Abstract
Indolines are important moieties present in various biologically significant molecules and have attracted considerable attention in synthetic chemistry. This paper describes a sequential Heck reaction/C–H activation/amination process to form indoline with di-tert-butyldiaziridinone. The reaction process likely proceeds via a pallada(II)cycle, which is converted to an indoline by oxidative addition to the diaziridinone and subsequent double C–N bond formation.
Keywords: indoline, Heck reaction, C–H activation, diaziridinone
Indolines are important moieties contained in various biologically and pharmaceutically active compounds,[1] and their syntheses have attracted considerable attention. The cyclization involving replacement of a leaving group by a nitrogen is among widely used methods to synthesize indolines.[2,3] Direct C–H amination presents an attractive strategy for the construction of this class of molecules and has become an active area in recent years.[4–6]
Recently, we have reported that α-methylstyrene can be efficiently transformed to spirocyclic indolines via a Pd(0)-catalyzed sequential C–N bond formation involving allylic and aromatic C–H amination with di-tert-butyldiaziridinone (1) (Scheme 1).[7] This reaction process likely proceeds via pallada(II)cycle A, which oxidatively inserts into the diaziridinone to form pallada(IV)cycle B.[8] Upon release of tert-butyl isocyanate (tBuNCO), intermediate B is converted to spirocyclic indoline 2 via two consecutive reductive eliminations.
Scheme 1.
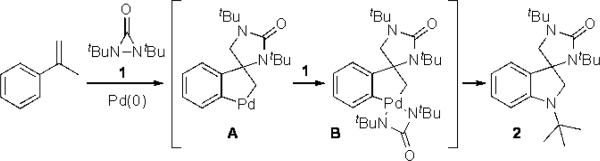
The formation of spirocyclic indoline from α-methylstyrene.
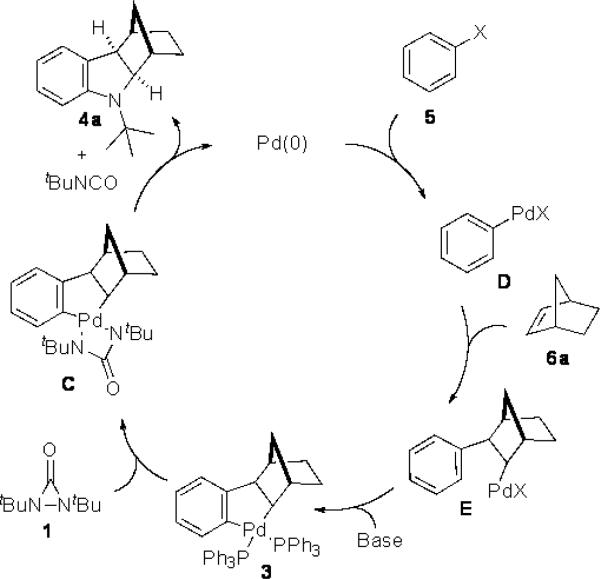
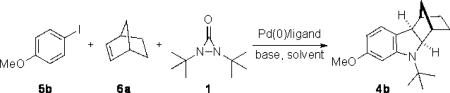
Previously, we have shown that Pd(0) can readily insert into the N–N bond of diaziridinone 1, and the resulting four-membered Pd(II) species reacts with dienes to form diamination products.[9] The oxidative insertion of a pallada(II)cycle to di-tert butyldiaziridinone (1) is mechanistically interesting. To further probe this process, known pallada(II)cycle 3[10] was thus prepared[11] and reacted with di-tert-butyldiaziridinone (1) (Scheme 2). The palladacycle was indeed smoothly transformed to indoline 4a in 66% yield at room temperature.[12] This result prompted us to investigate if the indoline could be directly formed from aryl halide (5), norbornene (6a), and di-tert-butyldiaziridinone (1) with catalytic amounts of Pd(0) by in situ formation of pallada(II)cycle 3 via Heck reaction and subsequent C–H activation (Scheme 3). The key issue for such transformation is whether aryl halide can compete with the diaziridinone for the oxidative addition by Pd(0) catalyst to initiate the catalytic cycle. Herein, we wish to report our preliminary studies on this subject.
Scheme 2.
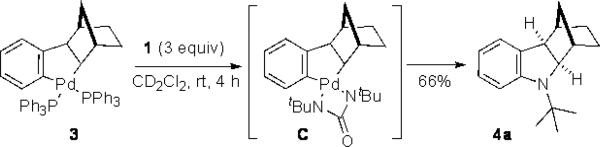
Synthesis of indoline 4a from pallada(II)cycle 3.
Scheme 3.
Proposed catalytic cycle.
Initial studies were carried out with p-iodoanisole (5b) as test substrate under various conditions. As shown in Table 1, the base had a significant effect on the reaction efficiency. No products were isolated with K2CO3, Na2CO3, and Et3N (Table 1, entries 1–3). To our delight, the reaction proceeded efficiently with base such as K3tPO4, NaO Bu, and Cs2CO3 (Table 1, entry 4–6). For example, treating p-iodoanisole (5b) and norbornene (6a) with 1.5 equiv of di-tert-butyldiaziridinone (1), 10 mol% Pd(PPh3)4, and 2.0 equiv of Cs2CO3 in toluene at 80 oC gave indoline 4b in 90% yield (Table 1, entry 6). Polar solvents such as DMF, MeCN, THF, and dioxane gave lower yields as compared to toluene (Table 1, entries 7–10). Similar yield (89%) was obtained when Pd(PPh3)4 was reduced from 10 mol% to 5 mol% (Table 1, entries 6 and 11). The effect of ligand was also briefly investigated with Pd2(dba)3 as catalyst (Table 1, entries 12–16). No product was formed with Pd2(dba)3 alone (Table 1, entry 12). PPh3, P(p-MeO-Ph)3, and P(p-CF3-Ph)3 were shown to be effective ligands for the reaction, giving indoline 4b in 81–90% yield (Table 1, entry 13–15). However, no desired product was obtained with BINAP (Table 1, entry 16).
Table 1.
Studies on the reaction conditions.[a]
| |||||
|---|---|---|---|---|---|
| entry | solvent | base | catalyst | ligand | yield [%][b] |
| 1 | PhMe | K2CO3 | Pd(PPh3)4 | - | 0 |
| 2 | PhMe | Na2CO3 | Pd(PPh3)4 | - | 0 |
| 3 | PhMe | Et3N | Pd(PPh3)4 | - | 0 |
| 4 | PhMe | K3PO4 | Pd(PPh3)4 | - | 79 |
| 5 | PhMe | NaOtBu | Pd(PPh3)4 | - | 82 |
| 6 | PhMe | Cs2CO3 | Pd(PPh3)4 | - | 90 |
| 7 | DMF | Cs2CO3 | Pd(PPh3)4 | - | 70 |
| 8 | MeCN | Cs2CO3 | Pd(PPh3)4 | - | 84 |
| 9 | THF | Cs2CO3 | Pd(PPh3)4 | - | 76 |
| 10 | Dioxane | Cs2CO3 | Pd(PPh3)4 | - | 74 |
| 11 [c] | PhMe | Cs2CO3 | Pd(PPh3)4 | - | 89 |
| 12 | PhMe | Cs2CO3 | Pd2(dba)3 | - | 0 |
| 13 | PhMe | Cs2CO3 | Pd2(dba)3 | PPh3 | 84 |
| 14 | PhMe | Cs2CO3 | Pd2(dba)3 | P(p-MeO-Ph)3 | 81 |
| 15 | PhMe | Cs2CO3 | Pd2(dba)3 | P(p-CF3-Ph)3 | 90 |
| 16 | PhMe | Cs2CO3 | Pd2(dba)3 | BINAP | 0 |
All reactions were carried out with p-iodoanisole (5b) (0.30 mmol), norbornene (6a) (0.60 mmol), di-tert-butyldiaziridinone (1) (0.45 mmol), Pd (0.030 mmol) (Pd/P = 1/4), base (0.60 mmol), and solvent (0.30 mL) at 80 °C under Ar for 36 h unless otherwise stated. For entry 4, the reaction time was 72 h.
Isolated yield.
The reaction was carried out with Pd(PPh3)4 (0.015 mmol) and di-tert-butyldiaziridinone (1) (0.35 mmol) at 80 °C for 48 h.
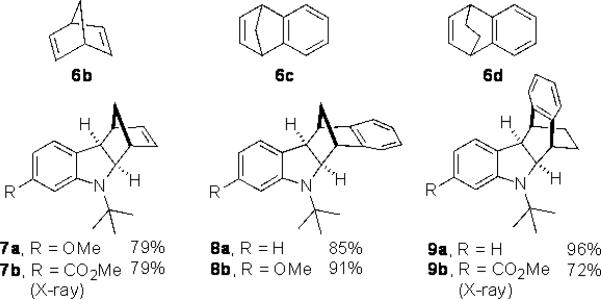
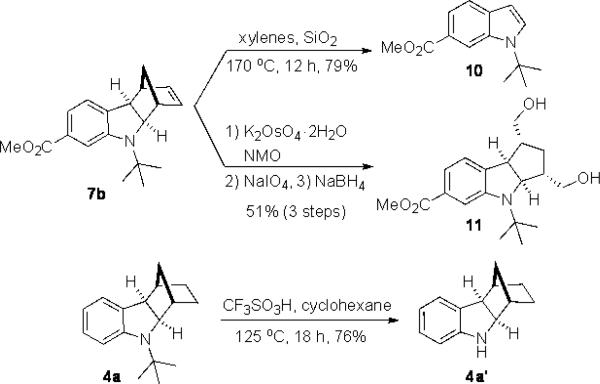
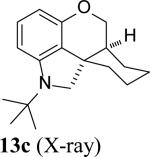
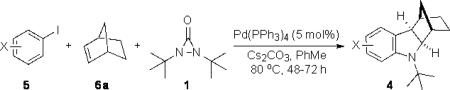
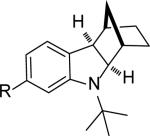
As shown in Table 2, the reaction process can be extended to various para-, meta-, ortho-, and disubstituted iodobenzenes, giving the corresponding indoline products in 67–97% yield (Table 2, entries 1–19). The Heck reaction occurred from the exo face of norbornene as indicated by the X-ray structure of 4g (see Supporting Information). For bromo-substituted iodobenzenes 5d and 5n, the reaction selectively occurred on iodide and gave indolines 4d and 4n in good yields (Table 2, entries 4 and 14). For meta-substituted iodobenzenes (Table 2, entries 9–12 and entry 18), the C–H amination regioselectively occurred at the sterically less hindered position. Good yields (72–96%) were also obtained for the corresponding indolines (7–9)[13] when norbornadiene (6b) and bridged olefins 6c, d were used (Figure 1).[14] As illustrated in the case of indoline 7b, the reaction process is amenable to gram scale. Indoline 7b can be converted to N-tert-butyl indole 10 in 79% yield with SiO2 in xylenes via retro-Diels-Alder reaction and to tricyclic indoline 11 in 51% yield over 3 steps via dihydroxylation, oxidative diol cleavage, and reduction (Scheme 4).[15] As shown in the case of indoline 4a, the removal of the tert-butyl group was accomplished with CF3SO3H−cyclohexane, giving deprotected indoline 4a’ in 76% yield (Scheme 4).
Table 2.
Substrate scope.[a]
| |||
|---|---|---|---|
| entry | substrate (5) | product (4) | yield [%][b] |

|
|
||
| 1 | 5a, R = H | 4a | 90 |
| 2 | 5b, R = OMe | 4b | 89 |
| 3 | 5c, R = n-Bu | 4c | 91 |
| 4 | 5d, R = Br | 4d | 68 |
| 5 | 5e, R = F | 4e | 72 |
| 6 | 5f, R = CF3 | 4f | 78 |
| 7 | 5g, R = CO2Me | 4g (X-ray) | 93 |
| 8 | 5h, R = NO2 | 4h | 84 |
|
|
||
| 9 | 5i, R = OMe | 4i | 82 |
| 10 | 5j, R = CH2OTBS | 4j | 97 |
| 11 | 5k, R = CO2Me | 4k | 85 |
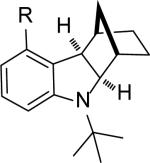
| 12 |
|
|
84 |
|
|
||
| 13 | 5m, R = OMe | 4m | 90[c] |
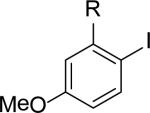
| 14 | 5n, R = Br | 4n | 81[c] |
| 15 | 5o, R = NO2 | 4o | 88[c] |
|
|
||
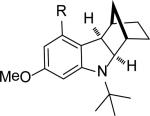
| 16 | 5p, R = OMe | 4p | 94[c] |
| 17 | 5q, R = NO2 | 4q | 80[c] |
| 18 |
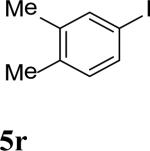
|
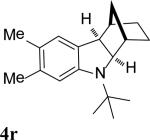
|
93 |
| 19 |
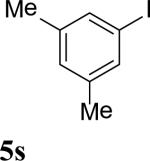
|
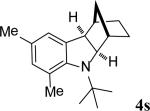
|
67 |
All reactions were carried out with iodobenzene 5 (0.30 mmol), norbornene (6a) (0.60 mmol), di-tert-butyldiaziridinone (1) (0.35 mmol), Pd(PPh3)4 (0.015 mmol), Cs2CO3 (0.60 mmol), and toluene (0.30 mL) at 80 °C under Ar for 48 h unless otherwise stated.
Isolated yield based on 5.
Reaction time, 72 h.
Figure 1.
Indolines from other bridged olefins
Scheme 4.
Synthetic transformations of indolines 7b and 4a.
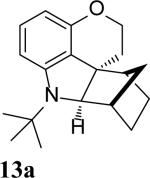
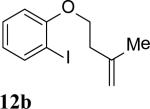
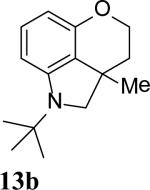
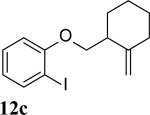
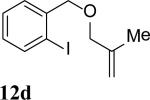
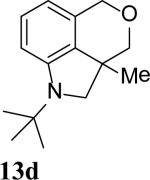
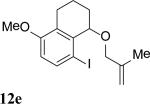
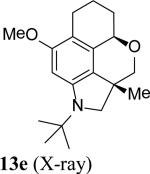
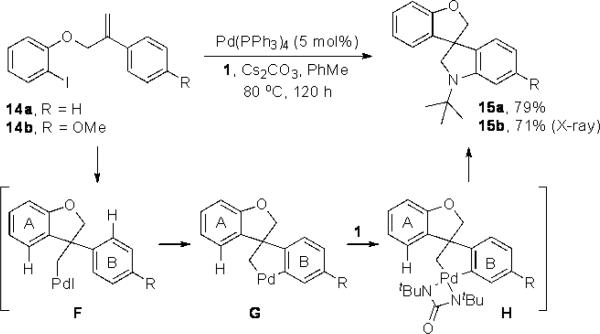
The intramolecular reaction process was also found to be feasible with alkene-tethered iodobenzenes.[16] As shown in Table 3, polycyclic fused indolines 13a–e[13] were readily obtained in 62– 93% yield (Table 3, entries 1–5). Iodobenzenes bearing non-bridged 1,1-disubstituted olefins were also effective substrates for the reaction (Table 3, entries 2–5). When o-iodophenyl allyl ethers 14 with phenyl substituents on the olefins were subjected to the reaction conditions, polycyclic spiro indolines 15 were obtained in 71–79% yield (Scheme 5). In these cases, the C–H activation occurred selectively at phenyl group B rather than phenyl group A to reduce the ring strain. Pentacyclic indoline 17 was obtained in 90% yield when iodobenzene 16 containing an exocyclic olefin was used as substrate (Scheme 6).[17]
Table 3.
Intramolecular process.[a]
All reactions were carried out with iodobenzene 12 (0.30 mmol), di-tert-butyldiaziridine (1) (0.35 mmol), Pd(PPh3)4 (0.015 mmol), Cs2CO3 (0.60 mmol), and toluene (0.30 mL) at 80 °C under Ar for 72 h unless otherwise stated.
Isolated yield based on compound 12.
0.030 mmol of Pd(PPh3)4 was used.
Reaction time, 120 h.
Scheme 5.
Synthesis of polycyclic spiro indoline 15.
Scheme 6.
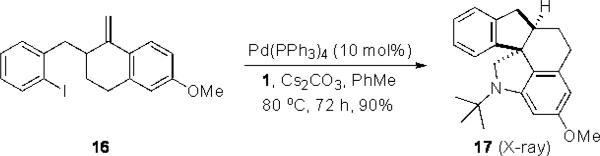
Synthesis of pentacyclic indoline 17.
In summary, we have developed a novel Pd(0)-catalyzed sequential Heck reaction/C–H activation/amination process with iodobenzenes, olefins, and di-tert-butyldiaziridinone (1), providing a variety of polycyclic indolines in good yields. The reaction process likely proceeds via a pallada(II)cycle, which is intercepted by the diaziridinone via oxidative addition (Scheme 3). The resulting pallada(IV)cycle is transformed to the indoline product after release of tert-butyl isocyanate and subsequent reductive elimination. The current work not only provides a new approach to indolines, which are contained in various biologically important molecules, but also further illustrates the versatile reactivity and synthetic utility of diaziridinone, which may open up new opportunity for development of other reaction processes.
Experimental Section
Representative procedure for intermolecular process (Table 2, entry 1): To a 1.5 mL vial equipped with a magnetic stir bar were added iodobenzene (5a) (0.0612 g, 0.30 mmol), norbornene (6a) (0.0564 g, 0.60 mmol), di-tert-butyldiaziridinone (1) (0.0595 g, 0.35 mmol), Pd(PPh3)4 (0.0173 g, 0.015 mmol), Cs2CO3 (0.1956 g, 0.60 mmol), and toluene (0.30 mL, distilled from sodium). The vial was flushed with argon for 20 s, sealed, and then immersed into an oil bath (80oC). The reaction mixture was vigorously stirred at 80 oC for 48 h, cooled to room temperature, and purified by flash chromatography on silica gel (hexanes: ethyl acetate = 100:1) to give indoline 4a (0.065 g, 90%).
Supplementary Material
Footnotes
We are grateful for generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-07).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.For selected examples, see: Bermudez J, Dabbs S, Joiner KA, King FD. J. Med. Chem. 1990;33:1929. doi: 10.1021/jm00169a017. Ontoria JM, Marco SD, Conte I, Francesco MED, Gardelli C, Koch U, Matassa VG, Poma M, Steinkühler C, Volpari C, Harper S. J. Med. Chem. 2004;47:6443. doi: 10.1021/jm049435d. Abdel-Rahman AH, Keshk EM, Hanna MA, El-Bady Sh. M. Bioorg. Med. Chem. 2004;12:2483. doi: 10.1016/j.bmc.2003.10.063. Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JEP, Dawson CE, Dourish CT, Duncton MAJ, Gaur S, George AR, Giles PR, Hamlyn RJ, Kennett GA, Knight AR, Malcolm CS, Mansell HL, Misra A, Monck NJT, Pratt RM, Quirk K, Roffey JRA, Vickers SP, Cliffe IA. Bioorg. Med. Chem. Lett. 2004;14:2367. doi: 10.1016/j.bmcl.2003.05.001. Noguchi T, Tanaka N, Nishimata T, Goto R, Hayakawa M, Sugidachi A, Ogawa T, Asai F, Ozeki T, Fujimoto K. Chem. Pharm. Bull. 2007;55:393. doi: 10.1248/cpb.55.393. Rode MA, Rindhe SS, Karale BK. J. Serb. Chem. Soc. 2009;74:1377. Poondra RR, Kumar NN, Bijian K, Prakesch M, Campagna-Slater V, Reayi A, Reddy PT, Choudhry A, Barnes ML, Leek DM, Daroszewska M, Lougheed C, Xu B, Schapira M, Alaoui-Jamali MA, Arya P. J. Comb. Chem. 2009;11:303. doi: 10.1021/cc8001525. Annedi SC, Ramnauth J, Maddaford SP, Renton P, Rakhit S, Mladenova G, Dove P, Silverman S, Andrews JS, Felice MD, Porreca F. J. Med. Chem. 2012;55:943. doi: 10.1021/jm201564u.
- 2.For leading reviews on the synthesis of indolines, see: Anas S, Kagan HB. Tetrahedron: Asymmetry. 2009;20:2193. Liu D, Zhao G, Xiang L. Eur. J. Org. Chem. 2010:3975. Zhang D, Song H, Qin Y. Acc. Chem. Res. 2011;44:447. doi: 10.1021/ar200004w.
- 3.For leading reviews on C–N bond formation, see: Hartwig JF. Angew. Chem. Int. Ed. 1998;37:2046. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. Wolfe JP, Wagaw S, Marcoux J-F, Buchwald SL. Acc. Chem. Res. 1998;31:805. Yang BH, Buchwald SL. J. Organomet. Chem. 1999;576:125. Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. Evano G, Blanchard N, Toumi M. Chem. Rev. 2008;108:3054. doi: 10.1021/cr8002505. Chemler SR. J. Organomet. Chem. 2011;696:150. doi: 10.1016/j.jorganchem.2010.08.041.
- 4.For recent leading reviews on C–H amination, see: Zalatan DN, Du Bois J. Top. Curr. Chem. 2010;292:347. doi: 10.1007/128_2009_19. Driver TG. Org. Biomol. Chem. 2010;8:3831. doi: 10.1039/c005219c. Cho SH, Kim JY, Kwak J, Chang S. Chem. Soc. Rev. 2011;40:5068. doi: 10.1039/c1cs15082k. Ramirez TA, Zhao B, Shi Y. Chem. Soc. Rev. 2012;41:931. doi: 10.1039/c1cs15104e. Gephart RT, III, Warren TH. Organometallics. 2012;31:7728. Roizen JL, Harvey ME, Bois J. Du. Acc. Chem. Res. 2012;45:911. doi: 10.1021/ar200318q. Zhang L, Deng L. Chin. Sci. Bull. 2012;57:2352. Jeffrey JL, Sarpong R. Chem. Sci. 2013;4:4092.
- 5.For leading references on synthesis of indolines via sp2 C–H activation, see: Houlden CE, Bailey CD, Ford JG, Gagné MR, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2008;130:10066. doi: 10.1021/ja803397y. Li J-J, Mei T-S, Yu J-Q. Angew. Chem. Int. Ed. 2008;47:6452. doi: 10.1002/anie.200802187. Wasa M, Yu J-Q. J. Am. Chem. Soc. 2008;130:14058. doi: 10.1021/ja807129e. Mei T-S, Wang X, Yu J-Q. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b. He G, Zhao Y, Zhang S, Lu C, Chen G. J. Am. Chem. Soc. 2012;134:3. doi: 10.1021/ja210660g. He G, Lu C, Zhao Y, Nack WA, Chen G. Org. Lett. 2012;14:2944. doi: 10.1021/ol301352v. Nadres ET, Daugulis O. J. Am. Chem. Soc. 2012;134:7. doi: 10.1021/ja210959p. Mei T-S, Leow D, Xiao H, Laforteza BN, Yu J-Q. Org. Lett. 2013;15:3058. doi: 10.1021/ol401246u.
- 6.For leading references on synthesis of indolines via sp3 C–H activation, see: Larock RC, Hightower TR, Hasvold LA, Peterson KP. J. Org. Chem. 1996;61:3584. doi: 10.1021/jo952088i. Watanabe T, Oishi S, Fujii N, Ohno H. Org. Lett. 2008;10:1759. doi: 10.1021/ol800425z. Neumann JJ, Rakshit S, Dröge T, Glorius F. Angew. Chem. Int. Ed. 2009;48:6892. doi: 10.1002/anie.200903035. Sun K, Sachwani R, Richert KJ, Driver TG. Org. Lett. 2009;11:3598. doi: 10.1021/ol901317j. Rousseaux S, Davi M, Sofack-Kreutzer J, Pierre C, Kefalidis CE, Clot E, Fagnou K, Baudoin O. J. Am. Chem. Soc. 2010;132:10706. doi: 10.1021/ja1048847. Nakanishi M, Katayev D, Besnard C, Kündig EP. Angew. Chem. Int. Ed. 2011;50:7438. doi: 10.1002/anie.201102639. Nguyen Q, Sun K, Driver TG. J. Am. Chem. Soc. 2012;134:7262. doi: 10.1021/ja301519q. Saget T, Lemouzy SJ, Cramer N. Angew. Chem. Int. Ed. 2012;51:2238. doi: 10.1002/anie.201108511.
- 7.Ramirez TA, Wang Q, Zhu Y, Zheng H, Peng X, Cornwall RG, Shi Y. Org. Lett. 2013;15:4210. doi: 10.1021/ol401935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For leading reviews on palladacycles derived from C–H activation, see: Dyker G. Angew. Chem. Int. Ed. 1999;38:1698. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6. Kakiuchi F, Chatani N. Adv. Synth. Catal. 2003;345:1077. M. Catellani. Synlett. 2003:298. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Catellani M, Motti E, Della Ca’ N. Acc. Chem. Res. 2008;41:1512. doi: 10.1021/ar800040u. Muñiz K. Angew. Chem. Int. Ed. 2009;48:9412. doi: 10.1002/anie.200903671. Ackermann L, Vicente R, Kapdi AR. Angew. Chem. Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. doi: 10.1002/chem.200902374. Xu L-M, Li B-J, Yang Z, Shi Z-J. Chem. Soc. Rev. 2010;39:712. doi: 10.1039/b809912j. Sehnal P, Taylor RJK, Fairlamb IJS. Chem. Rev. 2010;110:824. doi: 10.1021/cr9003242. Shi F, Larock RC. Top. Curr. Chem. 2010;292:123. doi: 10.1007/128_2008_46. McMurray L, O'Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885. doi: 10.1039/c1cs15013h. Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. Malinakova HC. Top. Organomet. Chem. 2011;35:85.
- 9.a Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]; b Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d. [DOI] [PubMed] [Google Scholar]; c Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]; d Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Zhao B, Du H, Cui S, Shi Y. J. Am. Chem. Soc. 2010;132:3523. doi: 10.1021/ja909459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Catellani M, Ferioli L. Synthesis. 1996:769. [Google Scholar]; b Catellani M, Frignani F, Rangoni A. Angew. Chem. Int. Ed. Engl. 1997;36:119. [Google Scholar]
- 11.Chai DI, Thansandote P, Lautens M. Chem. Eur. J. 2011;17:8175. doi: 10.1002/chem.201100210. [DOI] [PubMed] [Google Scholar]
- 12.For leading references on indoline formation from a nickel(II)cycle and an azide, see: Koo K, Hillhouse GL. Organometallics. 1995;14:4421. Koo K, Hillhouse GL. Organometallics. 1996;15:2669.
- 13.The stereochemistry of compounds 8 and 13a were tentatively assigned by analogy to 7 and 4.
- 14.No reactions were observed when styrene and α-methylstyrene were used. It appears that the reaction might be facilitated by the strain of bicyclic olefins.
- 15.Thansandote P, Hulcoop DG, Langer M, Lautens M. J. Org. Chem. 2009;74:1673. doi: 10.1021/jo802604g. [DOI] [PubMed] [Google Scholar]
- 16.For leading references on tandem intramolecular Heck reaction/C–H activation processes, see: Huang Q, Fazio A, Dai G, Campo MA, Larock RC. J. Am. Chem. Soc. 2004;126:7460. doi: 10.1021/ja047980y. Ruck RT, Huffman MA, Kim MM, Shevlin M, Kandur WV, Davies IW. Angew. Chem. Int. Ed. 2008;47:4711. doi: 10.1002/anie.200800549. Lu Z, Hu C, Guo J, Li J, Cui Y, Jia Y. Org. Lett. 2010;12:480. doi: 10.1021/ol902672a.
- 17.Bridged and 1,1-disubstituted olefins were used to avoid the β-hydride elimination.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.