Abstract
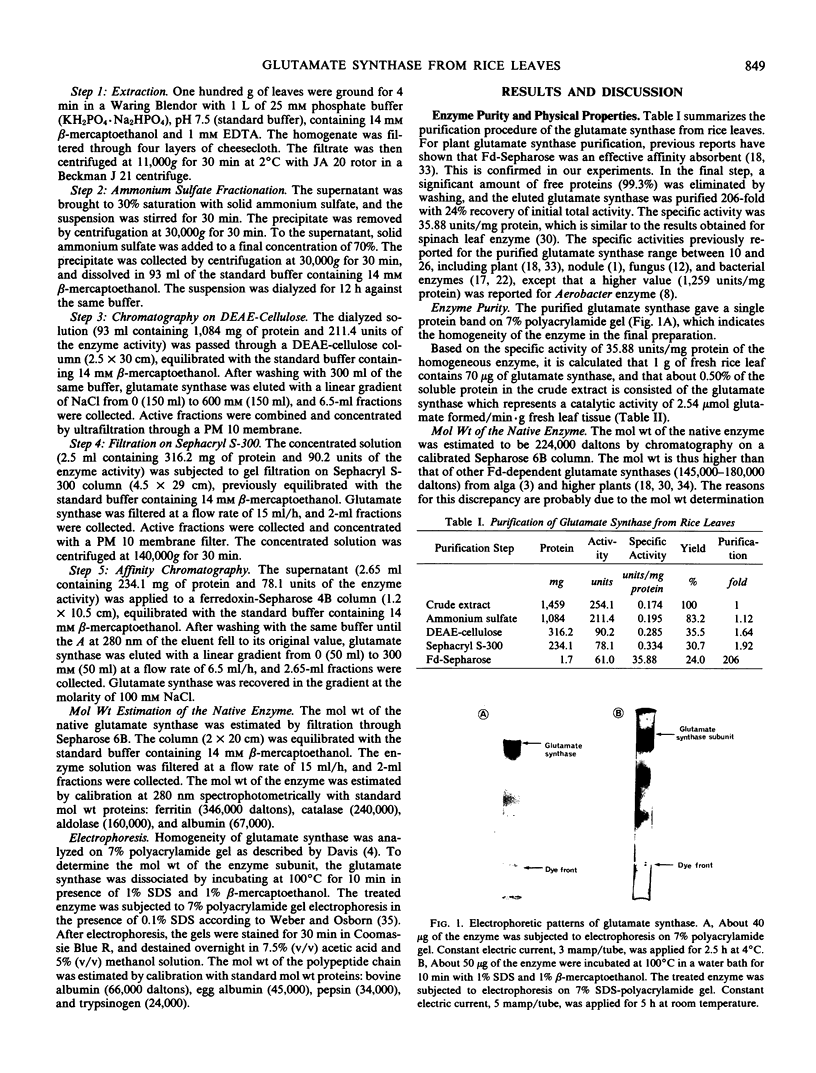
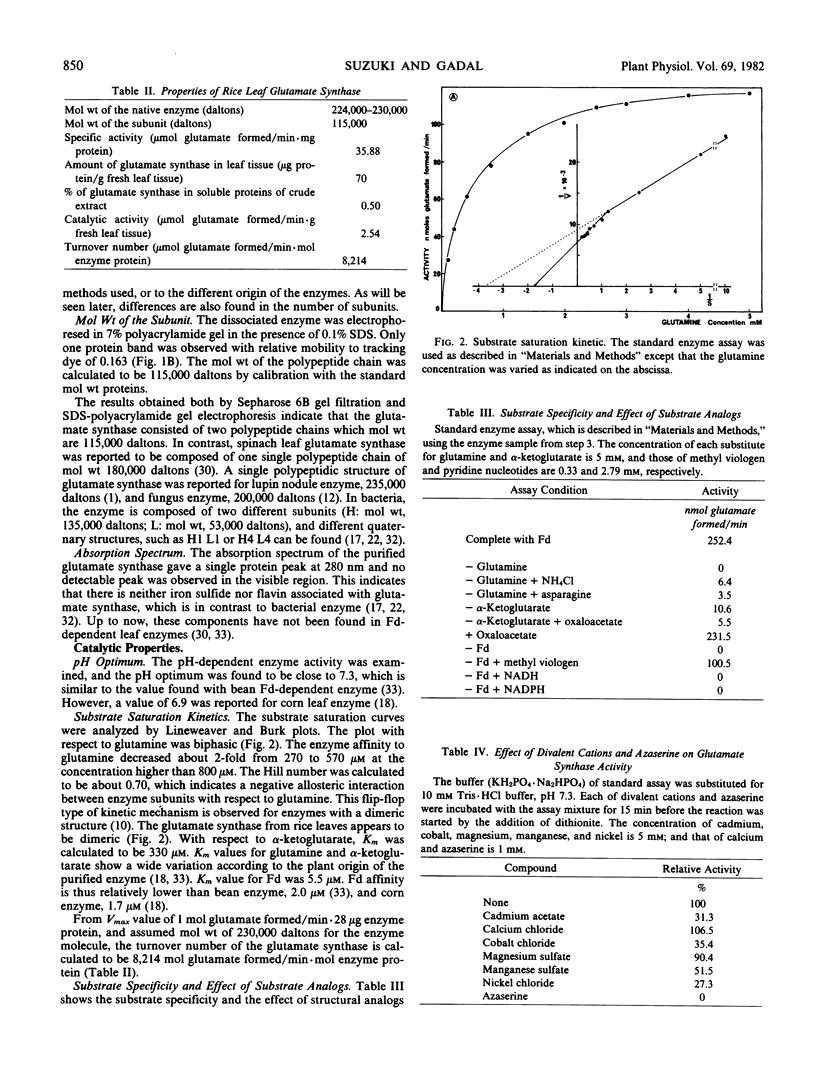
Ferredoxin-dependent glutamate synthase (EC 1.4.7.1) from rice leaves (Oryza sativa L. cv Delta) was purified 206-fold with a final specific activity of 35.9 μmoles glutamate formed per min per milligram protein by a procedure including ammonium sulfate fractionation, DEAE-cellulose chromatography, Sephacryl S-300 gel filtration, and ferredoxin-Sepharose affinity chromatography. The purified enzyme yielded a single protein band on polyacrylamide gel electrophoresis. Molecular weight of the native enzyme was estimated to be 224,000 daltons by Sepharose 6B gel filtration. Electrophoresis of the dissociated enzyme in sodium dodecyl sulfate-polyacrylamide gel gave a single protein band which corresponds to the subunit molecular weight of 115,000 daltons. Thus, it is concluded that the glutamate synthase is composed of two polypeptidic chains exhibiting the same molecular weight. Spectrophotometric analysis indicated that the enzyme is free of iron-sulfide and flavin. The pH optimum was 7.3. The enzyme had a negative cooperativity (Hill number of 0.70) for glutamine, and its Km value increased from 270 to 570 μm at a glutamine concentration higher than 800 μm. Km values for α-ketoglutarate and ferredoxin were 330 and 5.5 μm, respectively. Asparagine and oxaloacetate could not be substituted for glutamine and α-ketoglutarate, respectively. Enzyme activity was not detected with pyridine nucleotides as electron donors. Azaserine and several divalent cations were potent inhibitors. The purified enzyme was stabilized by dithiothreitol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland M. J., Benny A. G. Enzymes of nitrogen metabolism in legume nodules. Purification and properties of NADH-dependent glutamate synthase from lupin nodules. Eur J Biochem. 1977 Oct 3;79(2):355–362. doi: 10.1111/j.1432-1033.1977.tb11816.x. [DOI] [PubMed] [Google Scholar]
- Chapman D. J., Leech R. M. Changes in Pool Sizes of Free Amino Acids and Amides in Leaves and Plastids of Zea mays during Leaf Development. Plant Physiol. 1979 Mar;63(3):567–572. doi: 10.1104/pp.63.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fowler M. W., Jessup W., Sarkissian G. S. Glutamate synthetase type activity in higher plants. FEBS Lett. 1974 Sep 15;46(1):340–342. doi: 10.1016/0014-5793(74)80401-1. [DOI] [PubMed] [Google Scholar]
- Geary L. E., Meister A. On the mechanism of glutamine-dependent reductive amination of alpha-ketoglutarate catalyzed by glutamate synthase. J Biol Chem. 1977 May 25;252(10):3501–3508. [PubMed] [Google Scholar]
- Harada K., Wolfe R. G. Malic dehydrogenase. VII. The catalytic mechanism and possible role of identical protein subunits. J Biol Chem. 1968 Aug 10;243(15):4131–4137. [PubMed] [Google Scholar]
- Hirel B., Gadal P. Glutamine Synthetase in Rice: A COMPARATIVE STUDY OF THE ENZYMES FROM ROOTS AND LEAVES. Plant Physiol. 1980 Oct;66(4):619–623. doi: 10.1104/pp.66.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelt G., Mora J. Regulation and function of glutamate synthase in Neurospora crassa. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1688–1694. doi: 10.1016/0006-291x(80)91368-6. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. The occurrence of glutamate synthase in algae. Biochem Biophys Res Commun. 1975 Jan 2;64(3):856–862. doi: 10.1016/0006-291x(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G. Nondenaturing procedure for rapid preparation of ferredoxin from Clostridium pasteurianum. Anal Biochem. 1971 Jul;42(1):191–194. doi: 10.1016/0003-2697(71)90025-x. [DOI] [PubMed] [Google Scholar]
- McKenzie G. H., Ch'ng A. L., Gayler K. R. Glutamine Synthetase/Glutamine: alpha-Ketoglutarate Aminotransferase in Chloroplasts from the Marine Alga Caulerpa simpliciuscula. Plant Physiol. 1979 Mar;63(3):578–582. doi: 10.1104/pp.63.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Glutamate synthase. Properties of the glutamine-dependent activity. J Biol Chem. 1976 Jun 10;251(11):3294–3299. [PubMed] [Google Scholar]
- Platt S. G., Anthon G. E. Ammonia accumulation and inhibition of photosynthesis in methionine sulfoximine treated spinach. Plant Physiol. 1981 Mar;67(3):509–513. doi: 10.1104/pp.67.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




