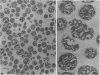
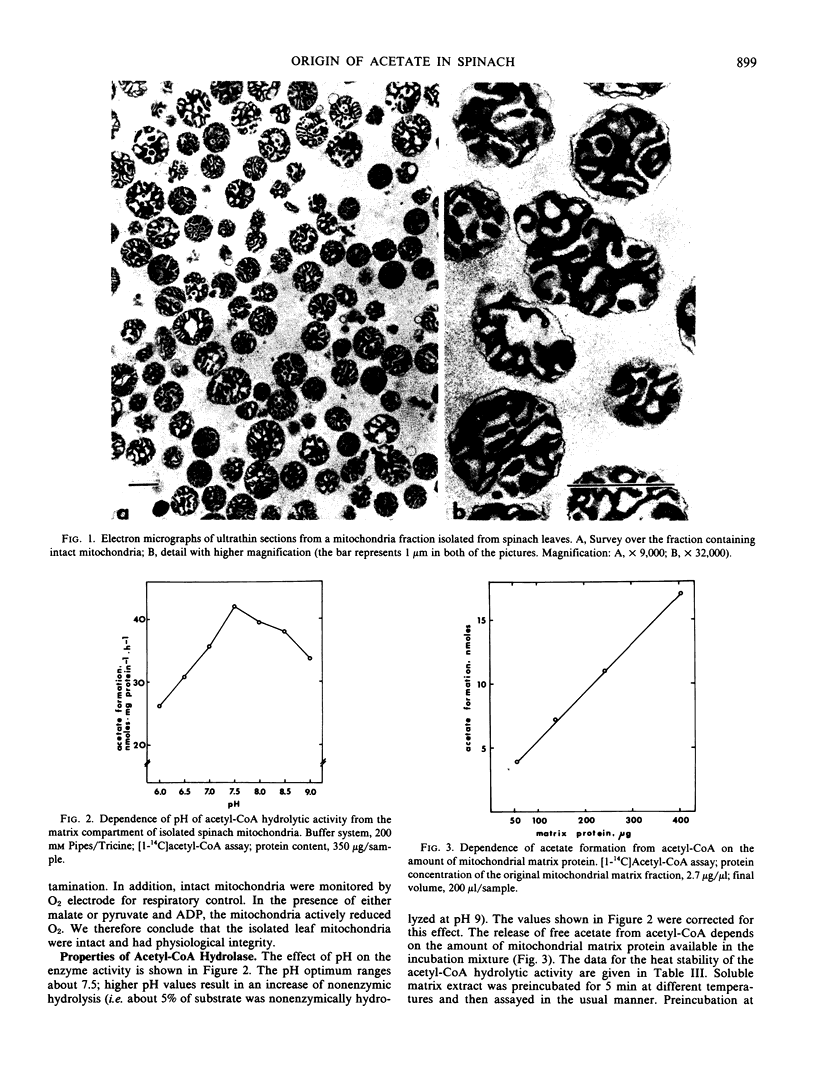
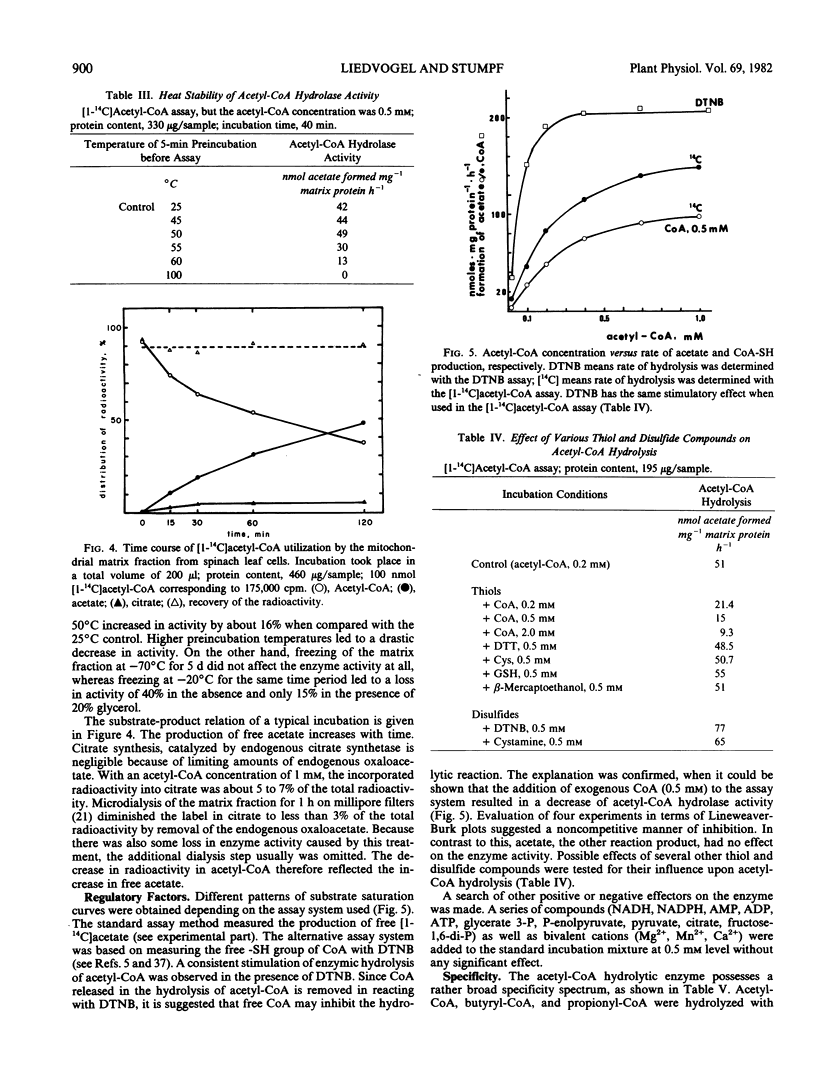
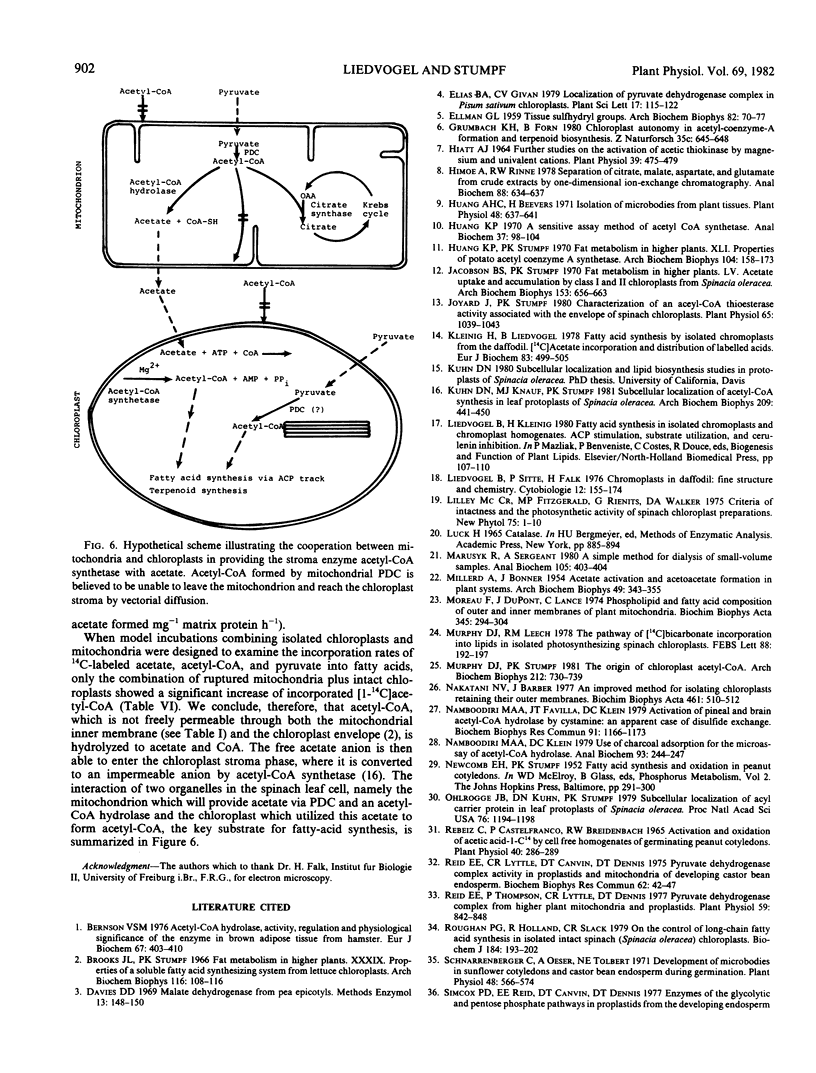
Abstract
Mitochondria were isolated from spinach (Spinacia oleracea L.) leaves using a Percoll gradient step. The high purity of the organelle fraction is demonstrated by electron microscopy and biochemical parameters. In the matrix space of these mitochondria, a short-chain acyl-coenzyme A hydrolase is present that converts acetyl-coenzyme A to acetate and coenzyme A with reasonable rates (Km, 150 micromolar; Vmax, 140 nanomoles acetate formed milligram1 protein hour−1). The enzyme is product inhibited by coenzyme A-sulfhydryl, other thiols are ineffective; however, the disulfides 5,5′-dithio-bis-(2-nitrobenzoate) and cystamine stimulate the hydrolysis. The possible role of this mitochondrial enzyme as a means of generating free acetate from pyruvate via acetyl-coenzyme A in the mitochondria of mature spinach leaves is discussed. It is suggested that free acetate moves rapidly from the mitochondrion to the chloroplast where acetyl-coenzyme A synthetase, solely localized in this organelle, converts the metabolically inert free acetate to the highly active acetyl-coenzyme A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernson S. M. Acetyl-CoA hydrolase; activity, regulation and physiological significance of the enzyme in brown adipose tissue from hamster. Eur J Biochem. 1976 Aug 16;67(2):403–410. doi: 10.1111/j.1432-1033.1976.tb10705.x. [DOI] [PubMed] [Google Scholar]
- Brooks J. L., Stumpf P. K. Fat metabolism in higher plants. XXXIX. Properties of a soluble fatty acid synthesizing system from lettuce chloroplasts. Arch Biochem Biophys. 1966 Sep 26;116(1):108–116. doi: 10.1016/0003-9861(66)90019-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Hiatt A. J. Further Studies on the Activation of Acetic Thiokinase by Magnesium and Univalent Cations. Plant Physiol. 1964 May;39(3):475–479. doi: 10.1104/pp.39.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himoe A., Rinne R. W. Separation of citrate, malate, aspartate, and glutamate in crude extracts by one-dimensional ion exchange thin-layer chromatography. Anal Biochem. 1978 Aug 1;88(2):634–637. doi: 10.1016/0003-2697(78)90466-9. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P. A sensitive assay method of acetyl CoA synthetase. Anal Biochem. 1970 Sep;37(1):98–104. doi: 10.1016/0003-2697(70)90263-0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Stumpf P. K. Fat metabolism in higher plants. XLI. Properties of potato acetyl coenzyme A synthetase. Arch Biochem Biophys. 1970 Sep;140(1):158–173. doi: 10.1016/0003-9861(70)90019-6. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Stumpf P. K. Fat metabolism in higher plants. LV. Acetate uptake and accumulation by class I and II chloroplasts from Spinacia oleracea. Arch Biochem Biophys. 1972 Dec;153(2):656–663. doi: 10.1016/0003-9861(72)90384-0. [DOI] [PubMed] [Google Scholar]
- Joyard J., Stumpf P. K. Characterization of an acyl-coenzyme a thioesterase associated with the envelope of spinach chloroplasts. Plant Physiol. 1980 Jun;65(6):1039–1043. doi: 10.1104/pp.65.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. Fatty acid synthesis by isolated chromoplasts from the daffodil. [14C]Acetate incorporation and distribution of labelled acids. Eur J Biochem. 1978 Feb;83(2):499–505. doi: 10.1111/j.1432-1033.1978.tb12116.x. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Knauf M., Stumpf P. K. Subcellular localization of acetyl-CoA synthetase in leaf protoplasts of Spinacia oleracea. Arch Biochem Biophys. 1981 Jul;209(2):441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- MILLERD A., BONNER J. Acetate activation and acetoacetate formation in plant systems. Arch Biochem Biophys. 1954 Apr;49(2):343–355. doi: 10.1016/0003-9861(54)90204-0. [DOI] [PubMed] [Google Scholar]
- Marusyk R., Sergeant A. A simple method for dialysis of small-volume samples. Anal Biochem. 1980 Jul 1;105(2):403–404. doi: 10.1016/0003-2697(80)90477-7. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. The origin of chloroplastic acetyl coenzyme A. Arch Biochem Biophys. 1981 Dec;212(2):730–739. doi: 10.1016/0003-9861(81)90417-3. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Namboodiri M. A., Favilla J. T., Klein D. C. Activation of pineal and brain acetyl-COA hydrolase by cystamine: an apparent case of disulfide exchange. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1166–1173. doi: 10.1016/0006-291x(79)92002-3. [DOI] [PubMed] [Google Scholar]
- Namboodiri M. A., Klein D. C. Use of charcoal adsorption for the microassay of acetyl-CoA-hydrolase. Anal Biochem. 1979 Mar;93(2):244–247. doi: 10.1016/s0003-2697(79)80145-1. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBEIZ C., CASTELFRANCO P., BREIDENBACH R. W. ACTIVATION AND OXIDATION OF ACETIC ACID-1-C14 BY CELL FREE HOMOGENATES OF GERMINATING PEANUT COTYLEDONS. Plant Physiol. 1965 Mar;40:286–289. doi: 10.1104/pp.40.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E. E., Lyttle C. R., Canvin D. T., Dennis D. T. Pyruvate dehydrogenase complex activity in proplastids and mitochondria of developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 6;62(1):42–47. doi: 10.1016/s0006-291x(75)80402-5. [DOI] [PubMed] [Google Scholar]
- Reid E. E., Thompson P., Lyttle C. R., Dennis D. T. Pyruvate dehydrogenase complex from higher plant mitochondria and proplastids. Plant Physiol. 1977 May;59(5):842–848. doi: 10.1104/pp.59.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Randall D. D. Pyruvate Dehydrogenase Complex from Chloroplasts of Pisum sativum L. Plant Physiol. 1979 Dec;64(6):1099–1103. doi: 10.1104/pp.64.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]