The results of this study suggest that patient factors and not breast MR imaging results significantly affect decisions by women with newly diagnosed breast cancer as to whether to undergo contralateral prophylactic mastectomy.
Abstract
Purpose
To assess which patient and magnetic resonance (MR) imaging factors are associated with the likelihood of contralateral prophylactic mastectomy (CPM) in patients with newly diagnosed breast cancer.
Materials and Methods
The American College of Radiology Imaging Network 6667 trial was compliant with HIPAA; institutional review board approval was obtained at each site. All patients provided written informed consent. This study was a retrospective review of data from 934 women enrolled in the trial who did not have a known contralateral breast cancer at the time of surgical planning. The authors assessed age, menopausal status, index breast cancer histologic results, contralateral breast histologic results, breast density, family history, race and/or ethnicity, MR imaging Breast Imaging Reporting and Data System (BI-RADS) assessment, and number of MR imaging lesions for association with CPM by using the Fisher exact test, exact χ2 test, and multivariate logistic regression analyses.
Results
Eighty-six of the 934 (9.2%) women underwent CPM and were more likely to be younger (mean age, 48 years [range, 27–78 years] vs mean age, 54 years [range, 25–86 years]; P < .0001), be premenopausal (55 of 86 [64%] vs 349 of 845 [41%], P < .0001), have ductal carcinoma in situ (DCIS) in the index breast (31% [27 of 86] vs 19% [164 of 848], P = .02), have greater breast density (71 of 86 [83%] vs 572 of 848 [68%], P = .004), and have a family history of breast cancer (44 of 86 [30%] vs 150 of 488 [18%], P = .01) than those who did not undergo CPM. Distributions of race and/or ethnicity, contralateral lesion pathologic results, and number of MR imaging lesions were similar in both groups. With multivariate modeling, younger age, greater breast density, DCIS index cancer, and family history remained significant, whereas menopausal status did not. Positive MR imaging assessments were not significantly more frequent in the CPM group than in the group of women who did not undergo CPM (14 of 86 [16.3%] vs 113 of 848 [13.3%], P = .43).
Conclusion
In patients with newly diagnosed breast cancer who underwent breast MR imaging at which a contralateral breast cancer was not identified, patient factors and not breast MR imaging BI-RADS scores were chief determinants in decisions regarding CPM.
© RSNA, 2014
Introduction
There is clear evidence that the incidence of contralateral prophylactic mastectomy (CPM) in patients newly diagnosed with breast cancer has increased during the past decade (1,2). Although the rate of CPM in patients with unilateral breast malignancy has increased more than 11-fold from 1998 (0.4%) to 2007 (4.7%) (3), the exact cause of this increase is debated. Results of many studies have shown that the greatest increase in CPM rates has been observed among relatively younger, more highly educated white women of advanced socioeconomic status with a positive family history of breast cancer (2–4). Several index cancer characteristics also have been identified to be associated with CPM rates, including large tumor size, preinvasive disease (eg, ductal carcinoma in situ [DCIS]), lobular subtype, and the presence of multicentric disease (2–5).
The use of breast magnetic resonance (MR) imaging for the preoperative determination of the extent of disease in patients with newly diagnosed breast cancer also has increased during this time frame (6). Authors of multiple studies have confirmed that preoperative breast MR imaging can show occult cancers in the ipsilateral and contralateral breasts of women with a recent unilateral breast cancer diagnosis (7–9); however, its effect on patient outcomes is less well understood. Although potential benefits of more accurate diagnosis of the extent of disease are acknowledged (10–12), there are concerns that false-positive breast MR images may lead to more aggressive therapy than otherwise indicated. Specifically, despite the potential advantage of breast MR imaging to help identify otherwise occult cancer in the contralateral breast, some authors have questioned whether its use also has increased CPM rates (13–15).
The question of whether false-positive MR imaging findings in the contralateral breast of patients with newly diagnosed breast cancer cause more women to undergo CPM has substantial clinical ramifications. There is inadequate evidence that CPM improves survival (16), and the annual risk of developing a contralateral breast cancer is low (from 0.3% to 1% per year [17–19]) and may be declining in the era of adjuvant hormonal therapy (20). Perhaps even more importantly, approximately 6% of women who choose to undergo CPM later experience regret regarding this decision (21). As a result, further elucidation of the relationships of false-positive breast MR imaging findings and other clinical factors with CPM rates will better allow physicians to counsel patients effectively about the benefits and risks of preoperative breast MR imaging. Thus, we sought to evaluate which patient and MR imaging factors are associated with a patient’s decision to undergo CPM.
Materials and Methods
Our study was an ancillary retrospective investigation, and we used the available prospectively collected American College of Radiology Imaging Network 6667 trial data regarding patient characteristics and MR imaging assessments. This trial was a multi-institutional study including 25 academic and private practice sites with international representation and 50 total readers (22–25). The data for the trial were collected prospectively, with the study protocol optimized for the primary aim of the study: to assess the diagnostic yield and accuracy of MR imaging for the evaluation of the contralateral breast in women recently diagnosed with breast cancer. Participants were recruited for the trial from April 1, 2003 to June 10, 2004. Women aged 18 years or older who had undergone mammography and clinical breast examination of the contralateral breast and received results negative for cancer within 90 days of receiving a diagnosis of breast cancer were eligible for the trial. All eligible women also underwent breast MR imaging of the contralateral breast within 60 days of their index breast cancer diagnosis. The means by which index breast cancers were diagnosed was not specified in the protocol; however, none of the index breast cancers were initially detected with breast MR imaging. Neither the clinicians treating the patients nor the patients themselves were blinded to the results of the MR imaging examinations. Clinicians were instructed to act on MR imaging findings according to the standard of care at their respective institutions. Institutional review board approval was obtained at each center before patient recruitment, and every patient enrolled provided written informed consent.
Study Cohort
Of the 969 women in the final contralateral breast screening trial study group, 31 had a contralateral breast cancer diagnosed as a result of a Breast Imaging Reporting and Data System (BI-RADS) category 0, 4, or 5 assessment and were excluded from our analyses. Four patients without definitive surgical or pathologic records confirming performance of CPM also were excluded. As a result, the final cohort included 934 women with newly diagnosed breast cancer who underwent breast MR imaging of the contralateral breast, which did not show cancer before surgical planning.
Breast MR Imaging Technique and MR Image Interpretation
Contrast material–enhanced breast MR imaging was performed at a magnetic field strength of at least 1.5 T with a dedicated breast coil. Images were obtained both before and after administration of a gadolinium-based contrast material, with imaging parameters as previously described (25). The 50 radiologists interpreting the MR images had interpreted at least 50 breast MR imaging examinations and performed at least five MR imaging–guided breast procedures before the study. Readers interpreted the breast MR images by using the American College of Radiology BI-RADS fourth edition criteria (26).
Data Collection, Patient Variables, and Statistical Analysis
The following patient variables were extracted from the trial database for each patient: patient age at MR imaging, menopausal status, breast tissue density at mammography, family history of breast cancer, ethnicity and race, tumor histologic results of the index breast malignancy, breast MR imaging BI-RADS assessment categories, number and sizes of lesions suspicious for cancer identified at MR imaging, and histopathologic results of biopsies (core needle or excisional) of lesions suspicious for cancer in the contralateral breast. Premenopausal status was defined as having had a menstrual period within 12 months of enrollment.
To create dichotomous variables to facilitate statistical analyses, the multiple categories of breast density, breast MR imaging BI-RADS assessment, ethnicity, race, and index tumor pathologic results were grouped. In the case of breast density, patients with breasts composed almost entirely of fat (<25% dense) or scattered fibroglandular tissue (25%–50%) were grouped and compared with those with heterogeneously dense (51%–75%) or extremely dense (>75%) breasts. For MR imaging BI-RADS assessment, categories 1 (negative for cancer), 2 (benign), and 3 (probably benign) were grouped together as negative and categories 0 (incomplete, additional imaging evaluation needed), 4 (abnormality suspicious for cancer), and 5 (highly suggestive of malignancy) were grouped together as positive, which was in keeping with the grouping in the original American College of Radiology Imaging Network 6667 protocol. To assess the effect that a less-certain negative assessment (probably benign) had on CPM rates, a second MR imaging BI-RADS assessment categorization was performed with categories 1 and 2 grouped as negative and categories 0, 3, 4, and 5 grouped as positive. Ethnicity was divided into Hispanic/Latino versus non-Hispanic, and race was divided into white versus nonwhite. Index breast malignancy pathologic results were grouped as DCIS, invasive (and invasive subtypes), and other categories. MR imaging pathologic findings in the contralateral breast were divided into high risk (containing atypical ductal hyperplasia, atypical lobular hyperplasia, and/or lobular carcinoma in situ) and benign findings.
Associations of CPM rates with MR imaging BI-RADS assessment positivity, patient factors, index breast cancer pathologic results, and contralateral breast lesion pathologic results were assessed by using the Fisher exact test. The association between the number of false-positive lesions suspicious for cancer identified in the contralateral breast at MR imaging and the choice to undergo CPM was examined by using the exact χ2 test. Differences in sizes of false-positive lesions suspicious for cancer identified in the contralateral breast at MR imaging were compared between the two cohorts by using the Satterthwaite t test. Those factors found at univariate analyses to be significantly associated with CPM rates were further assessed with multivariate modeling. The accuracy of the univariate and multivariate models to be associated with CPM status was compared by using the area under the receiver operating characteristic curve (Az). Correlation of factors that are at least in part dependent on age (breast density and menopausal status) was performed by using Spearman correlation analyses, and visual inspection was performed by using histograms of age versus breast density and menopausal status. All computations were performed with software (SAS 9.3; SAS Institute, Cary, NC). P < .05 was considered indicative of a significant difference.
Results
The average age ± standard deviation of the 934 patients in this cohort at the time of their MR examinations was 53.3 years ± 11.4 (range, 25–86 years). Eighty-six of the 934 (9.2%) patients underwent CPM.
MR Imaging BI-RADS Assessment and Number of Lesions and Associations with CPM Rates
There was a relatively even distribution of MR imaging BI-RADS assessments in both the CPM and non-CPM cohorts (Table 1). Patients who opted to undergo CPM did not have a significantly greater likelihood of receiving a positive or abnormal MR imaging BI-RADS assessment of that breast than did the non-CPM cohort (Table 2). This lack of correlation was true whether BI-RADS category 3 assessments were included as negative (14 of 86 [16.3%] patients positive in CPM cohort vs 113 of 848 [13.3%] positive in non-CPM cohort, P = .43) or as positive (24 of 86 [27.9%] positive in CPM cohort vs 205 of 848 [24.2%] positive in the non-CPM cohort, P = .41). The number of false-positive lesions suspicious for cancer identified at MR imaging in the contralateral breast ranged from zero to five in the non-CPM cohort and from zero to three in the CPM cohort (Table 3), and there was no significant difference in the number of false-positive lesions between the two cohorts (P = .16). In addition, there was no significant difference in false-positive mass lesion size in the contralateral breast between the two cohorts (P = .74) at MR imaging; however, nonmass lesions could not be compared because of limitations in the original study protocol (Table E1 [online]).
Table 1.
Distribution of MR Imaging BI-RADS Assessments
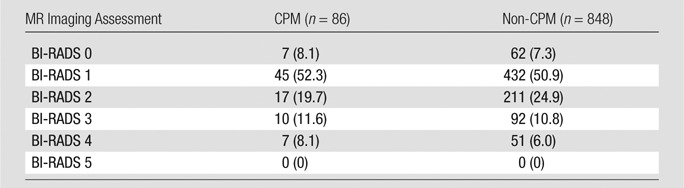
Note.—Data are numbers of patients, with percentages in parentheses.
Table 2.
BI-RADS Assessments Grouped as Positive versus Negative
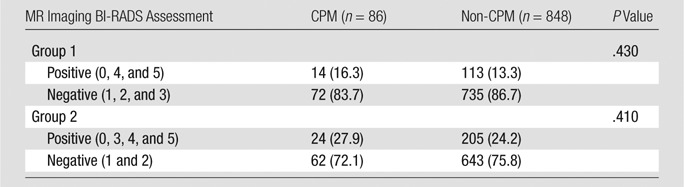
Note.—Unless otherwise indicated, data are numbers of patients, with percentages in parentheses.
Table 3.
False-Positive Lesions Detected in the Contralateral Breast at MR Imaging
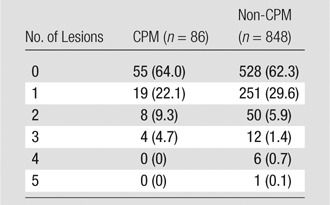
Note.—Data in parentheses are percentages.
Patient Factors and Association with CPM Rates
The mean age of women who opted for CPM was lower than that for the non-CPM cohort (48 years [range, 27–78 years] vs 54 years [range, 25–86 years], P < .001). Similarly, patients in the CPM cohort were more likely to be premenopausal (55 of 86 [64%] vs 349 of 845 [41%], P < .0001) and have greater breast density (71 of 86 [82.6%] vs 571 of 845 [67.5%], P = .004) (Table 4).
Table 4.
Distribution of Patient Factors
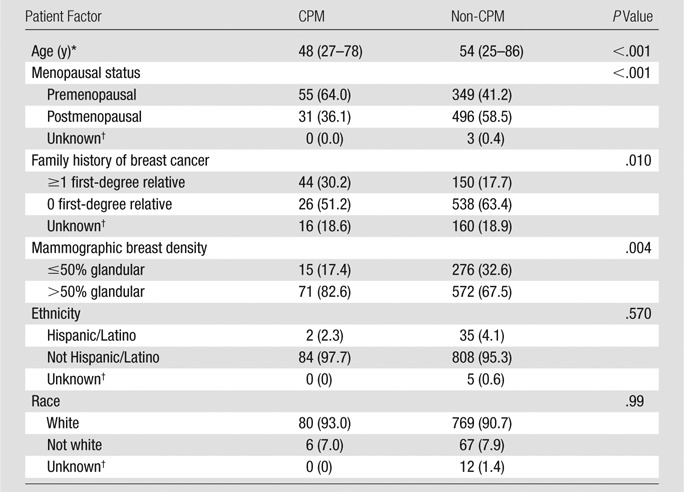
Note.—Unless otherwise indicated, data are numbers of patients, with percentages in parentheses.
Data are average, with range in parentheses.
Excluded from analyses.
Of the women for whom a family history could be obtained, those who opted for CPM were more likely to have a first-degree relative with a history of breast cancer (26 of 70 [37.1%] vs 150 of 688 [21.8%], P = .01) (Table 4). In fact, the presence of any family history of breast cancer also was associated with a patient choosing CPM (42 of 86 [48.8%] vs 300 of 838 [35.8%], P = .02). There was no association of Hispanic/Latino ethnicity (two of 84 [2.3%] Hispanic vs 35 of 843 [4.1%] non-Hispanic, P = .57) or nonwhite race (five of 84 [7.0%] nonwhite vs 67 of 836 [8.0%] white, P = .99) with CPM rates among the women for whom race and/or ethnicity was known (Table 4).
Index Malignancy and Contralateral Breast Pathologic Factors and Associations with CPM Rates
Patients who had an index breast cancer diagnosis of DCIS without an invasive component were more likely to choose CPM than those who had invasive or other types of breast cancer (27 of 86 [31.4%] patients vs 164 of 848 [19.3%] patients, P = .02, Table 5). Patients with a diagnosis of invasive lobular carcinoma in the index breast were no more likely to undergo CPM than were those with a diagnosis of other malignancies (10 of 86 [11.6%] patients vs 149 of 848 [17.6%] patients, P = .99; Table 5).
Table 5.
Index Breast Cancer Pathologic Results
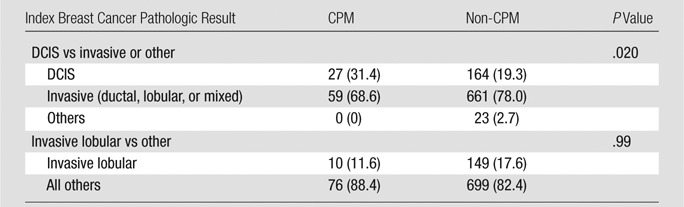
Note.—Data in parentheses are percentages.
In the non-CPM cohort, 11 biopsies (either core needle or excisional) were performed within the first 6 months after the MR imaging study that yielded a high-risk pathologic result (four atypical lobular hyperplasia, five atypical ductal hyperplasia, and two lobular carcinoma in situ lesions). In the CPM cohort, one patient received a diagnosis of a high-risk lesion (atypical ductal hyperplasia) on the basis of a core needle biopsy in the contralateral breast that corresponded to a BI-RADS category 4 lesion. In two other patients with MR imaging results that were positive for cancer (one BI-RADS category 0 and one BI-RADS category 3), a high-risk lesion was identified at mastectomy (atypical ductal hyperplasia and atypical lobular hyperplasia, respectively). Because the original study protocol only required that the most definitive pathologic results be reported, it cannot be excluded that these patients underwent biopsies before mastectomy. Nonetheless, even when these two lesions are included, there was no significant difference in the fraction of women in the CPM (3.5%, three of 86 patients) versus non-CPM (1.3%, 11 of 848 patients) cohorts with a high-risk lesion in the contralateral breast (P = .13).
Correlation and Multivariate Analyses of Factors Associated with CPM Rates
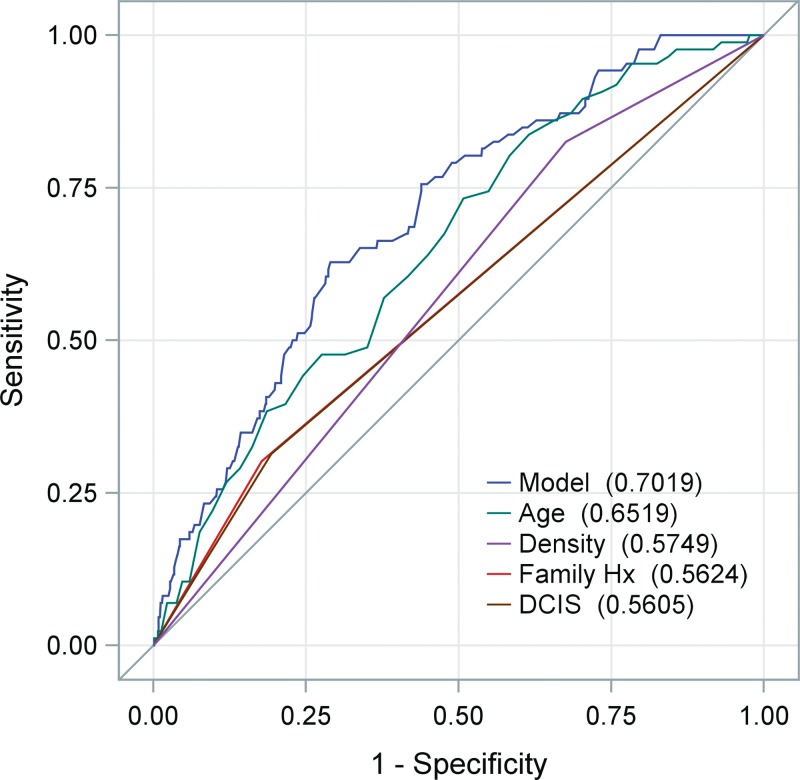
Menopausal status was correlated more highly with patient age (Spearman rank correlation coefficient, ρ = 0.75; P < .001) than breast density was correlated with age (ρ = 0.24, P < .001) or menopausal status (ρ = 0.21, P < .001). A visual inspection of the relationship among age, menopausal status, and breast density showed that although age and menopausal status do appear to be closely related, a fair proportion of young women had lower density breasts (21% with density ≤50% vs 79% with density >50% among those aged <40 years) and older women had dense breasts (54% with density >50% vs 46% with density >50% among those aged ≥65 years), suggesting that the relationship between age and density was not strong in this cohort. The factors found in univariate analyses to be significantly associated with CPM rates (including patient age, menopausal status, breast density, index tumor pathologic results [DCIS vs invasive], and family history of breast cancer) were further assessed by means of multivariate modeling (Table 6). All factors assessed except menopausal status remained significant. With receiver operating characteristic curve analyses, it was found that a model incorporating age, breast density, index tumor pathology, and family history provided a greater discriminative accuracy (Az = 0.70) than did the individual variables alone (Az = 0.65, 0.56, 0.57, and 0.56, respectively) (Figure).
Table 6.
Individual and Multivariable Logistic Regression Analyses
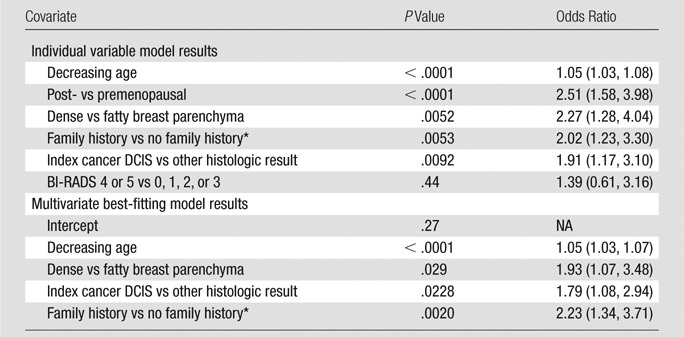
Note.—Data in parentheses are 95% confidence intervals. NA = not applicable.
In a first-degree relative.
Graph of receiver operator characteristic curves shows accuracy of individual and multivariate models for identifying patients who underwent CPM. Model incorporating age, mammographic breast density, index breast DCIS pathologic results, and family history (Hx) of a first-degree relative with breast cancer provide the greatest Az. Note that curves for DCIS and family history are so similar that they are virtually indistinguishable.
Discussion
We found that MR imaging BI-RADS assessment (after exclusion of patients in whom MR imaging showed a cancer) did not have a significant association with CPM rates, whereas patient factors of younger age, greater breast density, family history, and index breast cancer pathologic results of DCIS showed a significant association with CPM rates.
The results of our study suggest that a false-positive MR imaging assessment of the contralateral breast is not an important factor when a patient with a newly diagnosed breast cancer decides whether or not to undergo CPM. The proposed relationship of breast MR imaging with CPM (27) is predicated on the assumption that the additional examinations and biopsies resulting from positive MR imaging assessments in the contralateral breast add anxiety for a patient already dealing with a new cancer diagnosis. This, in turn, influences women to choose CPM even if biopsy results from this chain of events yield benign pathologic results. Although this hypothesized link often has been cited as a potential explanation for increasing CPM rates, authors of the few published studies who assert this causality did not control for known confounding clinical factors, nor did they assess the effect of specific MR imaging recommendations (13–15). The fact that CPM rates have been increasing since 1998 (1), at least 3 years before the time at which breast MR imaging use became more prevalent, also suggests that the relationship of breast MR imaging with increasing CPM rates is not likely causal. Our results demonstrate that abnormal but false-positive breast MR imaging assessments of the contralateral breast in patients with newly diagnosed breast cancer were not associated with CPM rates.
In all likelihood, a patient’s decision to undergo CPM is based on multiple factors, and our results based on examining additional patient factors associated with CPM rates support this concept. In this study, those who opted for CPM were more likely to be younger and premenopausal, which is in agreement with previously published findings. Patients in the CPM cohort were also more likely to have greater breast density.
We also found that women who have a family history of breast cancer (whether in a first-degree or more distant relative) are more likely to opt for CPM, which is consistent with previously published findings. We did not find an elevated rate of CPM based on ethnicity or race in our cohort, which conflicts with previously reported increased rates of CPM among white women. However, our study included relatively few nonwhite patients (73 of 922 [7.9%]), with race unknown for 12 patients; thus, our study was underpowered to fully allow assessment this relationship.
The relationship of increased CPM rates in patients with DCIS in the index breast is in agreement with the results of two previously published studies (2,3); however, the exact cause of this relationship remains unclear. The incidence of DCIS has increased most in the population older than 50 years (28), making it unlikely that this relationship correlates with younger age. Because the cure rates for this heterogeneous disease approach 100% with current therapies (29), it is surprising that a greater fraction of patients with DCIS opt for CPM than do patients with invasive breast cancer. It is possible that many patients with DCIS lack a clear understanding of their prognosis and the long-term effect on their health and future risk. Finally, although lobular histologic results did not correlate with CPM rates in our cohort, our study did not have sufficient power to detect a statistically significant relationship (power <40%). Further study on patient attitudes regarding index breast cancer diagnoses, particularly DCIS diagnoses, is needed to better understand their effect on patient decisions regarding the contralateral breast.
In our study, 9.2% of women underwent CPM, which is nearly twice the rate that was reported in 2007 by Yao et al (3). In that study, CPM rates were determined on the basis of data from the American College of Surgeon’s National Cancer Database, and the average age of patients in that cohort was 61.2 years (vs 53.3 years in our study), which likely contributed to this difference. Furthermore, it is possible that the patients included in our study either elected to or were recommended to undergo breast MR imaging in part owing to a perceived greater risk of breast cancer. In our patient cohort, 352 of the 934 (37.7%) patients had a family history of breast cancer, and at least 194 of the 934 (21.7%) patients had a first-degree relative with breast cancer. This value is higher than the average value (15%) of new breast cancer patients who have a first-degree relative with personal history of breast cancer (30–32). Thus, it is likely that our higher observed overall CPM rate is the result of studying a cohort of younger women with a greater proportion of high-risk patients than has been previously examined.
Our study had several limitations. This study was a retrospective review of data from a prospective trial designed for primary and secondary aims distinct from this study’s objective. As a result, we were limited to studying the MR imaging and patient factors for which data were acquired for the original trial. Because all patients in the study underwent MR imaging, we cannot confirm that the MR imaging process itself does not contribute to increased CPM rates. We also could not assess or control for factors that have been implicated in CPM rates in other studies (2–4), such as index tumor size, presence of multicentric disease, socioeconomic status, partner status, and quality of health insurance, because these parameters were not recorded during the prospective trial. Similarly, we could not assess the effects of having a genetic mutation or undergoing genetic counseling, which also have been associated with CPM rates (1,17,33). Other proposed but not well-studied factors, such as the availability of advanced reconstruction options, patient attitudes regarding symmetry and cosmesis, and surgeons’ attitudes or recommendations to the patients, were not included in this study.
In summary, our results suggest that patient factors and not breast MR imaging results significantly affect decisions by women with newly diagnosed breast cancer regarding CPM. Any further research on the effect of breast MR examinations on CPM rates should control for these important factors. Furthermore, additional investigations on the effect of patient attitudes regarding their individual breast cancer risk, physicians’ roles in discussing management choices with patients, and the availability of advanced reconstruction options on CPM decision-making are warranted.
Advance in Knowledge
■ The rate of contralateral prophylactic mastectomy in patients with newly diagnosed breast cancer does not appear to be associated with false-positive breast MR imaging results (16.3% in the women who underwent contralateral prophylactic mastectomy vs 13.3% in those who did not, P = .43).
Implication for Patient Care
■ Clinicians counseling patients with newly diagnosed breast cancer should not be deterred from recommending bilateral breast MR imaging on the basis that a false-positive result could influence decisions regarding contralateral prophylactic mastectomy.
SUPPLEMENTAL TABLE
Received August 27, 2013; revision requested October 11; revision received March 25, 2014; accepted April 8; final version accepted April 18.
Funding: This research was supported by the National Institutes of Health (grants U01 CA079778 and U01 CA080098).
From the 2012 RSNA Annual Meeting.
Supported by an ACRIN Young Investigator Initiative Grant.
Disclosures of Conflicts of Interest: H.R. disclosed no relevant relationships. L.G.H. disclosed no relevant relationships. C.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Wilex, Endocyte, Genentech, and Biomimetics; receives payment for medical advisory board membership from Wilex. Other relationships: disclosed no relevant relationships. M.C.M. disclosed no relevant relationships. M.D.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Siemens; institution receives royalties from MedRad. Other relationships: disclosed no relevant relationships. W.B.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution has grants/grants pending from Philips Medical and GE Healthcare. Other relationships: disclosed no relevant relationships. C.D.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from GE Healthcare and Philips; receives personal fees from GE Healthcare, Philips, and Bayer. Other relationships: disclosed no relevant relationships.
Abbreviations:
- Az
- area under the receiver operating characteristic curve
- BI-RADS
- Breast Imaging Reporting and Data System
- CPM
- contralateral prophylactic mastectomy
- DCIS
- ductal carcinoma in situ
References
- 1.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25(33):5203–5209. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 2009;27(9):1362–1367. [DOI] [PubMed] [Google Scholar]
- 3.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol 2010;17(10):2554–2562. [DOI] [PubMed] [Google Scholar]
- 4.Jones NB, Wilson J, Kotur L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol 2009;16(10):2691–2696. [DOI] [PubMed] [Google Scholar]
- 5.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol 2009;16(10):2697–2704. [DOI] [PubMed] [Google Scholar]
- 6.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw 2009;7(2):193–201. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008;26(19):3248–3258. [DOI] [PubMed] [Google Scholar]
- 8.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257(2):249–255. [DOI] [PubMed] [Google Scholar]
- 9.Plana MN, Carreira C, Muriel A, et al. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: systematic review of diagnostic accuracy and meta-analysis. Eur Radiol 2012;22(1):26–38. [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Cambic A, Hansen NM, Bethke KP. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg 2007;142(5):441–445; discussion 445–447. [DOI] [PubMed] [Google Scholar]
- 11.Obdeijn IM, Tilanus-Linthorst MM, Spronk S, et al. Preoperative breast MRI can reduce the rate of tumor-positive resection margins and reoperations in patients undergoing breast-conserving surgery. AJR Am J Roentgenol 2013;200(2):304–310. [DOI] [PubMed] [Google Scholar]
- 12.Pediconi F, Miglio E, Telesca M, et al. Effect of preoperative breast magnetic resonance imaging on surgical decision making and cancer recurrence rates. Invest Radiol 2012;47(2):128–135. [DOI] [PubMed] [Google Scholar]
- 13.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009;27(25):4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorbero ME, Dick AW, Beckjord EB, Ahrendt G. Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol 2009;16(6):1597–1605. [DOI] [PubMed] [Google Scholar]
- 15.Stucky CC, Gray RJ, Wasif N, Dueck AC, Pockaj BA. Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol 2010;17(Suppl 3):330–337. [DOI] [PubMed] [Google Scholar]
- 16.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev 2010 (11):CD002748. [DOI] [PubMed] [Google Scholar]
- 17.Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Ann Surg Oncol 2012;19(8):2600–2606. [DOI] [PubMed] [Google Scholar]
- 18.Healey EA, Cook EF, Orav EJ, Schnitt SJ, Connolly JL, Harris JR. Contralateral breast cancer: clinical characteristics and impact on prognosis. J Clin Oncol 1993;11(8):1545–1552. [DOI] [PubMed] [Google Scholar]
- 19.Kollias J, Ellis IO, Elston CW, Blamey RW. Clinical and histological predictors of contralateral breast cancer. Eur J Surg Oncol 1999;25(6):584–589. [DOI] [PubMed] [Google Scholar]
- 20.Nichols HB, Berrington de González A, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol 2011;29(12):1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery LL, Tran KN, Heelan MC, et al. Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol 1999;6(6):546–552. [DOI] [PubMed] [Google Scholar]
- 22.DeMartini WB, Hanna L, Gatsonis C, Mahoney MC, Lehman CD. Evaluation of tissue sampling methods used for MRI-detected contralateral breast lesions in the American College of Radiology Imaging Network 6667 trial. AJR Am J Roentgenol 2012;199(3):W386–W391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356(13):1295–1303. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology 2012;264(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein SP, Hanna LG, Gatsonis C, Schnall MD, Rosen MA, Lehman CD. Frequency of malignancy seen in probably benign lesions at contrast-enhanced breast MR imaging: findings from ACRIN 6667. Radiology 2010;255(3):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda DM, Hylton NM, Kuhl CK, et al. BI-RADS: Magnetic Resonance Imaging. In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al., eds. Breast Imaging Reporting and Data System: ACR BI-RADS–Breast Imaging Atlas. Reston, Va: American College of Radiology, 2003. [Google Scholar]
- 27.Tuttle TM. Magnetic resonance imaging and contralateral prophylactic mastectomy: the “no más” effect? Ann Surg Oncol 2009;16(6):1461–1462. [DOI] [PubMed] [Google Scholar]
- 28.Virnig BA, Shamliyan T, Tuttle TM, Kane RL, Wilt TJ. Diagnosis and management of ductal carcinoma in situ (DCIS). Evid Rep Technol Assess (Full Rep) 2009;(185):1–549. [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow M, Strom EA, Bassett LW, et al. Standard for the management of ductal carcinoma in situ of the breast (DCIS). CA Cancer J Clin 2002;52(5):256–276. [DOI] [PubMed] [Google Scholar]
- 30.Collaborative Group on Hormonal Factors in Breast Cancer . Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 2001;358(9291):1389–1399. [DOI] [PubMed] [Google Scholar]
- 31.Buist DS, Abraham LA, Barlow WE, et al. Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res Treat 2010;124(3):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations), based on November 2011 SEER data submission. Bethesda, Md: National Cancer Institute. http://seer.cancer.gov/csr/1975_2009_pops09/. Published April 2012.. [Google Scholar]
- 33.Briasoulis E, Roukos DH. Contralateral prophylactic mastectomy: mind the genetics. J Clin Oncol 2008;26(11):1909–1910; author reply 1910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.