Abstract
Temperature sensitive copolymer systems were previously studied using modified diffusion cells in vitro for intratympanic injection, and the PLGA-PEG-PLGA copolymer systems were found to provide sustained drug delivery for several days. The objectives of the present study were to assess the safety of PLGA-PEG-PLGA copolymers in intratympanic injection in guinea pigs in vivo and to determine the effects of additives glycerol and poloxamer in PLGA-PEG-PLGA upon drug release in the diffusion cells in vitro for sustained inner ear drug delivery. In the experiments, the safety of PLGA-PEG-PLGA copolymers to inner ear was evaluated using auditory brainstem response (ABR). The effects of the additives upon drug release from PLGA-PEG-PLGA hydrogel were investigated in the modified Franz diffusion cells in vitro with cidofovir as the model drug. The phase transition temperatures of the PLGA-PEG-PLGA copolymers in the presence of the additives were also determined. In the ABR safety study, the PLGA-PEG-PLGA copolymer alone did not affect hearing when delivered at 0.05-mL dose but caused hearing loss after 0.1-mL injection. In the drug release study, the incorporation of the bioadhesive additive, poloxamer, in the PLGA-PEG-PLGA formulations was found to decrease the rate of drug release whereas the increase in the concentration of the humectant additive, glycerol, provided the opposite effect. In summary, the PLGA-PEG-PLGA copolymer did not show toxicity to the inner ear at the 0.05-mL dose and could provide sustained release that could be controlled by using the additives for inner ear applications.
Keywords: Sustained release, drug delivery, auditory brainstem response, hydrogel, ear, cidofovir
INTRODUCTION
Effective drug delivery to the inner ear remains a challenge due to the intrinsic complexity of the ear. Although transtympanic delivery such as intratympanic injection offers better therapeutic efficacy than systemic delivery and eliminates systemic side effects [1], the short drug residence time in the middle ear after intratympanic delivery reduces its effectiveness; drugs deposited in the middle ear are rapidly cleared via the Eustachian tube, e.g., when the patient swallows. Multiple transtympanic administrations are therefore required to maintain drugs at their therapeutic concentrations in the inner ear, and this leads to temporary partial hearing loss associated with tympanic membrane perforations and other adverse effects. It has been hypothesized that a sustained release drug delivery system with long residence time in the middle ear can provide an advantage over conventional intratympanic injections.
Previous studies have examined poloxamer [2–4], chitosan glycerophosphate [5], hyaluronic acid [6–8], and poly lactic/glycolic acid systems [9] as drug delivery systems for the ear. Hydrogel has been investigated extensively for sustained drug delivery in other routes of administration [10]. Temperature sensitive hydrogel can be permanently cross-linked hydrogel with a defined three-dimensional structure for temperature controlled drug delivery such as in heat-triggered subdermal implants (triggered by an increase in body temperature in a disease state or an external source) or can be thermally reversible hydrogel such as triblock copolymers Pluronics (poloxamers) [11, 12]. A temperature sensitive hydrogel (sol-gel) that is a liquid at room temperature and becomes solid at higher temperature can be utilized in inner ear delivery. In this case, the temperature sensitive gel solution can be placed in the middle ear by intratympanic injection, conform on the round window niche, solidify at body temperature, and provide sustained inner ear delivery via the round window membrane (RWM).
A previous study to evaluate formulations for sustained inner ear drug delivery using a modified diffusion cell setup in vitro has demonstrated that temperature sensitive PLGA-PEG-PLGA triblock copolymer systems could provide sustained drug delivery over several days [13]. The objectives of the present study were to assess the safety of PLGA-PEG-PLGA copolymers for intratympanic injection in guinea pigs in vivo using auditory brainstem response (ABR) and to examine the effects of additives glycerol and poloxamer in PLGA-PEG-PLGA upon drug release from the copolymer system in the diffusion cells in vitro for sustained drug delivery. The phase transition temperatures of PLGA-PEG-PLGA copolymers with the additives were also determined. It was believed that the duration of drug delivery could be extended by the incorporation of these additives.
MATERIAL AND METHODS
Materials
Polyethylene glycol (PEG 1450), D,L-lactide and glycolide were purchased from Polysciences Inc (Warrington, PA). Stannous 2-ethylhexanoate (stannous octoate) was obtained from Sigma Aldrich (St. Louis, MO). Phosphate buffered saline (PBS, pH 7.4 containing 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride) was prepared by PBS tablets purchased from Sigma Aldrich and distilled deionized water. Sterile saline USP was from Baxter (Round Lake, IL). Cidofovir 75 mg/mL Injection (Vistide®) was obtained from Gilead Sciences (Foster City, CA) and was diluted to the required concentration with PBS or saline. Poloxamer (Pluronic® F 127 Prill) was from BASF Chemical Company (Mt. Olive, NJ, US). Poly(lactic-co-glycolic acid)-poly(ethylene glycol)-poly(lactic-co-glycolic acid) triblock copolymer (PLGA-PEG-PLGA), AK24, of PEG 1,000 and 3:1 lactide:glycolide, Mn = 3,552 and Mw = 4,286, was purchased from Akina, Inc. (West Lafayette, IN). Tetrabutylammonium phosphate monobasic and sodium phosphate dibasic heptahydrate were purchased from Acros Organics (Fair Lawn, NJ, US). Glycerol was from Humco Lab (Texarkana, TX). High performance liquid chromatography (HPLC) grade acetonitrile was purchased from Pharmaco-AAPER (Shelbyville, KY, US). All other chemicals were of analytical grades.
Animals
Guinea pigs, weighing 250–600 grams, cytomegalovirus (CMV) free colony were bred at the Cincinnati Children’s Hospital Medical Center, an AAALAC approved facility. The use of animals was approved by Institutional Animal Care and Use Committee at both the Children’s Hospital Cincinnati and University of Cincinnati.
Synthesis of Temperature-sensitive Copolymer
Triblock copolymers (PLGA-PEG-PLGA, 8/1) were synthesized according to ring opening polymerization procedure using stannous octanoate as the catalyst [13, 14]. Briefly, 1.5 g polyethylene glycol (PEG 1450) was dried in a flask under vacuum at 120 °C for 3 h. Appropriate amounts of DL-lactide (~ 3.2 g) and glycolide (~0.32 g) were then added to the dried PEG in the flask to obtain a lactide to glycolide molar ratio of 8/1 and total weight ratio of PEG 1450 of 30% w/w. After mixing the reagents in the flask, stannous octanoate was added and the mixture was heated and maintained at 155 °C overnight. The copolymer product was subsequently dissolved in water and the solution was heated at 80 °C to precipitate the copolymer. Then, the copolymer was subjected to three cycles of purification to remove low-molecular weight impurities and unreacted monomers. Purification was performed by dissolving the copolymer in cold water followed by heating to precipitate the copolymer. The supernatant water was decanted. The resulting copolymer after purification was lyophilized and stored in a dessicator. The average molecular structure of the copolymer was checked using 1H-NMR. Briefly, 1H-NMR spectra of PLGA-PEG-PLGA were obtained in CDCl3 using Varian (Inova-300) at 300 MHz. The DL-lactide to glycolide ratio was determined from the NMR spectra [15].
Auditory Brainstem Response (ABR) Assessment in vivo
All guinea pigs underwent a baseline ABR on Day 0. Weekly ABR evaluations were then performed on Day 7, Day 14, Day 21, and Day 28 following intratympanic injections of the PLGA-PEG-PLGA copolymer solution on Day 0. Intratympanic injection of sterile saline was the control. The copolymer solution was prepared by dissolving 20% polymer in PBS at cool temperature and sterilized by filtration. In addition to the PLGA-PEG-PLGA copolymer synthesized in the present study, a commercial PLGA-PEG-PLGA copolymer was also used for comparison. The purpose of testing a commercial PLGA-PEG-PLGA copolymer was to check whether the hearing loss found in the ABR study was a result of impurities introduced in the copolymer synthesis procedure in the present study or the copolymer itself.
In the experiments, the animals were anesthetized with 1–3% isoflurane for the intratympanic injection and ABR examination. The ABR needle electrodes were placed in the vertex and behind each pinnae. Frequency specific tone pips at 8, 16, and 32 kHz were delivered in 10dB steps using high frequency transducers (MO14600) inserted into the ear canal. Waveforms were recorded using an Intelligent Hearing Systems Smart-EP (Miami, FL, USA). All waveforms were duplicated and the threshold was defined as the lowest level at which a repeatable response was obtained.
Drug Release from Copolymer System in vitro
Two additives glycerol and poloxamer, a humectant and bioadhesive, respectively, were evaluated with the synthesized PLGA-PEG-PLGA copolymer. These additives are generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA) and are commonly used in the pharmaceutical industry. Table 1 lists the compositions of the formulations tested in the modified Franz diffusion cells in the drug release study. Formulations #1–6 consisted of PLGA-PEG-PLGA and one or both of the additives, and Formulations #7–8 consisted of only PLGA-PEG-PLGA. PBS without PLGA-PEG-PLGA and the additives was the control (PBS control). In these experiments, the copolymer solution was prepared in a vial and mixed with the additives. Cidofovir solution (Vistide®) was then added to the mixture to obtain a final cidofovir concentration of 5.0 mg/mL. The modified Franz-type diffusion cells had a diffusion surface area of 0.2 cm2 with a rubber membrane containing a hole of approximately 1 mm in diameter and were maintained at 37 °C in a water-bath. A Millipore filter membrane (0.45 μm) was mounted between the diffusion half-cells under the rubber membrane to serve as the support and to separate the donor and receiver chambers. The solution in the receiver compartment was PBS containing 0.02% w/v sodium azide. The donor compartment was 0.1 mL of cidofovir formulations (Table 1). At the start of the experiments, the donor compartment was sealed by Parafilm to maintain the temperature of the system above the copolymer lower critical solution temperature. The release of cidofovir from the copolymer gel through the filter membrane support was measured. At predetermined time points, 1-mL samples were withdrawn from the receiver compartment and replaced with fresh receiver solution. The samples were analyzed for cidofovir using high-performance liquid chromatography (HPLC). The drug release vs. time profiles of the formulations were analyzed under the assumption of first order release from the hydrogel through the small diffusional area of the diffusion cells into the receiver chamber:
| (1) |
where Ct is the concentration of the drug remaining in the donor chamber at time t, C0 is the initial concentration of the drug, and k is the apparent first order release rate constant. The apparent first order release rate constants were calculated using Eq. 1 and Linest function fitting in Microsoft Excel (Redwood, WA) by setting the y-intercept to zero. The apparent first order rate constants were then analyzed by a regression analysis:
| (2) |
where ko is the rate constant of PBS control, p, h, and b are the coefficients, and P, H, and B are the concentrations of PLGA-PEG-PLGA, glycerol, and poloxamer in the formulations, respectively. The relationships between drug release rates (dependent variable k) and the concentrations of the copolymer and additives (independent variables P, H, and B) of the formulations were determined with least squares-fitting software Scientist (MicroMath, Salt Lake City, UT). The main purpose of this regression analysis was to evaluate the impact of the concentration of PLGA-PEG-PLGA, glycerol, and poloxamer in the formulations upon the upward or downward trends of changes in drug release rates. It should be emphasized that this analysis is not a quantitative model for drug release from the hydrogels and is not related to the drug release mechanism.
Table 1.
Formulations tested in the drug release study in vitro.
| Formulation # | Concentration (%) | ||
|---|---|---|---|
| PLGA-PEG-PLGAa | Glycerol | Poloxamer | |
| 1 | 15.0 | 3.9 | 2.1 |
| 2 | 16.9 | 8.0 | 1.5 |
| 3 | 18.8 | 2.1 | 1.1 |
| 4 | 20.8 | 6.1 | 0.6 |
| 5 | 20.0 | 10.0 | 1.0 |
| 6 | 22.9 | 10.0 | 0 |
| 7 | 15.0 | 0 | 0 |
| 8 | 24.6 | 0 | 0 |
| PBS (control) | 0 | 0 | 0 |
Synthesized PLGA-PEG-PLGA.
HPLC Analysis of Cidofovir
HPLC assay of cidofovir was performed using a liquid chromatography system (Prominence system; Shimadzu, Columbia, MD, US) equipped with CBM-20A system controller, LC-20AT solvent delivery module, SIL-20A auto-sampler, SPD-20A UV-Vis detector, and an Eclipse XDB-C8 column (150 × 4.6 mm, 5 μm; Agilent Technologies, Santa Clara, CA, US). The mobile phase consisted of 3.5 mM sodium phosphate dibasic and 1.5 mM tetrabutylammonium phosphate monobasic in water and acetonitrile at a ratio of 90:10 (v/v). The mobile phase was delivered at 1.0 mL/min and the detection wavelength was 274 nm. Calibration curves were constructed using standards of cidofovir concentrations between 1.5 – 75 μg/ml and found to be linear in this range.
Copolymer Sol-Gel Transition Temperature in vitro
The gelation behavior of the copolymer formulations was studied by physical observation. Copolymer solutions of different concentrations were prepared by dissolving the copolymer in PBS in a small test tube and allowed to completely go into solution in an ice-bath. PBS was used because it could maintain the pH of the copolymer solution. The physical form and the change in solution viscosity and turbidity (i.e., solution, gel, or precipitated form) were then monitored in a temperature controlled water-bath to determine the transition temperatures of the formulations. The relationships between the transition temperatures and the concentrations of the copolymer and additives of the formulations were then analyzed using an equation similar to Eq. 2.
RESULTS AND DISCUSSION
ABR Study in vivo
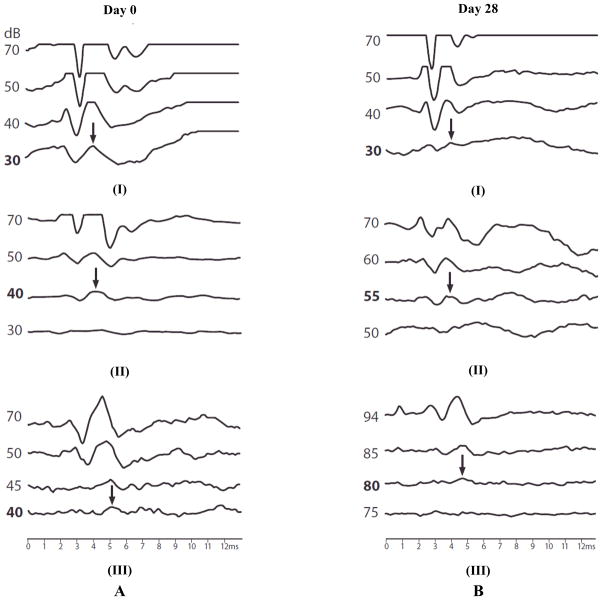
The safety of the PLGA-PEG-PLGA delivery system was evaluated by ABR. Prestudy ABR evaluations were performed at Day 0, followed by the intratympanic injection of PLGA-PEG-PLGA copolymer solution into the middle ear. Post-injection ABR evaluations were performed on Day 7, Day 14, Day 21, and Day 28. Fig. (1) shows the representative ABR tracings with their threshold values before the injection on Day 0 and after the injection on Day 28. Fig. (2) presents the ABR results after intratympanic injection of 0.1 mL copolymer gel solution. In this figure, the threshold values after intratympanic injections were divided by the initial threshold values of the same animal before the injections and the ratios were plotted against time. These threshold ratios are shown as their means and 95% confidence intervals. Hearing loss is indicated by a ratio significantly higher than unity (> 1.0). The data in the figure show that the 0.1-mL injection of the synthesized PLGA-PEG-PLGA copolymer gel solution could induce hearing loss. Particularly, hearing loss was more noticeable at the higher frequency region. To investigate whether the hearing loss observed was specifically related to the method of polymer synthesis in the present study or a general phenomenon of PLGA-PEG-PLGA, a commercial PLGA-PEG-PLGA copolymer was also examined. As shown in Fig. (2), hearing loss was more significant for the commercial PLGA-PEG-PLGA copolymer. This suggests that the ABR results are not specific to the synthesized PLGA-PEG-PLGA in the present study, but are related to general influence of 0.1-mL injection of PLGA-PEG-PLGA on ABR.
Fig. 1.
Representative ABR tracings at 32 kHz obtained on (A) Day 0 before intratympanic injections and (B) Day 28 after the injections in guinea pigs in vivo. The differences among the Day 0 tracings are due to experimental variability. ABR tracings are from (I) control, (II) 0.05 mL, and (III) 0.1 mL injection experiments. The arrows and bold dB values indicate the ABR threshold values.
Fig. 2.
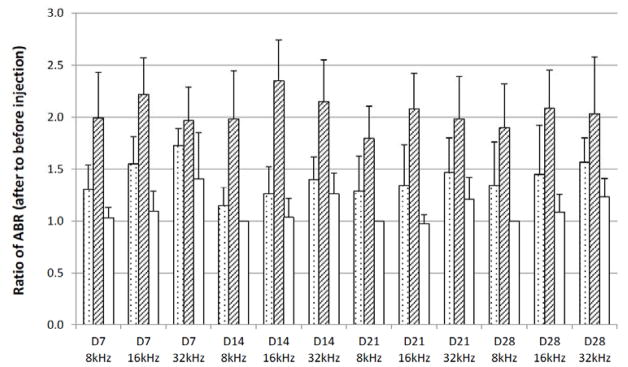
Ratios of ABR threshold values after 0.1 mL intratympanic injections of copolymer systems to those before injections in guinea pigs over 28 days in vivo. The average threshold values of ABR were 30–45 dB before the injections. Injection of 0.1 mL saline was the control. Symbols: dotted bars, synthesized PLGA-PEG-PLGA; striped bars, commercial PLGA-PEG-PLGA; open bars, saline (control). D0, D7, D14, D21, and D28 indicate ABR data on 7 days, 14 days, 21 days, and 28 days after the injection, respectively. 8kHz, 16kHz, and 32kHz indicate the frequency. Mean ± 95% confidence interval (n ≥ 4). Hearing loss is indicated by a ratio significantly higher than 1.0.
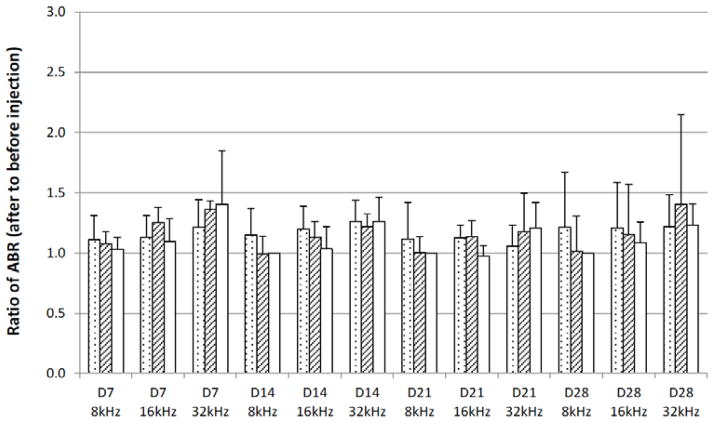
A review of previous inner ear drug delivery studies of temperature sensitive gels shows that smaller injection volume (0.05 mL) was used [2–4]. Since 0.05-mL intratympanic injection instead of 0.1 mL was previously studied and found to be nontoxic, smaller injection volume was examined in the present study. Fig. (3) presents the ABR results after intratympanic injection of 0.05 mL copolymer gel solution. At the lower injection volume of 0.05 mL, there is no statistical difference between the ABR results of 0.05 mL PLGA-PEG-PLGA injection and saline control. Although the cause of the different responses after 0.05 and 0.1 mL injections is not clear, the ABR results suggest that the PLGA-PEG-PLGA copolymer does not affect hearing when injected at 0.05-mL dose. Further investigation is required to determine the cause of hearing loss at 0.1-mL injection dose of the PLGA-PEG-PLGA solutions.
Fig. 3.
Ratios of ABR threshold values after 0.05 mL intratympanic injections of copolymer systems to those before injections in guinea pigs over 28 days in vivo. The average threshold values of ABR were 35–50 dB before the injections. Injection of 0.1 mL saline was the control. Symbols: dotted bars, synthesized PLGA-PEG-PLGA; striped bars, commercial PLGA-PEG-PLGA; open bars, saline (control). D0, D7, D14, D21, and D28 indicate ABR data on 7 days, 14 days, 21 days, and 28 days after the injection, respectively. 8kHz, 16kHz, and 32kHz indicate the frequency. Mean ± 95% confidence interval (n ≥ 4).
Drug Release from Copolymer System in vitro
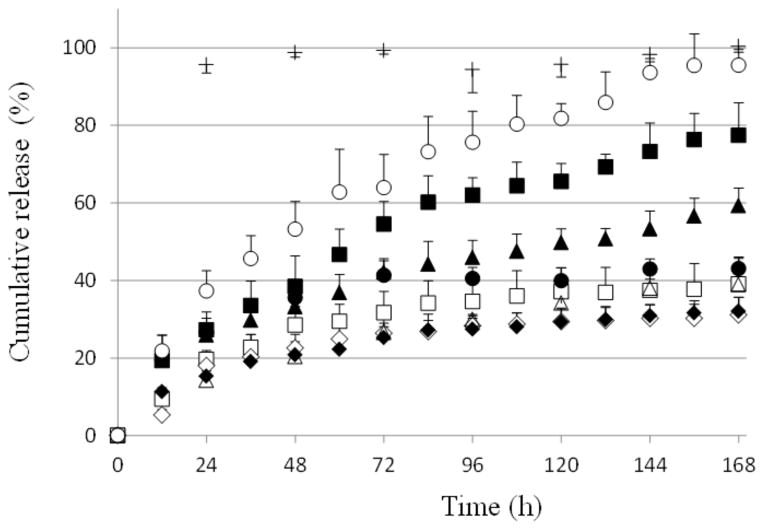
Fig. (4) shows the drug release vs. time profiles of cidofovir from the formulations in the diffusion cell experiments in vitro. Diffusion-controlled drug release from hydrogels is commonly described by the square root of time release function or time-dependent power law function of exponent equal to ~0.5. However, a previous study has shown that a first order release process is more appropriate to describe drug release in the modified diffusion cells employed in the present study due to the small diffusional area as the transport rate-limiting step [13]. As the goal of the present study was to evaluate the effects of additives upon drug release from the PLGA-PEG-PLGA systems, the first order release model was the focus in the analyses of the present drug release testing to obtain the apparent rate constants (Eq. 1) for comparison and for empirical regression fitting (Eq. 2).
Fig. 4.
Cidofovir release profiles from formulations of PLGA-PEG-PLGA with and without additives in Franz diffusion cell experiments in vitro. PBS was the control. Symbols: closed diamonds, closed squares, closed triangles, open squares, open circles, open diamonds, closed circles, open triangles, and crosses represent Formulation #1 to #8 and PBS, respectively. Mean ± SD (n = 3).
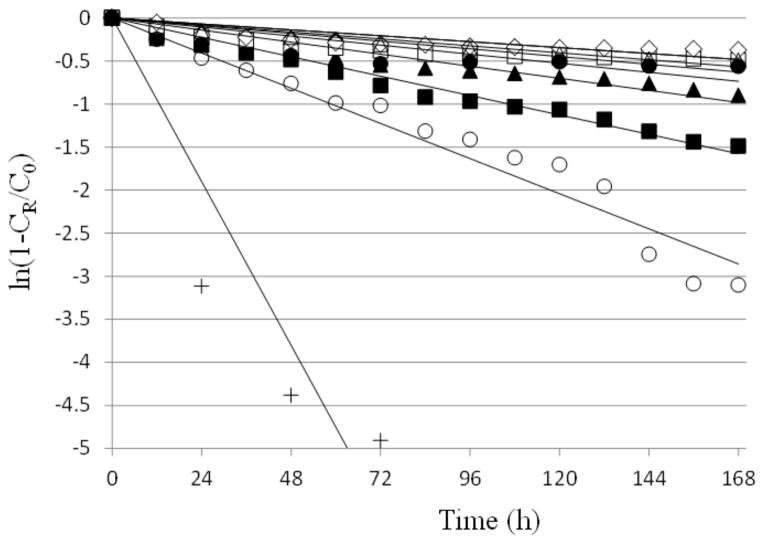
Fig. (5) presents the natural logarithm of the fraction of drug remaining in the formulation vs. time profiles to determine the apparent rate constants of drug release from the formulations (Eq. 1). Table 2 summarizes the apparent rate constants of drug release from the formulations and PBS control under the first order release assumption. The rate constants were then analyzed by a regression analysis using Eq. 2 to deduce the relationships between the rate constants and the concentrations of PLGA-PEG-PLGA, glycerol, and poloxamer. The analysis found that the coefficients p and b were negative and the coefficient h was positive. This indicates that the rate of drug release from the sustained delivery system in general decreased when the concentration of PLGA-PEG-PLGA increased and/or with the incorporation of bioadhesive poloxamer, but the rate of drug release increased with the addition of humectant glycerol. Also examined in this rate constant analysis was the impact of other parameters such as those of the second order functions (i.e., P2, H2, B2, P × H, P × B, and H × B) by the extension of Eq. 2. The addition of these parameters did not affect the conclusion of the analysis: negative coefficients p and b and positive coefficient h. In addition to the first order release model, the square root of time release model was also examined for comparison (data not shown). Overall, the rank orders of the rate constants determined using the first order and square root of time release models for the formulations are essentially the same, and the use of the first order release model should not affect the conclusion in the present study.
Fig. 5.
Apparent first-order fitting of cidofovir release from formulations of PLGA-PEG-PLGA with and without additives in Franz diffusion cell experiments in vitro. PBS was the control. Symbols: closed diamonds, closed squares, closed triangles, open squares, open circles, open diamonds, closed circles, open triangles, and crosses represent Formulation #1 to #8 and PBS, respectively. CR is the concentration representing the cumulative amount of drug in the receiver at time t.
Table 2.
Apparent rate constants of drug release from the formulations.
| Formulation # | First order rate constant (h−1) a, regression analysis R2 |
|---|---|
| 1 | 0.0029±0.0002, 0.941 |
| 2 | 0.0094±0.0003, 0.994 |
| 3 | 0.0058±0.0003, 0.970 |
| 4 | 0.0037±0.0003, 0.936 |
| 5 | 0.017±0.001, 0.984 |
| 6 | 0.0029±0.0003, 0.922 |
| 7 | 0.0044±0.0007, 0.893 |
| 8 | 0.0034±0.0002, 0.978 |
| PBS (control) | 0.079±0.008, 0.954 |
Rate constant, k, calculated from the slope of ln(Ct/C0) vs. t; mean ± standard error.
In general, drug release from PLGA-PEG-PLGA sol-gel systems can be controlled by the concentration of PLGA-PEG-PLGA in the systems [12, 16]. However, because the transition temperatures of the sol-gel systems are affected by PLGA-PEG-PLGA concentration, this could present a problem to control the rates of drug release from the sol-gel systems using solely this method. Previous studies have shown the effects of additives in temperature sensitive polymer systems upon their drug release behavior. For example, polymer additives were observed to affect drug release rates in the development of pluronic-containing formulations for ophthalmic and other applications [17–19]. The present study investigated the effects of additives upon the rates of drug release from the PLGA-PEG-PLGA hydrogel formulations. The in vitro drug release results demonstrate that sustained release of cidofovir from PLGA-PEG-PLGA can be controlled by the additives. This allows the manipulation of the drug release rates of the PLGA-PEG-PLGA systems with two other factors (i.e., concentrations of poloxamer and glycerol). The present results also illustrate that the duration of sustained drug delivery can be extended to more than 168 hr (7 days) with these formulations in the diffusion cell release study. For example, approximately 30% drug was released in 168 h with Formulation #1, which was significantly slower than those reported in a previous study: 40–70% drug release in 96 h [13]. Also, this was more than five times longer drug release than that from PBS control, in which drug release was close to 100% in 24 h. The longer sustained release is a potential advantage in inner ear drug delivery.
Copolymer Sol-Gel Temperature in vitro
Table 3 presents the lower critical solution temperatures (i.e., solution to gel phase transition temperatures) of the PLGA-PEG-PLGA formulations. At the lower critical solution temperature of the PLGA-PEG-PLGA copolymer, the copolymer solution went through a sol-gel phase-transition from a solution into a gel when the temperature increased. The transition temperatures of the copolymer systems were then analyzed by a regression analysis similar to Eq. 2 to deduce the effects of PLGA-PEG-PLGA, glycerol, and poloxamer on the transition temperatures. The analysis showed that when the concentration of PLGA-PEG-PLGA in the solution increased, the transition temperature decreased. This result is consistent with those observed in previous studies of PLGA-PEG-PLGA systems [14, 20]. The analysis also indicated that, in general, both glycerol and poloxamer reduced the transition temperatures of the sol-gel systems with poloxamer having a stronger impact on the transition temperatures than glycerol.
Table 3.
Transition temperatures of the formulations.
| Formulation # | Transition temperature (°C) a |
|---|---|
| 1 | 27 |
| 2 | 22 |
| 3 | 28 |
| 4 | 23 |
| 5 | 20 |
| 6 | 22 |
| 7 | 30 |
| 8 | 24 |
The transition temperatures represent average values of solution to gel transitions when temperature increases (n = 2).
PLGA-PEG-PLGA Systems for Inner Ear Delivery in Practice
Sustained drug delivery and accurate dosing to the inner ear remain a technologically challenging task, and the current methods to deliver drugs to the inner ear have a number of drawbacks [1, 21, 22]. The increasing significance of ear diseases such as sensorineural hearing loss, Meniere’s disease, and tinnitus has led to recent efforts to develop therapeutic compounds and sustained local drug delivery systems to treat these conditions. For example, in intratympanic injection, the simple deposition of therapeutic agents in the middle ear on the RWM by intratympanic injections of drug solutions in inner ear disease treatments is hampered by the short residence time of the drugs on the RWM and clearance (of the injection solutions) through the Eustachian tube. Several hydrogel systems were previously investigated for sustained drug delivery to the ear [2–7]. The present study illustrated the effects of two additives, a bioadhesive and humectant, to modify the sustained delivery of cidofovir from PLGA-PEG-PLGA hydrogels. This could allow a tailored-made design of PLGA-PEG-PLGA using these excipients for sustained drug release from the hydrogel in the middle ear after intratympanic administration. The present study also identified the intratympanic injection condition for PLGA-PEG-PLGA sol-gel (0.05 mL) that could be safe for sustained drug delivery to the inner ear.
CONCLUSIONS
The safety of PLGA-PEG-PLGA copolymers in intra-tympanic injection was assessed in guinea pigs in vivo. In the safety study, the PLGA-PEG-PLGA copolymers were found to be relatively safe in intratympanic injection as they did not affect hearing in the present study when 0.05-mL intratympanic injections of PLGA-PEG-PLGA were used. The effects of additives glycerol and poloxamer in PLGA-PEG-PLGA upon the transition temperatures of the hydrogels and drug release were investigated in the diffusion cells in vitro. In these drug release experiments, it was shown that the rate of drug release from PLGA-PEG-PLGA generally was reduced by the incorporation of poloxamer as the bioadhesive additive but was increased by the addition of glycerol as the humectant. PLGA-PEG-PLGA could provide sustained release of cidofovir that can be controlled by using the additives.
Acknowledgments
This research was supported by NIH Grant DC011062. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDCD or NIH. The authors thank Alisa Reece for her help in the experiments.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
PATIENT CONSENT
Declared none.
References
- 1.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Ye Q, Sweet J, Tapp R, Dolan DF, Altschuler RA, Lichter J, LeBel C. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32:171–179. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- 3.Salt AN, Hartsock J, Plontke S, LeBel C, Piu F. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16:323–335. doi: 10.1159/000322504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Dellamary L, Fernandez R, Harrop A, Keithley EM, Harris JP, Ye Q, Lichter J, LeBel C, Piu F. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393–401. doi: 10.1159/000241896. [DOI] [PubMed] [Google Scholar]
- 5.Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–711. doi: 10.1097/MLG.0b013e31815f8e41. [DOI] [PubMed] [Google Scholar]
- 6.Borden RC, Saunders JE, Berryhill WE, Krempl GA, Thompson DM, Queimado L. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol Neurootol. 2011;16:1–11. doi: 10.1159/000313506. [DOI] [PubMed] [Google Scholar]
- 7.Saber A, Laurell G, Bramer T, Edsman K, Engmer C, Ulfendahl M. Middle ear application of a sodium hyaluronate gel loaded with neomycin in a Guinea pig model. Ear Hear. 2009;30:81–89. doi: 10.1097/AUD.0b013e31818ff98e. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RM, Meyer JD, Matsuura JE, Shefter E, Hart MJ, Malone DJ, Manning MC. In vitro release kinetics of gentamycin from a sodium hyaluronate gel delivery system suitable for the treatment of peripheral vestibular disease. Drug Dev Ind Pharm. 1999;25:15–20. doi: 10.1081/ddc-100102137. [DOI] [PubMed] [Google Scholar]
- 9.Horie RT, Sakamoto T, Nakagawa T, Tabata Y, Okamura N, Tomiyama N, Tachibana M, Ito J. Sustained delivery of lidocaine into the cochlea using poly lactic/glycolic acid microparticles. Laryngoscope. 2010;120:377–383. doi: 10.1002/lary.20713. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 11.Shim MS, Lee HT, Shim WS, Park I, Lee H, Chang T, Kim SW, Lee DS. Poly(D,L-lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly (D,L-lactic acid-co-glycolic acid) tri-block copolymer and thermoreversible phase transition in water. J Biomed Mater Res. 2002;61:188–196. doi: 10.1002/jbm.10164. [DOI] [PubMed] [Google Scholar]
- 12.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Feng L, Tolia G, Liddell MR, Hao J, Li SK. Evaluation of intratympanic formulations for inner ear delivery: methodology and sustained release formulation testing. Drug Dev Ind Pharm. doi: 10.3109/03639045.2013.789054. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294:103–112. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Jeong B, Choi YK, Bae YH, Zentner G, Kim SW. New biodegradable polymers for injectable drug delivery systems. J Control Release. 1999;62:109–114. doi: 10.1016/s0168-3659(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Pieper R, Webster DC, Singh J. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288:207–218. doi: 10.1016/j.ijpharm.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Desai SD, Blanchard J. In vitro evaluation of pluronic F127-based controlled-release ocular delivery systems for pilocarpine. J Pharm Sci. 1998;87:226–230. doi: 10.1021/js970090e. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 2002;85:73–81. doi: 10.1016/s0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 19.Shastri DH, Prajapati ST, Patel LD. Design and development of thermoreversible ophthalmic in situ hydrogel of moxifloxacin HCl. Curr Drug Deliv. 2010;7:238–243. doi: 10.2174/156720110791560928. [DOI] [PubMed] [Google Scholar]
- 20.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 21.Rivera T, Sanz L, Camarero G, Varela-Nieto I. Drug delivery to the inner ear: strategies and their therapeutic implications for sensorineural hearing loss. Curr Drug Deliv. 2012;9:231–242. doi: 10.2174/156720112800389098. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Ito J. Local drug delivery to the inner ear using biodegradable materials. Ther Deliv. 2011;2:807–814. doi: 10.4155/tde.11.43. [DOI] [PubMed] [Google Scholar]