.108 | −0.25 | 5.43 | 0.029* | 0.00 | 45.88 | 0.724 | 0.78 | 1.98 | 0.027* |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05,
P < 0.01.
Table 17.
Full results–OLS regression model controlling for demographics: CSHQ subscale–sleep disturbed breathing
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socio-economic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std Coeff. | Raw Coeff. | P-value | Std Coeff. | Raw Coeff. | P-value | |
| DHA (22 : 6) | −0.07 | −0.04 | 0.447 | 0.18 | −0.04 | 0.048* | 0.00 | −0.07 | 0.985 | 0.01 | −0.55 | 0.321 | 0.13 | −0.02 | 0.388 |
| DPA (22 : 5) | 0.31 | 0.10 | 0.056 | 0.16 | 0.17 | 0.073 | 0.01 | 0.31 | 0.875 | 0.01 | 2.58 | 0.365 | 0.12 | 0.11 | 0.45 |
| EPA (20 : 5) | 0.00 | 0.00 | 0.996 | 0.17 | 0.00 | 0.051 | 0.00 | 0.00 | 0.998 | 0.01 | −0.01 | 0.315 | 0.14 | 0.00 | 0.347 |
| ALA (18 : 3) | −0.13 | −0.04 | 0.347 | 0.17 | −0.07 | 0.052 | 0.00 | −0.13 | 0.995 | 0.01 | −1.07 | 0.297 | 0.14 | −0.05 | 0.355 |
| Total omega-3 | 0.00 | 0.00 | 0.973 | 0.17 | 0.00 | 0.055 | 0.00 | 0.00 | 0.999 | 0.01 | 0.01 | 0.311 | 0.14 | 0.00 | 0.347 |
| DPA (n-6) (22 : 5) | −0.22 | −0.02 | 0.719 | 0.18 | −0.12 | 0.055 | 0.00 | −0.21 | 0.981 | 0.01 | −1.81 | 0.362 | 0.15 | −0.08 | 0.328 |
| Adrenic (22 : 4) | 0.29 | 0.07 | 0.226 | 0.15 | 0.16 | 0.126 | 0.00 | 0.29 | 0.968 | 0.01 | 2.41 | 0.308 | 0.14 | 0.10 | 0.355 |
| AA (20 : 4) | 0.02 | 0.03 | 0.654 | 0.16 | 0.01 | 0.085 | 0.00 | 0.02 | 0.961 | 0.01 | 0.16 | 0.309 | 0.14 | 0.01 | 0.344 |
| DGLA (20 : 3) | −0.08 | −0.03 | 0.567 | 0.18 | −0.04 | 0.044* | 0.00 | −0.07 | 0.982 | 0.01 | −0.63 | 0.292 | 0.14 | −0.03 | 0.359 |
| GLA (18 : 3) | −0.14 | −0.04 | 0.353 | 0.18 | −0.08 | 0.047* | 0.00 | −0.14 | 0.989 | 0.01 | −1.19 | 0.308 | 0.14 | −0.05 | 0.365 |
| LA (18 : 2) | −0.02 | −0.04 | 0.588 | 0.17 | −0.01 | 0.051 | 0.00 | −0.02 | 0.973 | 0.01 | −0.13 | 0.377 | 0.15 | −0.01 | 0.332 |
| Total omega-6 | 0.00 | −0.01 | 0.835 | 0.18 | 0.00 | 0.06 | 0.00 | 0.00 | 0.983 | 0.01 | −0.03 | 0.349 | 0.14 | 0.00 | 0.344 |
| Omega-3 index | −0.04 | −0.03 | 0.534 | 0.18 | −0.02 | 0.049* | 0.00 | −0.04 | 0.982 | 0.01 | −0.35 | 0.312 | 0.14 | −0.02 | 0.373 |
| Ratio DHA : AA | −0.83 | −0.06 | 0.289 | 0.17 | −0.45 | 0.054 | 0.00 | −0.82 | 0.96 | 0.01 | −6.91 | 0.306 | 0.13 | −0.30 | 0.401 |
| Ratio EPA : AA | −0.13 | 0.00 | 0.944 | 0.17 | −0.07 | 0.055 | 0.00 | −0.13 | 0.998 | 0.01 | −1.06 | 0.32 | 0.14 | −0.05 | 0.346 |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05.
Table 18.
Full results–OLS regression model controlling for demographics: CSHQ subscale–day sleepiness
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socioeconomic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | |
| DHA (22 : 6) | −0.32 | −0.06 | 0.225 | −0.83 | −0.05 | 0.004** | 0.37 | −0.10 | 0.053 | 0.00 | −0.81 | 0.997 | 1.17 | −0.04 | 0.019* |
| DPA (22 : 5) | 0.54 | 0.05 | 0.463 | −0.89 | 0.09 | 0.003** | 0.39 | 0.16 | 0.042* | 0.00 | 1.38 | 0.957 | 1.16 | 0.06 | 0.015* |
| EPA (20 : 5) | 0.05 | 0.00 | 0.948 | −0.86 | 0.01 | 0.003** | 0.37 | 0.02 | 0.048* | 0.00 | 0.13 | 0.991 | 1.21 | 0.01 | 0.014* |
| ALA (18 : 3) | −0.17 | −0.01 | 0.789 | −0.86 | −0.03 | 0.003** | 0.37 | −0.05 | 0.047* | 0.00 | −0.42 | 0.976 | 1.21 | −0.02 | 0.014* |
| Total omega-3 | −0.06 | −0.02 | 0.741 | −0.85 | −0.01 | 0.004** | 0.37 | −0.02 | 0.052 | 0.00 | −0.16 | 0.974 | 1.21 | −0.01 | 0.014* |
| DPA (n-6) (22 : 5) | 0.40 | 0.01 | 0.781 | −0.88 | 0.07 | 0.004** | 0.37 | 0.12 | 0.049* | 0.00 | 1.02 | 0.956 | 1.20 | 0.04 | 0.015* |
| Adrenic (22 : 4) | −0.29 | −0.02 | 0.64 | −0.83 | −0.05 | 0.005** | 0.37 | −0.09 | 0.05 | 0.00 | −0.75 | 0.987 | 1.22 | −0.03 | 0.014* |
| AA (20 : 4) | 0.00 | 0.00 | 0.993 | −0.86 | 0.00 | 0.004** | 0.37 | 0.00 | 0.051 | 0.00 | 0.00 | 0.988 | 1.21 | 0.00 | 0.014* |
| DGLA (20 : 3) | 0.20 | 0.02 | 0.661 | −0.88 | 0.03 | 0.003** | 0.37 | 0.06 | 0.046* | 0.00 | 0.50 | 0.989 | 1.22 | 0.02 | 0.013* |
| GLA (18 : 3) | 0.07 | 0.01 | 0.914 | −0.86 | 0.01 | 0.003** | 0.37 | 0.02 | 0.047* | 0.00 | 0.17 | 0.988 | 1.22 | 0.01 | 0.014* |
| LA (18 : 2) | −0.03 | −0.02 | 0.658 | −0.86 | −0.01 | 0.003** | 0.37 | −0.01 | 0.051 | 0.00 | −0.08 | 0.964 | 1.22 | 0.00 | 0.013* |
| Total omega-6 | −0.01 | −0.02 | 0.779 | −0.85 | 0.00 | 0.004** | 0.37 | 0.00 | 0.053 | 0.00 | −0.03 | 0.987 | 1.22 | 0.00 | 0.013* |
| Omega-3 index | −0.20 | −0.05 | 0.351 | −0.84 | −0.03 | 0.004** | 0.37 | −0.06 | 0.054 | 0.00 | −0.51 | 0.981 | 1.18 | −0.02 | 0.017* |
| Ratio DHA : AA | −3.06 | −0.06 | 0.185 | −0.87 | −0.51 | 0.003** | 0.38 | −0.93 | 0.042* | 0.00 | −7.82 | 0.969 | 1.16 | −0.34 | 0.019* |
| Ratio EPA : AA | −1.03 | −0.01 | 0.864 | −0.86 | −0.17 | 0.003** | 0.37 | −0.31 | 0.049* | 0.00 | −2.62 | 0.973 | 1.21 | −0.11 | 0.014* |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05,
P < 0.01.
Table 19.
Full results–OLS regression model controlling for demographics: CSHQ total sleep disturbance score
| Fatty acid | Gender (f/m) | Age (years) | Weight (kg) | Socio-economic status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std coeff. | Raw coeff. | P-value† | Std coeff. | Raw coeff. | P-value | Std coeff. | Raw coeff. | P-value | Std Coeff. | Raw Coeff. | P-value | Std Coeff. | Raw Coeff. | P-value | |
| DHA (22 : 6) | −1.35 | −0.11 | 0.026* | −0.23 | −0.10 | 0.738 | −0.39 | −0.18 | 0.39 | 0.06 | −1.50 | 0.208 | 4.08 | −0.07 | 0.002** |
| DPA (22 : 5) | 2.24 | 0.09 | 0.057 | −0.46 | 0.16 | 0.505 | −0.30 | 0.30 | 0.505 | 0.06 | 2.49 | 0.252 | 4.07 | 0.11 | 0.002** |
| EPA (20 : 5) | 2.07 | 0.06 | 0.284 | −0.38 | 0.15 | 0.588 | −0.34 | 0.27 | 0.438 | 0.06 | 2.30 | 0.219 | 4.28 | 0.10 | 0.001** |
| ALA (18 : 3) | 0.08 | 0.00 | 0.96 | −0.35 | 0.01 | 0.619 | −0.37 | 0.01 | 0.407 | 0.06 | 0.08 | 0.195 | 4.27 | 0.00 | 0.001** |
| Total omega-3 | −0.09 | −0.01 | 0.848 | −0.33 | −0.01 | 0.635 | −0.37 | −0.01 | 0.407 | 0.06 | −0.10 | 0.191 | 4.27 | 0.00 | 0.001** |
| DPA (n-6) (22 : 5) | 2.47 | 0.04 | 0.48 | −0.44 | 0.18 | 0.525 | −0.39 | 0.32 | 0.391 | 0.07 | 2.74 | 0.169 | 4.21 | 0.12 | 0.001** |
| Adrenic (22 : 4) | 1.00 | 0.03 | 0.539 | −0.43 | 0.07 | 0.542 | −0.36 | 0.13 | 0.423 | 0.06 | 1.11 | 0.197 | 4.26 | 0.05 | 0.001** |
| AA (20 : 4) | 0.06 | 0.01 | 0.816 | −0.37 | 0.00 | 0.595 | −0.36 | 0.01 | 0.425 | 0.06 | 0.07 | 0.192 | 4.28 | 0.00 | 0.001** |
| DGLA (20 : 3) | 0.86 | 0.04 | 0.37 | −0.46 | 0.06 | 0.521 | −0.36 | 0.11 | 0.423 | 0.06 | 0.95 | 0.211 | 4.31 | 0.04 | 0.001** |
| GLA (18 : 3) | 0.49 | 0.02 | 0.748 | −0.37 | 0.04 | 0.596 | −0.37 | 0.06 | 0.413 | 0.06 | 0.55 | 0.197 | 4.29 | 0.02 | 0.001** |
| LA (18 : 2) | −0.04 | −0.01 | 0.827 | −0.34 | 0.00 | 0.624 | −0.37 | −0.01 | 0.407 | 0.06 | −0.04 | 0.209 | 4.29 | 0.00 | 0.001** |
| Total omega-6 | 0.02 | 0.01 | 0.852 | −0.36 | 0.00 | 0.607 | −0.36 | 0.00 | 0.421 | 0.06 | 0.02 | 0.188 | 4.27 | 0.00 | 0.001** |
| Omega-3 index | −0.67 | −0.07 | 0.215 | −0.28 | −0.05 | 0.688 | −0.39 | −0.09 | 0.391 | 0.06 | −0.74 | 0.191 | 4.18 | −0.03 | 0.002** |
| Ratio DHA : AA | −13.39 | −0.12 | 0.009** | −0.37 | −0.96 | 0.59 | −0.32 | −1.76 | 0.478 | 0.06 | −14.86 | 0.174 | 4.05 | −0.64 | 0.002** |
| Ratio EPA : AA | 11.94 | 0.05 | 0.43 | −0.32 | 0.86 | 0.645 | −0.37 | 1.57 | 0.407 | 0.06 | 13.26 | 0.22 | 4.26 | 0.57 | 0.001** |
CSHQ, Child Sleep Habits Questionnaire; OLS, ordinary least squares; ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (both omega-3 and omega-6); DHA, docosahexaenoic acid; LA, linoleic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid.
On the basis of bootstrapped standard errors, 500 repetitions, seed 14 141.
P < 0.05,
P < 0.01.
Author contributions
The study was conceived and designed by: PM and AJR. The study was operationalized and implemented by JRB and RPS. The data were analysed by TFS and PM. The paper was written by PM, AJR, TFS and JRB. ‘Major revisions and final approval by PM, AJR, JRB, TFS and RPS.
Conflicts of interest
JRB, RPS and TFS have no competing interests. PM and AJR have been and are involved in occasional paid consultancy work (lectures and advice) for companies and organizations which produce, sell or promote foods or supplements containing omega-3 fatty acids. None of these competing interests have influenced this study's conduct, analyses or reporting.
References
- Bailey-Hall E, Nelson EB, Ryan AS. Validation of a rapid measure of blood PUFA levels in humans. Lipids. 2008;43:181–186. doi: 10.1007/s11745-007-3140-7. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J. Am. Acad. Child Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am. J. Clin. Nutr. 2000;71(Suppl. 1):327S–330S. doi: 10.1093/ajcn/71.1.327S. [DOI] [PubMed] [Google Scholar]
- Catala A. The function of very long chain polyunsaturated fatty acids in the pineal gland. Biochim. Biophys. Acta. 2010;1801:95–99. doi: 10.1016/j.bbalip.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am. J. Clin. Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- Cornu C, Remontet L, Noel-Baron F, et al. A dietary supplement to improve the quality of sleep: a randomized placebo controlled trial. BMC Complement. Altern. Med. 2010;10:29. doi: 10.1186/1472-6882-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- Department of Education. Department of Education Special Educational Needs Code of Practice—DfES/581/2001. London: Department of Education; 2001. [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med. Rev. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Harris WS, Klurfeld DM. Twentieth-century trends in essential fatty acid intakes and the predicted omega-3 index: evidence versus estimates. Am. J. Clin. Nutr. 2011;93:907–908. doi: 10.3945/ajcn.111.014365. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr. Top. Med. Chem. 2011;11:1047–1057. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- Huss M, Volp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems—an observational cohort study. Lipids Health Dis. 2010;9:105. doi: 10.1186/1476-511X-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Waku K, Yamaguchi C, et al. A convenient method for determination of the C20-22 PUFA composition of glycerolipids in blood and breast milk. Lipids. 2002;37:523–526. doi: 10.1007/s11745-002-0927-x. [DOI] [PubMed] [Google Scholar]
- Ivanhoe JR, Lefebvre CA, Stockstill JW. Sleep disordered breathing in infants and children: a review of the literature. Pediatr. Dent. 2007;29:193–200. [PubMed] [Google Scholar]
- Judge MP, Cong X, Harel O, Courville AB, Lammi-Keefe CJ. Maternal consumption of a DHA-containing functional food benefits infant sleep patterning: an early neurodevelopmental measure. Early Hum. Dev. 2012;88:531–537. doi: 10.1016/j.earlhumdev.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Kopasz M, Loessl B, Hornyak M, et al. Sleep and memory in healthy children and adolescents—a critical review. Sleep Med. Rev. 2010;14:167–177. doi: 10.1016/j.smrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ladesich JB, Pottala JV, Romaker A, Harris WS. Membrane level of omega-3 docosahexaenoic acid is associated with severity of obstructive sleep apnea. J. Clin. Sleep Med. 2011;7:391–396. doi: 10.5664/JCSM.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M, Champeil-Potokar G, Alessandri JM, et al. An (n-3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J. Nutr. 2008;138:1719–1724. doi: 10.1093/jn/138.9.1719. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang A, Li L. Sleep duration and overweight/obesity in children: review and implications for pediatric nursing. J. Spec. Pediatr. Nurs. 2012;17:193–204. doi: 10.1111/j.1744-6155.2012.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–166. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: a cross-sectional analysis from the DOLAB study. PLoS One. 2013;8:e66697. doi: 10.1371/journal.pone.0066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- Papandreou C. Independent associations between fatty acids and sleep quality among obese patients with obstructive sleep apnoea syndrome. J. Sleep Res. 2013;22:569–572. doi: 10.1111/jsr.12043. [DOI] [PubMed] [Google Scholar]
- Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr. Res. 2012;32:309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Price AM, Wake M, Ukoumunne OC, Hiscock H. Five-year follow-up of harms and benefits of behavioral infant sleep intervention: randomized trial. Pediatrics. 2012;130:643–651. doi: 10.1542/peds.2011-3467. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int. Rev. Psychiatry. 2006;18:155–172. doi: 10.1080/09540260600583031. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Montgomery P. The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 Years: a randomized, controlled trial (The DOLAB Study) PLoS One. 2012;7:e43909. doi: 10.1371/journal.pone.0043909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep–wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation's children. Pediatrics. 2007;119(Suppl. 1):S29–S37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- Standards and Testing Agency. National Curriculum Teacher Assessments and Key Stage Tests. London: Department of Education; 2009. [Google Scholar]
- Stevens LJ, Zentall SS, Deck JL, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am. J. Clin. Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- Tan ML, Ho JJ, Teh KH. Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders. Cochrane Database Syst. Rev. 2012;12:CD009398. doi: 10.1002/14651858.CD009398.pub2. [DOI] [PubMed] [Google Scholar]
- Tilmanne J, Urbain J, Kothare MV, Wouwer AV, Kothare SV. Algorithms for sleep–wake identification using actigraphy: a comparative study and new results. J. Sleep Res. 2009;18:85–98. doi: 10.1111/j.1365-2869.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:512–519. [PubMed] [Google Scholar]
- Wiggs L, Montgomery P, Stores G. Actigraphic and parent reports of sleep patterns and sleep disorders in children with subtypes of attention-deficit hyperactivity disorder. Sleep. 2005;28:1437–1445. doi: 10.1093/sleep/28.11.1437. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and sleep: mini-review and hypothesis. Med. Hypotheses. 1998;50:139–145. doi: 10.1016/s0306-9877(98)90200-6. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz-Shenkar S, Carasso RL. Effects of essential fatty acids in iron deficient and sleep-disturbed attention deficit hyperactivity disorder (ADHD) children. Eur. J. Clin. Nutr. 2011;65:1167–1169. doi: 10.1038/ejcn.2011.80. [DOI] [PubMed] [Google Scholar]
- Zaouali-Ajina M, Gharib A, Durand G, et al. Dietary docosahexaenoic acid-enriched phospholipids normalize urinary melatonin excretion in adult (n-3) polyunsaturated fatty acid-deficient rats. J. Nutr. 1999;129:2074–2080. doi: 10.1093/jn/129.11.2074. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hamilton JH, Salem N, Jr, Kim HY. N-3 fatty acid deficiency in the rat pineal gland: effects on phospholipid molecular species composition and endogenous levels of melatonin and lipoxygenase products. J. Lipid Res. 1998;39:1397–1403. [PubMed] [Google Scholar]


