Abstract
Early identification of chronic hepatitis B is important for optimal disease management and prevention of transmission. Cost and lack of access to commercial hepatitis B surface antigen (HBsAg) immunoassays can compromise the effectiveness of HBV screening in resource-limited settings and among marginalized populations. High-quality point-of-care (POC) testing may improve HBV diagnosis in these situations. Currently available POC HBsAg assays are often limited in sensitivity. We evaluated the NanoSign® HBs POC chromatographic immunoassay for its ability to detect HBsAg of different genotypes and with substitutions in the ‘a’ determinant. Thirty-seven serum samples from patients with HBV infection, covering HBV genotypes A–G, were assessed for HBsAg titre with the Roche Elecsys HBsAg II quantification assay and with the POC assay. The POC assay reliably detected HBsAg at a concentration of at least 50 IU/mL for all genotypes, and at lower concentrations for some genotypes. Eight samples with substitutions in the HBV ‘a’ determinant were reliably detected after a 1/100 dilution. The POC strips were used to screen serum samples from 297 individuals at risk for HBV in local clinical settings (health fairs and outreach events) in parallel with commercial laboratory HBsAg testing (Quest Diagnostics EIA). POC testing was 73.7% sensitive and 97.8% specific for detection of HBsAg. Although the POC test demonstrated high sensitivity over a range of genotypes, false negatives were frequent in a clinical setting. Nevertheless, the POC assay offers advantages for testing in both developed and resource-limited countries due to its low cost (0.50$) and immediately available results.
Keywords: diagnosis, Hepatitis B S antigen, point-of-care, variant
Introduction
Chronic infection with hepatitis B virus (HBV) results in an estimated 786 000 deaths annually worldwide 1. Infected individuals may remain asymptomatic for long periods but are at risk of progressive liver disease, and can transmit the virus to other susceptible individuals. Early identification of infection is thus important. The primary marker for screening and laboratory diagnosis of HBV infection is hepatitis B surface antigen (HBsAg), a component of the virus envelope that is also found in the blood in great excess as noninfectious subviral particles. Immunoassays with high sensitivity and specificity for HBsAg detection are used in developed countries and are subject to strict regulatory requirements and quality assurance. While HBsAg testing has been at the forefront of blood screening and subsequent improvements to public health, the cost and the lack of access to commercial immunoassays can limit their usefulness in resource-limited settings and among marginalized high-risk populations including injection drug users, immigrants and the homeless. The time and expense involved in blood processing and patient call-back and counselling can be considerable, and many patients are lost to follow-up. High-quality point-of-care (POC) testing may overcome some of these hurdles and reduce costs.
A number of rapid POC assays for HBsAg are commercially available. These tests have a range of diagnostic accuracy and generally do not have the same sensitivity and specificity as commercial screening immunoassays, potentially returning false-negative or false-positive results leading to subsequent inappropriate clinical management. With ten different HBV genotypes (A–J), genetic diversity presents a major challenge, and genotype-dependent reduced sensitivity has been shown with some assays 2. In addition, HBV variants carrying amino acid substitutions in the ‘a’ determinant of HBsAg can escape detection 2–4; such amino acid substitutions can emerge under the selective pressure of antiviral therapy (e.g. lamivudine) or immune response (e.g. postvaccination). We evaluated a POC test for the detection of HBsAg – the NanoSign® HBs POC strips – using samples from patients infected with different HBV genotypes and defined specimens with substitutions in the ‘a’ determinant. We also used the test to screen a panel of at-risk patients and compared the results with standard-of-care (SOC) serologic testing.
Methods
The NanoSign HBs POC strip (Bioland, Seoul, South Korea) is a chromatographic immunoassay. A mouse monoclonal antibody to HBsAg (capture reagent) is immobilized on a nitrocellulose membrane. A second mouse monoclonal antibody to HBsAg, conjugated to colloidal gold, is in a fibre pad in contact with the membrane (detector reagent). When the strip is placed into the serum sample, the solubilized conjugate binds to any HBsAg present. The resulting complex migrates by passive diffusion along the test strip until it is captured by the antibody immobilized on the nitrocellulose membrane and forms a visible coloured band. A coloured control band, confirming that the colloidal gold conjugate is functional, must also appear for the test to be valid. The manufacturer claims the assay can detect HBsAg concentrations as low as 1.4 IU/mL.
Forty-five HBsAg-positive serum samples from patients with chronic HBV infection were obtained from a bio-repository at the Victorian Infectious Diseases Reference Laboratory, Melbourne, Australia. HBV genotype was determined using the online HBV genome analysis program SeqHepB, as previously described 5,6. Thirty-seven samples, covering HBV genotypes A–G, were assessed for HBsAg titre with the Roche Elecsys HBsAg II quantification assay (Roche Diagnostics, Mannheim, Germany). The dead volume remaining in the Elecsys sample cup after quantification (≈100 μL) was used to evaluate the NanoSign® HBs POC strips. Serial tenfold dilutions of samples representing each HBV genotype were tested with both assays. Eight samples with known substitutions in the HBV ‘a’ determinant were tested with both assays, undiluted and at a dilution of 1/100.
NanoSign® HBs POC strips were used to screen serum samples from 297 individuals at risk of HBV in local clinical settings (health fairs and outreach events) in parallel with SOC serologic testing for HBsAg through a commercial laboratory (Quest Diagnostics EIA; Madison, NJ, USA). Trained technicians under the supervision of a pharmacist or physician performed and read the results of the POC tests. All participants provided informed consent.
Results and Discussion
Genotype sensitivity
The 37 samples tested included HBV genotypes A (n = 4), B (n = 4), C (n = 6), D (n = 4), E (n = 8), F (n = 4) and G (n = 7). Two HBV genotype C samples were the divergent subgenotype C4, found only in Indigenous Australians, which has a surface gene region most closely related to HBV genotype J 7. All 37 samples tested positive for HBsAg using the POC strips (sensitivity 100%). HBsAg-negative samples did not produce any signal (specificity 100%), even when samples were seropositive for hepatitis A virus or hepatitis C virus. Testing serially diluted serum samples (Fig.1a) showed that the POC strips reliably detected HBsAg at a concentration of at least 50 IU/mL for all genotypes and at lower concentrations for some genotypes. We acknowledge that this detection limit is similar to or slightly higher than most HBsAg enzyme immunoassays 3; however, POC strips appear to be sensitive enough to detect HBsAg levels in serum from chronically infected patients across a range of genotypes.
Fig 1.
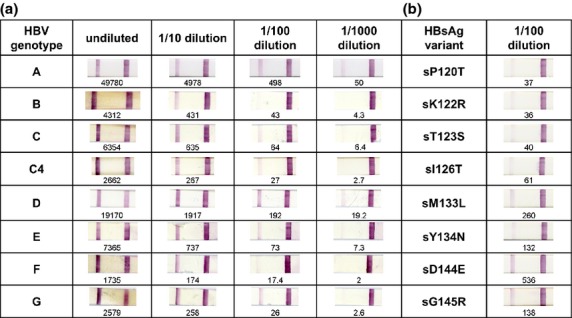
NanoSign HBs POC test strip results for a panel of serum samples with known HBV genotypes (panel a) and for eight samples with ‘a’ determinant mutations (panel b). HBsAg titres measured by Elecsys HBsAg II assay are indicated under each strip in IU/mL. The assay control band is on the right of each strip with the sample band on the left.
Detection of HBsAg variants
All of the eight samples with known substitutions in the HBV ‘a’ determinant were reliably detected after a 1/100 dilution (Fig.1b). Common HBsAg variants surface (s)D144E and sG145R, in particular, showed clear reactivity. This is notable because the ‘a’ determinant fragment 137–147 constitutes the antigen domain recognized by neutralizing antibodies. Immune selection pressure following extensive vaccination programmes has led to increased frequency of these variants, particularly sG145R 8. The sM133L variant appears as a false negative in several HBsAg test kits, even at concentrations above the detection limit established for wild-type HBV samples 3. The sM133L variant was clearly detectable with the HBs POC strips. Our results suggest that NanoSign HBs POC strips can detect a broad range of HBsAg variants. It would be informative to evaluate the POC strips using additional mutants known to escape detection with other tests.
Patient screening
Among the 297 patients tested, 72% were Vietnamese attending community outreach events at churches and health fairs, 66% were female and the mean age was 54 years. Only 4% reported being born in the United States and 42.4% reported having access to health care. Overall, 19 (6.4%) tested by SOC were positive for HBsAg. POC testing was 73.7% sensitive and 97.8% specific for the detection of HBsAg (Table1). The positive predictive value was 70%, and the negative predictive value was 98.2%, thus POC testing failed to detect 26% of positive cases of HBV infection, but identified 98% of those requiring vaccination. The cost of SOC was five times higher than POC, costing $126.43 vs. $25.30 per positive case. The high rate of false negatives could have been related to operator error, low HBsAg levels, assay degeneration or lot variation.
Table 1.
Results of HBsAg screening with NanoSign HBs POC test strips and commercial standard-of-care (SOC) testing*
| HBsAg detection | SOC positive | SOC negative | Total |
|---|---|---|---|
| POC positive | 14 | 6 | 20 |
| POC negative | 5 | 272 | 277 |
| Total | 19 | 278 | 297 |
SOC serologic testing performed by Quest Diagnostics.
Conclusion
In conclusion, in a laboratory setting, we demonstrated that NanoSign HBs POC strips can detect HBsAg at levels much lower than normally found in the blood of infected individuals and that the strips were nonreactive for samples previously shown to be HBsAg negative. In addition, the POC assay detected a range of HBV genotypes and ‘a’ determinant variants. In a clinical setting, although the POC assay demonstrated high specificity, false negatives were frequent. As HBV genotype and HBsAg variants do not appear to alter the results based on our study, the high number of false negatives could be explained by operator or batch variation. Therefore, this test is not appropriate for HBV screening in blood banks. Nevertheless, the POC assay still offers advantages for testing in both developed and resource-limited countries due to its low cost (0.50$) and immediately available results (20 min from phlebotomy to laboratory test assessment). This affordable and rapid test could provide a significant improvement in HBsAg screening options for high-prevalence groups, drastically lowering screening costs and reducing the barriers to testing.
Acknowledgments
All authors had full access to the study data, contributed to the writing and or review of the manuscript and approved the manuscript for submission. The study was funded in part by the Gilead and Bristol-Myers Squibb Foundations. The authors acknowledge the help of members of the Asian Pacific Health Foundation and pharmacy student volunteers during the patient testing phase of the study.
Glossary
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- POC
point-of-care
Conflict of Interests
The authors have no conflict of interests to declare. B Tran is the Executive Director of the Asian Pacific Health Foundation, a non-profit organization.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheiblauer H, El-Nageh M, Diaz S, et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang. 2010;98:403–414. doi: 10.1111/j.1423-0410.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger KM, Bauer T, Böhm D, Jilq W. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J Gen Virol. 2000;81:1165–1174. doi: 10.1099/0022-1317-81-5-1165. [DOI] [PubMed] [Google Scholar]
- 4.Ly TD, Servant-Delmas A, Bagot S, et al. Sensitivities of four new hepatitis B virus surface antigen (HBsAg) assays in detection of HBsAg mutant forms. J Clin Microbiol. 2006;44:2321–2326. doi: 10.1128/JCM.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuen LK, Ayres A, Littlejohn M, et al. SeqHepB: a sequence analysis program and relational database system for chronic hepatitis B. Antiviral Res. 2007;75:64–74. doi: 10.1016/j.antiviral.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Ayres A, Locarnini S, Bartholomeusz A. HBV genotyping and analysis for unique mutations. Methods Mol Med. 2004;95:125–149. doi: 10.1385/1-59259-669-X:125. [DOI] [PubMed] [Google Scholar]
- 7.Davies J, Littlejohn M, Locarnini SA, et al. The molecular epidemiology of hepatitis B in the Indigenous people of northern Australia. J Gastroenterol Hepatol. 2013;28:1234–1241. doi: 10.1111/jgh.12177. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman JN, Zuckerman AJ. Mutations of the surface protein of hepatitis B virus. Antiviral Res. 2003;60:75–78. doi: 10.1016/j.antiviral.2003.08.013. [DOI] [PubMed] [Google Scholar]


