Abstract
Background
Axillary reverse mapping (ARM) is a novel technique for preserving the upper extremity lymphatic pathways during axillary lymph node surgery. However, there is no evidence of the usefulness of ARM for patients undergoing sentinel lymph node biopsy (SNB).
Methods
Between August 2009 and July 2012, 372 patients who underwent the SNB procedure for breast cancer were enrolled in this study. Using the indocyanine green fluorescence technique and indigocarmine blue dye method, we studied the relationship between the upper extremity lymphatic flow and breast sentinel node (SN). Our aim of this study was the probability of postoperative lymphedema with respect to whether the upper extremity lymphatics corresponded to the breast SN.
Results
Among the 327 patients who underwent the SNB procedure, the upper extremity lymphatics drainage into the breast SN in 76 (23.2%; corresponding group), and only 5 patients in this group developed lymphedema. In contrast, none of the patients in the noncorresponding group developed lymphedema.
Conclusions
ARM during SN biopsy can identify the group of patients who are at high risk for developing lymphedema. More risk-focused guidance should be used for these patients. J. Surg. Oncol. 2014 109:612–615.
Keywords: breast cancer, axillary reverse mapping, sentinel lymphnode biopsy, lymphedema, fluorescence image
INTRODUCTION
The sentinel lymph node biopsy (SNB) has been established as a less invasive approach for axillary staging; however, this procedure continues to have a risk of postoperative lymphedema.
Axillary reverse mapping (ARM) is a novel technique for preserving the upper extremity lymphatic pathways during axillary lymph node surgery. However, there is no evidence of the usefulness of ARM in patients undergoing the SNB procedure. We used the indocyanine green (ICG, Diagnogreen: Daiichi Sankyo, Co., Ltd., Tokyo, Japan) fluorescence technique to perform ARM during the SNB procedure and found this method to be useful for identifying the risk group of postoperative lymphedema.
PATIENTS AND METHODS
Patients
Between August 2009 and July 2012, 432 SNB procedures for breast cancer were performed at our hospital. Among these, 372 patients who underwent surgery for Stage 0–IIB (Tis–T3, N0, and M0) primary breast cancer, without preoperative chemotherapy or bilateral disease, were enrolled in this study. Forty-five patients who revealed sentinel lymph node (SN) metastasis (pN1 mi and pN1) were excluded. Finally 327 patients were studied and they were divided into 2 groups (corresponding and noncorresponding groups). We defined the corresponding group as those with upper extremity lymphatic drainage into the breast SN (ICG fluorescence “shine” at the breast SN and/or upper extremity blue lymphatic drainage into the breast SN) and the noncorresponding group as those with both “do not shine” and “do not drain from the upper extremity.” Six of the 327 patients were unsuitable for analysis because of recurrence or loss to follow-up; thus, 321 patients who had undergone the SNB procedure were finally analyzed (Fig. 1). This study was approved by the institutional review board of our hospital, and all patients agreed to participate after providing written informed consent.
Fig 1.
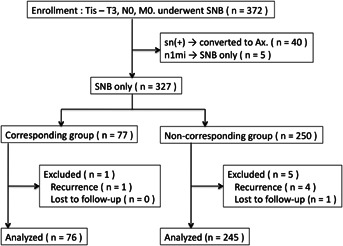
Trial profile of this study.
The SNB Procedure
We performed the SNB procedure using preoperative lymphoscintigraphy and an intraoperative radioisotope (RI) in addition to the dye method (triple mapping procedure). 99mTc-phytate was used at a dose of 18.5 MBq/body for RI, and indigocarmine (Daiichi Sankyo) was used at a dose of 1.5 ml/body. RI was intradermally injected more than 2 hr before surgery at the perialeolar site, and the blue dye was subcutaneously injected immediately before surgery at the perialeolar site.
The ARM Technique
We combined the ICG fluorescence imaging and indigo carmine blue dye methods. More than 2 hr before surgery, 0.15 ml of ICG was subcutaneously injected into the interdigital area, and immediately before surgery, 1.5 ml of blue dye was subcutaneously injected in the upper one third of the arm. While performing the SNB procedure, we scrutinized the presence (corresponding group) or absence (noncorresponding group) of the lymphatics drainage into the breast SN using ARM with a fluorescence imaging camera (Photodynamic Eye, Hamamatsu Photonics K.K., Hamamatsu, Japan) and the naked eye (Fig. 2).
Figure 2.
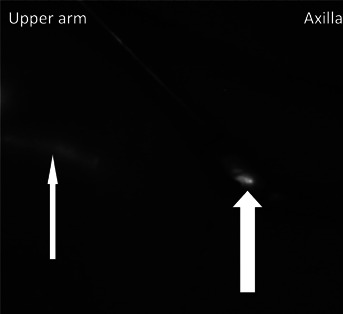
Indocyanine green fluorescence image during the sentinel node biopsy procedure (right axilla). Fluorescence image of the subcutaneous lymphatic channel through the skin (narrow white arrow). Axillary lymphatic flow into the breast sentinel lymph node (corresponding) group (thick white arrow).
The Location of SNs and ARM Lymphatics
We studied the location of SNs and ARM lymphatics. We divided the axillary region into three groups: (1) apparent cranial side of the second intercostobrachial nerve, (2) around the second intercostobrachial nerve, and (3) apparent caudal side of the nerve.
The Lymphedema Assessment
The bilateral arm circumference was measured in all patients before and at 1, 6, and 12 months after surgery and every 6 months thereafter. The measurement point was determined based on the international consensus of best practices for the management of lymphedema 1. Taking into account the average body size of a Japanese individual, we considered a 1- to 2-cm expansion of the arm as mild edema and >2-cm expansion as severe edema based on the Japanese Breast Cancer Society study squad report 2.
Statistical Analysis
The chi-square test was used to estimate intergroup differences (corresponding or noncorresponding to SN). Two-tailed P values <0.05 were considered to be statistically significant.
RESULTS
Identifying the upper extremity lymphatics was possible in 120/372 (32.3%) patients who underwent the SNB procedure. In a total of 77/372 (20.7%) patients, the lymphatics drained into the breast SN (corresponding group). The median follow-up period was 28 months (range, 12–47 months).
No upper extremity lymphatics were observed at the apparent caudal side of the second intercostobrachial nerve.
Characteristics of the two groups are shown in Table1. The median age of the patients in the corresponding and noncorresponding groups were 59 years (range, 24–89) and 58 years (range, 28–88), respectively, and mean body mass index (BMI) was 22.5 kg/m3 (range, 17.1–32.9 kg/m3) and 22.4 kg/m3 (range, 15.7–40.7 kg/m3), respectively. The mean number of lymph nodes removed was 1.51 (range, 1–6) and 1.80 (range, 1–6), respectively. Postoperative chemotherapy was administered in 5 and 27 patients, and postoperative whole breast irradiation because of partial mastectomy was performed in 60 and 212 patients, respectively. No statistical differences were observed between the two groups.
TABLE I.
A Comparison of Clinical Features Between the Patients With (Corresponding Group) or Without (Noncorresponding Group) Lymphatics Flowing Into the Breast Sentinel Node as Detected Using Axillary Reverse Mapping
| Corresponding group (n = 76) | Noncorresponding group (n = 245) | P-Value | |
|---|---|---|---|
| Age median (range) | 59 (24–89) | 58 (28–88) | NS |
| BMI mean (range) | 22.5 (17.1–32.9) | 22.4 (15.7–40.7) | NS |
| Number of removed nodes mean (range) | 1.51 (1–6) | 1.80 (1–6) | NS |
| Clinical stage (T stage) | |||
| 0 (Tis) | 16 | 54 | 0.91 |
| IA (T1) | 39 | 131 | |
| IIA (T2) | 19 | 52 | |
| IIB (T3) | 2 | 8 | |
| Chemotherapy | |||
| No | 71 | 218 | 0.26 |
| Yes | 5 | 27 | |
| Whole breast irradiation | |||
| No | 16 | 33 | 0.11 |
| Yes | 60 | 212 | |
BMI, body mass index; NS, not significant.
During follow-up, only five patients in the corresponding group developed lymphedema, whereas none of the patients in the noncorresponding group developed lymphedema (Table2).
TABLE II.
Occurrence of Lymphedema in the Two Groups
| Corresponding group (n = 76) | Noncorresponding group (n = 245) | P-Value | |
|---|---|---|---|
| Postoperative lymphedema | |||
| + | 5 | 0 | <0.0001 |
| − | 71 | 245 | |
Lymphedema in four of our patients was mild, whereas it was severe in one patient. Two patients developed lymphedema within 1 month, whereas the remaining three developed lymphedema within 6 months of surgery.
Patient characteristics in the corresponding group with and without lymphedema were compared (Table3). The median age of the patients with and without lymphedema was 47 years (range, 41–64 years) and 59 years (range, 24–89 years), respectively. The mean BMI was 20.6 kg/m3 (range, 18.3–22.3 kg/m3) and 22.6 kg/m3 (range, 17.1–32.9 kg/m3), respectively. The mean number of lymph nodes removed was 1.60 (range, 1–2) and 1.62 (range, 1–6), respectively. Postoperative chemotherapy was administered in two and three patients, and postoperative whole breast irradiation was performed in two and 58 patients, respectively.
TABLE III.
Characteristics of Patients With and Without Lymphedema in the Group With Lymphatics Flowing Into the Breast Sentinel Node (Corresponding Group) as Detected Using Axillary Reverse Mapping
| Patients with lymphedema (n = 5) | Patients without lymphedema (n = 71) | P-Valuea | |
|---|---|---|---|
| Age median (range) | 47 (41–64) | 59 (24–89) | NS |
| BMI mean (range) | 20.6 (18.3–22.3) | 22.6 (17.1–32.9) | NS |
| Number of removed nodes mean (range) | 1.6 (1–2) | 1.62 (1–6) | NS |
| Clinical stage (T stage) | |||
| 0 (Tis) | 0 | 16 | 0.57 |
| IA (T1) | 3 | 36 | |
| IIA (T2) | 2 | 16 | |
| IIB (T3) | 0 | 3 | |
| Postoperative chemotherapy | |||
| No | 3 | 68 | 0.002 |
| Yes | 2 | 3 | |
| Whole breast irradiation | |||
| No | 3 | 13 | 0.03 |
| Yes | 2 | 58 | |
BMI, body mass index; NS, not significant.
aReference values.
DISCUSSION
In 2007, Thompson et al. 3 and Nos et al. 4 reported their analysis of the relations on the upper extremity and breast lymphatics, and they named the procedure as ARM. Several studies have reported this concept, including the analyses of the upper extremity lymphatic flow, relationship among the draining lymph nodes from the upper extremity and breast, consideration of preserving the upper extremity lymphatic flow, and the efficacy of reconstructing the upper extremity lymphatic flow by microscopic surgery 5–12.
ICG releases an 830-nm fluorescence that is caused by an excitation light of 760 nm. This characteristic allowed the practical observation of the microcirculation. ICG is currently being widely used in the field of ophthalmology and brain surgery. It is also useful for evaluating the impaired blood flow in a graft during breast reconstructive surgery 11–15. The SNB procedure and ICG fluorescence technique are performed to evaluate the lymphatic pathways 16,17. Professor Noguchi first described fluorescence imaging in ARM procedure and reported its usefulness for detecting lymphatic drainage from the upper extremities. This procedure also permits ARM nodes and/or lymphatics from blue and/or hot SLNs 18,19.
Because the SNB procedure is performed at our institute using the triple mapping technique, introducing ICG during ARM did not cause any particular problems.
Several reports on detecting the upper extremity lymphatic flow using the dye method alone are available; however, according to our investigation, the detection rate during axillary dissection was approximately 60%; therefore, we believed that the concomitant use of ICG was necessary for higher sensitivity 9,18.
When we started using the ICG fluorescence technique, no data on the difference in lymphatic detection rate were available between the fluorescence and blue dye methods. We used both ICG and indigocarmine because we wanted to know the difference in detection rates. When we located the blue nodes during the procedure, we traced the blue lymphatic drainage into the nodes to clarify whether the lymphatics originated from the breast or upper extremities. Thus, we could distinguish whether the blue nodes were SLNs or ARM nodes.
In our study of using ICG during the axillary dissection, the detection rate for the upper extremity lymphatics and drained lymph nodes was 97.7% (125/128 procedures). Because of the ICG fluorescence, the upper extremity lymphatics could be clearly observed during the axillary dissection. All four patients in whom the lymphatics could not be detected had cancers involving the axillary lymph node and severe lymph stasis on the affected side before surgery.
All patients enrolled in this study had no lymph node metastasis clinically and had no lymphatic stasis before surgery, we included patients without fluorescence at the breast SN as noncorresponding group.
No adverse side effects caused by ICG were observed other than the dye remaining at the injection site for several weeks.
The low detection rate of the upper extremity lymphatics in this study was caused by ARM that was performed as a part of the techniques necessary during the SNB procedure. Areas other than the breast SN were not assessed. The SNB procedure is a less invasive surgery, and we believe that the additional ARM procedure acts contrary to this objective.
The median follow-up period was 28 months. There are several reports that approximately 50% patients who developed lymphedema occurred within 12–18 months after the axillary dissection, and we believe that this observation period is sufficient for evaluation 20,21.
We classified the location of the SN and ARM lymphatics into three groups, including around the second intercostobrachial nerve or the cranial or caudal side. The reason for classifying the locations into three groups is because the positional relationship between the second intercostobrachial nerve and the SN cannot be clearly divided into upper and lower positions. For example, we sometimes observed that this nerve runs across the SN. In the corresponding group, all SN was located around and/or the cranial side of the second intercostobrachial nerve. However, no upper extremity lymphatics was present at the caudal side of the second intercostobrachial nerve. This means that when the SN was located on the caudal side of the second intercostobrachial nerve, we did not have to worry about postoperative lymphedema.
Although the SNB procedure carries lower morbidity rates compared with the axillary lymph node dissection, the rate of postoperative lymphedema is 0–13% with SNB alone, even when experienced surgeons performed the procedure; this rate averaged approximately 7% in two large cooperative group trials 6,22,23. In our study, lymphedema was found in 5 of total 321 cases (1.6%), and limited to the corresponding group, 5 of 76 cases (6.6%). Postoperative lymphedema was not observed in the noncorresponding group, and the outcome was significant (P ≤ 0.0001).
The characteristics of patients with and without lymphedema in the corresponding group are presented in Table3. The P-value was calculated as a reference because the number of patients in the lymphedema group was small and could not be included in the statistical analysis. However, a tendency to develop lymphedema was observed in patients who received postoperative chemotherapy. Chemotherapy is one of the risk factors for developing lymphedema, potentially damaged lymphatics in the corresponding group were easily developed lymphedema undergoing chemotherapy 1.
The cost of this technique is very low, including the ICG drug costs, and only few minutes were required to identify the lymphatic pathways. Moreover, identifying the pathways is very simple, and it can be performed at any institute who has a fluorescence imaging camera.
All patients who developed lymphedema improved by early intervention with appropriate guidance and physical therapy. Because lymphedema tends to aggravate if it recurs, it is necessary for patients to acquire knowledge such as avoiding the trigger and promptly consulting the surgeon when lymphedema occurs 24,25. If this guidance is provided only to the corresponding group, which is the risk group, instead of all patients who undergo the SNB procedure, human resources can be effectively used, and patients in the noncorresponding group can experience relief from worry for not requiring unnecessary care.
Our hospital provides guidance to the corresponding group alone among all patients who have undergone the SNB procedure. The noncorresponding group is explained that they may continue their normal daily activities without any particular guidance following surgery.
CONCLUSIONS
Performing ARM during the SNB procedure was useful for identifying the group of patients who are at a high risk for developing lymphedema. More risk-focused guidance should be used for these limited patients.
Acknowledgments
This study was funded by a research grant from the Japan Surgical Association, Saitama Branch (Saitamaken Gekaikai). The authors would like to thank Enago (www.enago.jp) for the English language review.
Glossary
- ARM
axillary reverse mapping
- SNB
sentinel lymph node biopsy
- SN
sentinel node
- ICG
indocyanine green
- RI
radioisotope
- BMI
body mass index
- NS
not significant
REFERENCES
- 1.The lymphoedema Framework. Best practice for the management of lymphoedema. International consensus. London: Medical Education Partnership. (MEP) Ltd; 2006. [Google Scholar]
- 2.Kitamura K, Akazawa K: Multi-center survey of breast cancer related arm lymphedema and future issues. J Jpn Coll Angiol. 2010;50:715–720. [Google Scholar]
- 3.Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14:1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 4.Nos C, Lesieur B, Clough KB, et al. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14:2490–2496. doi: 10.1245/s10434-007-9450-4. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T, Sakoda T, Yoshimizu N, et al. Axillary reverse mapping (ARM) may be useful in preventing lymphedema during axillary lymph node dissection for breast cancer patients. Jpn J Breast Cancer. 2009;24:737–740. [Google Scholar]
- 6.Boneti C, Korourian S, Bland K, et al. Axillary reverse mapping: Mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg. 2008;206:1038–1042. doi: 10.1016/j.jamcollsurg.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Boneti C, Korourian S, Diaz Z, et al. Scientific Impact Award: Axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am J Surg. 2009;198:482–487. doi: 10.1016/j.amjsurg.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Bedrosian I, Babiera GV, Mittendorf EA, et al. A phase I study to assess the feasibility and oncologic safety of axillary reverse mapping in breast cancer patients. Cancer. 2010;116:2543–2548. doi: 10.1002/cncr.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda K, Ogawa Y, Komatsu H, et al. Evaluation of the metastatic status of lymph nodes identified using axillary reverse mapping in breast cancer patients. World J Surg Oncol. 2012;10:233–239. doi: 10.1186/1477-7819-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011;18:2500–2505. doi: 10.1245/s10434-011-1624-4. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery: Indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2012 doi: 10.1097/SAP.0b013e3182605580. Dec 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Mihara M, Murai N, Hayashi Y, et al. Using indocyanine green fluorescent lymphography and lymphatic-venous anastomosis for cancer-related lymphedema. Ann Vasc Surg. 2012;26:278. doi: 10.1016/j.avsg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg. 2004;53:205–209. doi: 10.1097/01.sap.0000116284.51679.ea. [DOI] [PubMed] [Google Scholar]
- 14.Komorowska-Timek E, Gurtner GC: Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1073. doi: 10.1097/PRS.0b013e3181d17f80. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa Y, Nakamura Y, Kawachi Y, et al. A custom-made, low-cost intraoperative fluorescence navigation system with indocyanine green for sentinel lymph node biopsy in skin cancer. Dermatology. 2011;222:261–268. doi: 10.1159/000327080. [DOI] [PubMed] [Google Scholar]
- 16.Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 17.Hojo T, Nagao T, Kikuyama M, et al. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210–213. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi M, Yokoi M, Nakano Y: Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J Surg Oncol. 2010;101:217–221. doi: 10.1002/jso.21473. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi M: Axillary reverse mapping for breast cancer. Breast Cancer Res Treat. 2010;119:529–535. doi: 10.1007/s10549-009-0578-8. [DOI] [PubMed] [Google Scholar]
- 20.Meric F, Buchholz TA, Mirza NQ, et al. Long-term complications associated with breast-conservation surgery and radiotherapy. Ann Surg Oncol. 2002;9:543–549. doi: 10.1007/BF02573889. [DOI] [PubMed] [Google Scholar]
- 21.Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: A study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17:138–147. doi: 10.1016/j.breast.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Cormier JN, Askew RL, Mungovan KS, et al. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 25.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, et al. Effectiveness of early physiotherapy to prevent lymphedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]


