Abstract
Changes in genome expression during normal cellular and plastid development in the first leaf of young (7-day-old) wheat (Triticum aestivum var. Maris Dove) were investigated by examining homogeneous populations of leaf cells and plastids of several developmental ages present in the same leaf. The cells were characterized over a period immediately following the last cell division. All of the leaf cells had cytoplasmic contents and nuclei, and between 44% (young tissue) and 54% (older tissue) of the leaf cells were mesophyll cells. Chloroplast development was complete 36 hours after the chloroplasts had ceased dividing. Extremely large changes occurred in cellular constituents over a very short period of leaf development. Maximum rates of accumulation of ribulose bisphosphate carboxylase per mesophyll cell (80 picograms/hour), chlorophyll per mesophyll cell (9 picograms/hour), and 70S ribosomes per mesophyll cell (19 × 105/hour) were recorded.
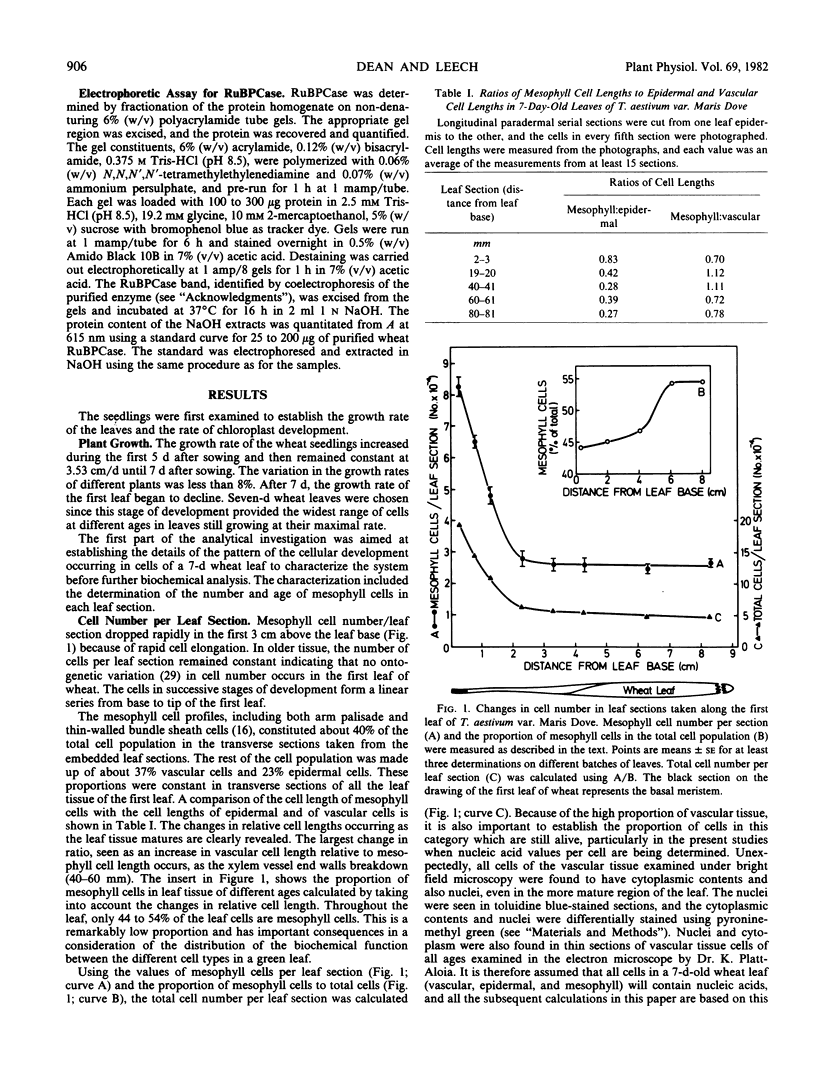
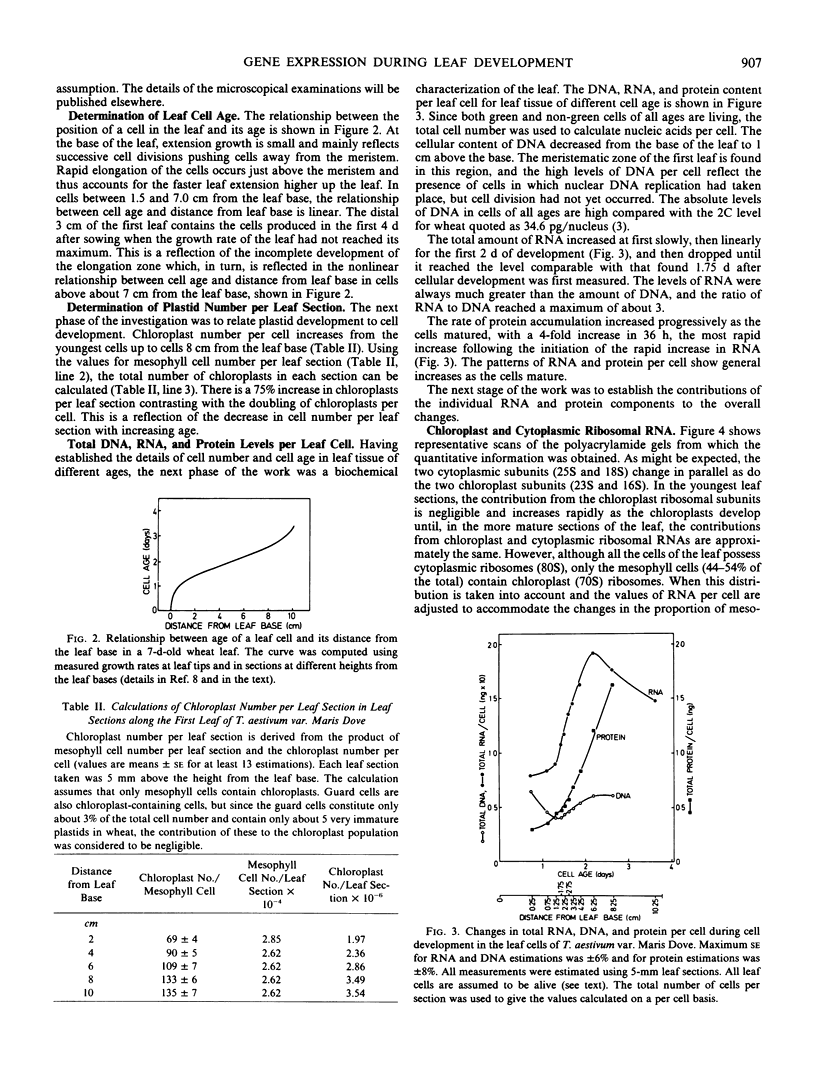
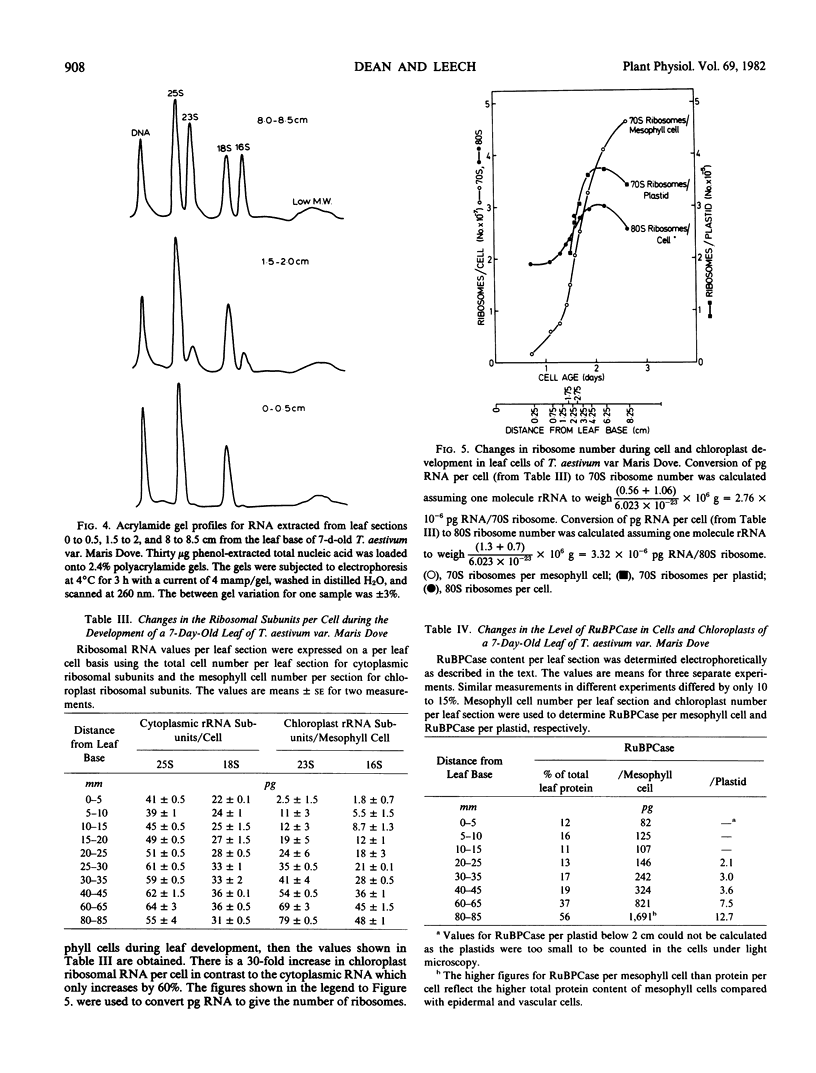
Total cellular DNA varied from 40 to 60 picograms/cell, reflecting the changes in nuclear and chloroplast DNA synthesis during different phases of cellular and chloroplast division. The period of maximum accumulation of protein, total RNA, and both 80S and 70S ribosomes occurred between 36 and 48 hours after the last cell division. Between 48 and 60 hours, 70S rRNA per cell and protein content per cell continued to increase as 80S rRNA per cell declined. Ribulose bisphosphate carboxylase per cell increased 20-fold between 15 and 60 hours.
Full text
PDF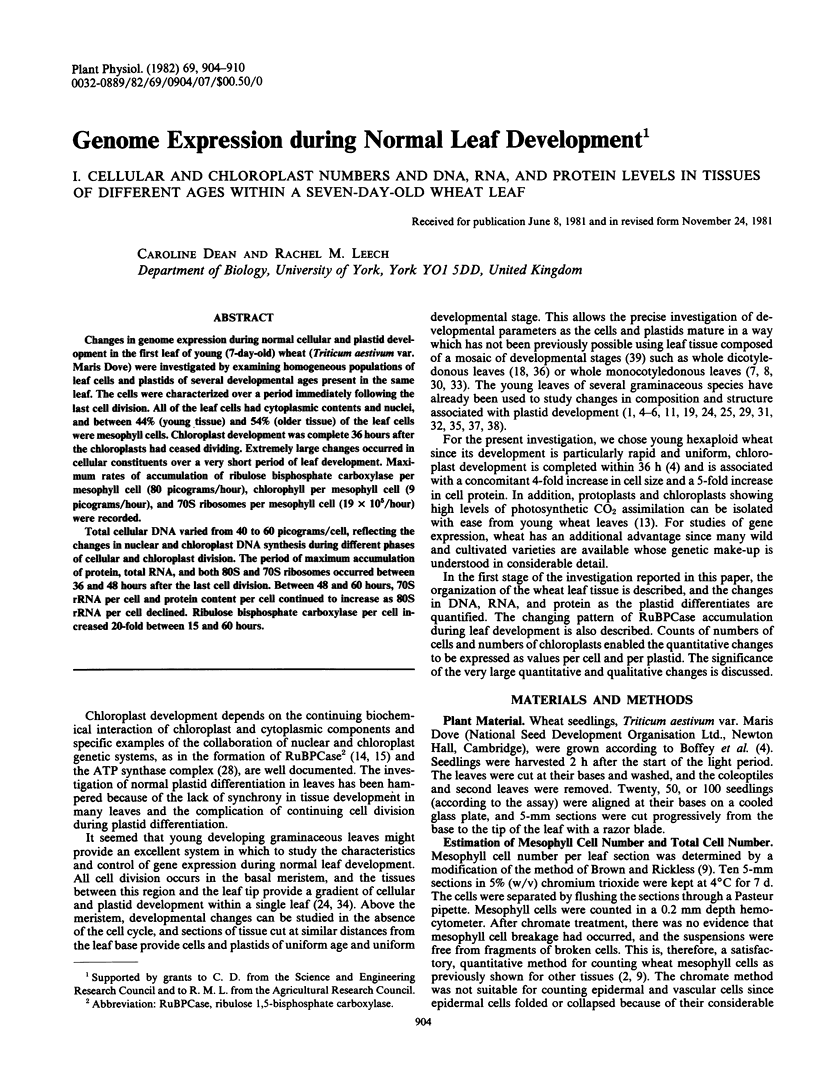



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W. M., Leaver C. J., Weir E. M., Riezman H. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: I. Developmental Changes in Cotyledonary Protein, RNA, and Enzyme Activities during Germination. Plant Physiol. 1978 Oct;62(4):542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. D., Smith J. B. Nuclear dna amounts in angiosperms. Philos Trans R Soc Lond B Biol Sci. 1976 May 27;274(933):227–274. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- Boffey S. A., Ellis J. R., Selldén G., Leech R. M. Chloroplast Division and DNA Synthesis in Light-grown Wheat Leaves. Plant Physiol. 1979 Sep;64(3):502–505. doi: 10.1104/pp.64.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffey S. A., Selldén G., Leech R. M. Influence of Cell Age on Chlorophyll Formation in Light-grown and Etiolated Wheat Seedlings. Plant Physiol. 1980 Apr;65(4):680–684. doi: 10.1104/pp.65.4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite J. J., Bogorad L. A one-step method for the isolation and determination of leaf ribulose-1,5-diphosphate carboxylase. Anal Biochem. 1971 May;41(1):57–66. doi: 10.1016/0003-2697(71)90191-6. [DOI] [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- Hawke J. C., Rumsby M. G., Leech R. M. Lipid biosynthesis in green leaves of developing maize. Plant Physiol. 1974 Apr;53(4):555–561. doi: 10.1104/pp.53.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J. Molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1973 Sep;135(1):237–240. doi: 10.1042/bj1350237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese B. M., Leech R. M. Sequential changes in the lipids of developing proplastids isolated from green maize leaves. Plant Physiol. 1976 May;57(5):789–794. doi: 10.1104/pp.57.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola-Morgenthaler L. R., Morgenthaler J. J., Price C. A. Synthesis of coupling factor CF1 protein by isolated spinach chloroplasts. FEBS Lett. 1976 Feb 1;62(1):96–100. doi: 10.1016/0014-5793(76)80025-7. [DOI] [PubMed] [Google Scholar]
- Patterson B. D., Smillie R. M. Developmental changes in ribosomal ribonucleic Acid and fraction I protein in wheat leaves. Plant Physiol. 1971 Feb;47(2):196–198. doi: 10.1104/pp.47.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul J. L., Brangeon J., Reyss A. Interaction between External and Internal Conditions in the Development of Photosynthetic Features in a Grass Leaf: I. REGIONAL RESPONSES ALONG A LEAF DURING AND AFTER LOW-LIGHT OR HIGH-LIGHT ACCLIMATION. Plant Physiol. 1980 Oct;66(4):762–769. doi: 10.1104/pp.66.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul J. L., Brangeon J., Reyss A. Interaction between External and Internal Conditions in the Development of Photosynthetic Features in a Grass Leaf: II. REVERSIBILITY OF LIGHT-INDUCED RESPONSES AS A FUNCTION OF DEVELOPMENTAL STAGES. Plant Physiol. 1980 Oct;66(4):770–774. doi: 10.1104/pp.66.4.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES M. J., YEMM E. W. DEVELOPMENT OF CHLOROPLASTS AND THE SYNTHESIS OF PROTEINS IN LEAVES. Nature. 1963 Dec 14;200:1077–1080. doi: 10.1038/2001077a0. [DOI] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selldén G., Leech R. M. Localization of DNA in Mature and Young Wheat Chloroplasts Using the Fluorescent Probe 4'-6-Diamidino-2-phenylindole. Plant Physiol. 1981 Sep;68(3):731–734. doi: 10.1104/pp.68.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Ellis R. J. Protein synthesis in chloroplasts. VIII. Differential synthesis of chloroplast proteins during spinach leaf development. Biochim Biophys Acta. 1980 Apr 30;607(2):319–330. doi: 10.1016/0005-2787(80)90084-2. [DOI] [PubMed] [Google Scholar]



