Abstract
State dependent learning effects have been widely studied in a variety of drugs of abuse. However, they have yet to be studied in relation to sexual motivation. The current study investigated state-dependent learning effects of cocaine in male Japanese quail (Coturnix japonica) using a sexual conditioning paradigm. Cocaine-induced state-dependent learning effects were investigated using a 2 × 2 factorial design with training state as one factor and test state as the other factor. During a 14-day training phase, male quail were injected once daily with 10 mg/kg cocaine or saline and then placed in a test chamber after 15 min. In the test chamber, sexual conditioning trials consisted of presentation of a light conditioned stimulus (CS) followed by sexual reinforcement. During the state dependent test, half of the birds received a shift in drug state from training to testing (Coc → Sal or Sal → Coc) while the other half remained in the same drug state (Coc → Coc or Sal → Sal). Results showed that male quail that were trained and tested in the same state (Coc → Coc or Sal → Sal) showed greater sexual conditioning than male quail that were trained and tested in different states (Sal → Coc) except when cocaine was administered chronically prior to the test (Coc → Sal). For the latter condition, sexual conditioning persisted from cocaine training to the saline test. The findings suggest that state dependent effects may alter sexual motivation and that repeated exposure to cocaine during sexual activity may increase sexual motivation which may, in turn, may lead to high risk sexual activities. An alternative explanation for the findings is also discussed.
Keywords: cocaine, Pavlovian conditioning, state-dependent learning, state-dependent memory, sexual behavior, Japanese quail
1. Introduction
State-dependent learning occurs when learned information is better recalled when an organism is in the same state of consciousness as when the memory was formed [1]. In a classic experiment by Goodwin and colleagues [2], male volunteers were asked to perform various memory tasks while under the influence of alcohol or while sober. The following day, they were retested under similar or opposite conditions. The results indicated that subjects who learned the material while intoxicated had difficulty recalling the task in a sober state. Conversely, subjects who were tested and retested under the influence performed as well as subjects who were tested and retested under sober conditions [2]. Thus, in accordance with state-dependent learning, memories appear to be best recalled in the same physiological state in which they were encoded.
State-dependent learning has been observed for several addictive drugs including ethanol [2, 3, 4, 5], pentobarbital [2], morphine [6, 7, 8, 9] methylphenidate [10], and amphetamine [11]. Romieu and colleagues [12] used a modified passive avoidance task and found that relatively low doses (0.1 and 0.3 mg/kg) of cocaine induced state-dependent learning in mice. Low doses were used because they did not affect acquisition or consolidation of the memory. In addition, it was found that the cocaine-induced state-dependent effect could be altered by GABA and opioid receptor modulation [12].
Several studies have shown that repeated pre-exposure to addictive drugs may facilitate sex seeking behavior [13, 14, 15, 16]. Levens and Akins [17] conducted an experiment to determine whether repeated preexposure to cocaine would increase sexual conditioning in male Japanese quail. Chronic cocaine administration (10 mg/kg, once a day for 6 days) resulted in increased locomotor activity compared to saline. After a 10 day withdrawal period, male quail received sexual conditioning trials that consisted of the presentation of an arbitrary conditioned stimulus (CS) followed by sexual reinforcement. Males that received repeated administration of cocaine demonstrated more conditioned approach behavior to the CS compared to males that received repeated administration of saline. The findings and others like it [e.g., 14, 15] suggest that cocaine and similar psychostimulants may facilitate sexual conditioning and thereby contribute to a drug-facilitated increase in sexual activity.
The current research used a well-studied Pavlovian sexual conditioning paradigm to test for state-dependent effects of cocaine in male Japanese quail. In the Pavlovian sexual conditioning paradigm, male quail are presented with a light CS and this is followed by a mating opportunity with a receptive female quail [18]. Males learn that the CS predicts a mating opportunity and as such, they approach and remain near the CS. This sexually conditioned approach behavior is viewed as an index of sexual motivation. As described above, pre-exposure to chronic cocaine has been shown to enhance this sexually conditioned approach behavior [17, 19]. In addition, Troisi & Akins [20] found that cocaine functioned as an interoceptive discriminative cue that predicted an association between a CS and a mating opportunity. Thus, cocaine may not only facilitate sexual conditioning but it may also function as a discriminative cue or occasion setter.
The purpose of the current experiment was to test state-dependent effects of cocaine using a 2 × 2 factorial design with training state (drug or no drug) as one factor and test state (drug or no drug) as the other factor [see 21, 22, and 23 for reviews]. Two groups will have a shift in drug state from training to testing while two groups will remain in the same state during training and testing. A state-dependent account would predict that sexually conditioned approach behavior would be attenuated in groups that receive a shift in drug state relative to those that do not receive a shift [21, 22, 23].
2. Materials and Methods
2.1. Animals
Thirty one (n = 31) experimentally and sexually naïve male quail approximately 6 months old served in the experiment. An equal number of female quail were used as copulation partners. Eggs were supplied by Northwest Gamebirds (Kennewick, WA) and quail chicks were hatched and then raised in mixed-sex groups until approximately 28 days of age. At 28 days of age, male quail were housed individually and female quail were group housed in wire mesh cages (supplied by GQF Manufacturing, Savannah, GA). Female quail were housed individually when selected for the experiment. Subjects were raised in long-daylight (16L: 8D) conditions to maintain reproductive readiness [24] with food and water available ad libitum. All subjects were drug naïve prior to the experiment.
Male quail were selected as subjects on the basis of a pretest for copulation. A female quail was placed in the home cage of each male for 5 min. Only males that successfully copulated within 5 minutes were used in the experiment. Male quail are not likely to copulate with a female bird if they have not done so within 5 minutes [25].
Female quail were assigned to male quail on a rotation basis across acquisition trials so that male quail were never paired with the same female quail more than once. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted under the guidelines of the Division of Laboratory Animal Research at the University of Kentucky.
2.2. Apparatus
Six large Plexiglas test chambers (91.4 cm wide × 61.0 cm deep × 30.5 cm tall) were used during the training phase. The side walls of the chambers were covered with white paper and the floors of the chambers were covered with white corrugated paper. A smaller Plexiglas side cage (30.5 cm wide × 61.0 cm deep × 27.9 cm tall) was attached to one end of each test chamber and was used to house a female bird during training. A door connecting the test chamber and the side cage allowed male quail to access the female's side cage. The door was covered with white paper so that males and females could not see each other unless the door was opened. A 25w red light (15.25 cm above the floor) was used as the conditioned stimulus (CS). The CS zone was marked on the corrugated floor (36.6 cm × 36.6 cm) in front of the females test cage door.
2.3. Drugs
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline (0.9% NaCl) at a dose of 10 mg/kg and injected intraperitoneally (i.p.). Drug was injected 15 min prior to testing.
2.4. Procedure
2.4.1. Training
The training phase consisted of fourteen trials, one per day, with each subject receiving the same treatment throughout training. During each training trial, male quail were injected with 10mg/kg of cocaine (n = 16) or saline (n = 15) intraperitoneally (i.p.). Males were then placed back in their home cage for 15 min. After the 15 min, they were transported to the test room and placed in the test chamber. Quail received a 30 sec pre-CS period, after which the CS light was illuminated for 30 sec and was then turned off. Subsequently, the door to the female's side cage was raised and males were allowed to enter the side cage for a copulatory opportunity with a female quail for 5 min. The amount of time (sec) male quail spent in the CS zone during the CS light presentation was recorded. Approach behavior (time in CS zone) was measured after both feet crossed into the CS zone.
2.4.2. Withdrawal period
The training phase was followed by a 10-day withdrawal period. During this phase, quail remained in their home cage and did not receive drug or saline.
2.4.3. State-dependent Test
Following a 10-day withdrawal period, quail were given a state-dependent test. During the test, half of the male quail that received repeated cocaine administration during the training phase were injected with saline and half of the males that received repeated administration of saline during training were injected with cocaine. The other half of the male quail that received repeated cocaine administration during the training phase were injected with cocaine and the other half of the males that received repeated administration of saline during training were injected with saline. Therefore, half of the birds received a shift in drug state from training to testing (Coc → Sal or Sal → Coc) while the other half remained in the same drug state from training and testing (Coc → Coc or Sal → Sal). The state-dependent test consisted of one test and was conducted on a single day.
2.4.4. Data analyses
Training data were analyzed with a two-way (training state × trials) repeated measures analysis of variance (ANOVA). Data for the state-dependent test were analyzed with a between-subjects ANOVA (training state × test state). Pair-wise comparisons were conducted with Fisher's LSD test where appropriate. For all analyses, 0.05 was chosen as the significance level.
3. Results and Discussion
Figure 1A shows the amount of time (sec) spent near the CS that predicted a female across training trials for males that received either cocaine or saline. An ANOVA revealed a significant main effect of treatment [F (1, 29) = 52.07] and a significant treatment × trials interaction [F (13, 377) = 4.32]. Male quail that received cocaine spent significantly more time near the CS that predicted a female, overall and across training trials, compared to males that received saline. There was also a significant main effect of trials [F (13, 377) = 130.33] suggesting that both cocaine and saline groups showed acquisition of learning across training trials.
Figure 1.
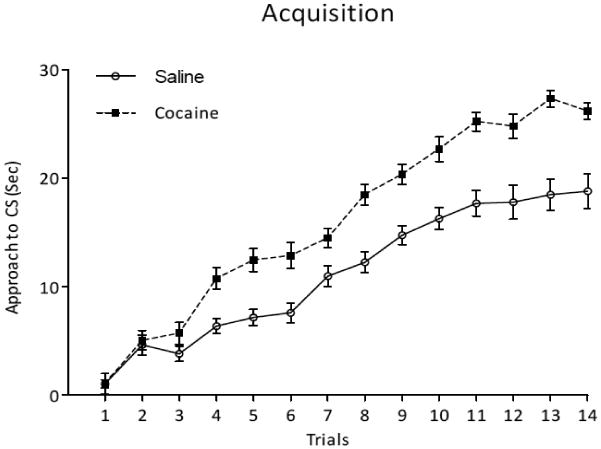
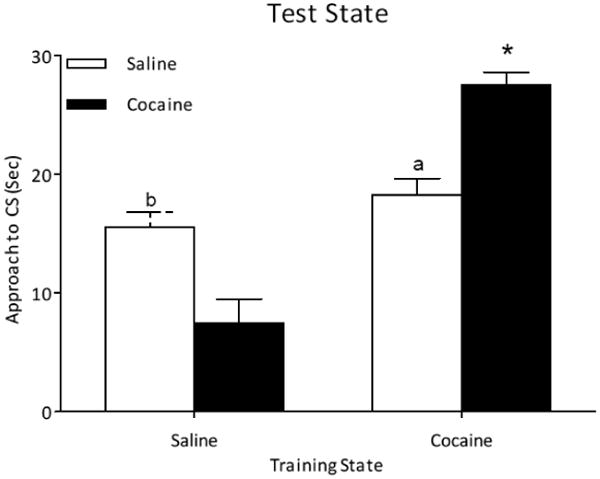
A. Time spent near the CS (sec) for male quail that received chronic cocaine or saline across training trials. B. Time spent near the CS (sec) during the state dependent test in which half of the subjects received a switch in treatment from training to test (Coc →Sal or Sal → Coc) and the other half were given the same treatment (Coc → Coc or Sal → Sal). “*” indicates Coc → Coc > all groups; “a” indicates Sal → Sal > Sal → Coc; “b” indicates Coc → Sal > Sal → Coc; p < 0.05
Figure 1B represents the amount of time (sec) male quail spent near the CS during the state-dependent test either when the state remained the same from training to testing (Coc → Coc or Sal → Sal) or when it was shifted (Coc → Sal or Sal → Coc). The ANOVA revealed a significant training state × test state interaction [F (1, 27) = 27.22]. Subsequent analyses indicated that the Coc → Coc group had greater conditioned approach than the other groups; that the Coc → Sal group had greater conditioned approach than the Sal → Coc group; and that the Sal → Sal group had greater conditioned approach than the Sal → Coc group.
The results of the current experiment indicate that male quail that were trained and tested in the same state (Coc → Coc or Sal → Sal) show greater sexual conditioning than male quail that were trained and tested in different states (Sal → Coc) except for the Coc → Sal (discussed below). The present findings indicate that cocaine may induce state-dependent learning. The findings support those of Romeiu and colleagues [12] in which they found that relatively lower doses of cocaine (0.03, 0.1, and 0.3 mg/kg) did not alter acquisition but induced a cocaine state-dependent effect. The current study used a dose of 10 mg/kg cocaine because this dose has been shown to induce behavioral sensitization and conditioned place preference in male Japanese quail [17, 26]. This dose has also been shown to facilitate sexual conditioning and performance [17] and function as a discriminative interoceptive stimulus for sexual reinforcement [20].
In the current experiment, a state-dependent effect was not evident when cocaine was given chronically during training prior to saline during the state dependent test (Coc → Sal). Previous studies indicate that chronic pre-exposure to cocaine facilitates sexual conditioning [17, 19, 20] and that this facilitative effect persists during extinction [unpublished data]. Therefore, it was not surprising that the shift from a cocaine training state to a saline test state (one administration of saline) did not decrease responding.
We have chosen to interpret the current findings as a state dependent effect. However, at least one other explanation is possible. In the current experiment, administration of drug in the saline-trained birds as well as the removal of drug in the drug-trained birds may have represented a novel state. Birds have a neophobic nature and fear has been shown to reduce exploration and cause behavioral inhibition in domestic fowl [27, 28, 29]. While state-dependent learning may be one explanation for the present findings, it is equally likely that quail perceived the change in drug state as a novel stimulus thereby decreasing exploration in the test chamber or inducing a competing response.
4. Conclusions
The current study is the first to demonstrate cocaine-induced state-dependent learning in a sexual behavior paradigm. The findings contribute to our understanding of how interoceptive drug cues may function to enhance learning and motivation and ultimately lead to high risk sexual activities. In humans, repeated cocaine use has been associated with high risk sexual activities, including sex with multiple partners and unprotected sex [30, 31, 32]. From a state-dependent learning viewpoint, memories are best recalled in the same physiological state in which they were encoded [33]. The current findings suggest that repeated exposure to cocaine during sexual activity may later increase sexual motivation and this may in turn, lead to high risk sexual behavior.
Highlights.
State-dependent learning effects of cocaine in a sexual conditioning paradigm
State dependent effects may alter sexual motivation
State dependent effects of cocaine may contribute to risky sex
Acknowledgments
Financial support for this research was provided by NIDA grant #DA022451 awarded to CKA. The authors would like to thank Luke Cornett and B. Levi Bolin for their help with data collection, animal care, and technical support. We would also like to thank reviewers for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overton DA. State-dependent learning produced by depressant and atropine-like drugs. Psychopharmacologia. 1966;10(1):6–31. doi: 10.1007/BF00401896. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: state-dependent effects in man. Science. 1969;163(3873):1358–1360. doi: 10.1126/science.163.3873.1358. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow NR, van der Kooy D, Koob GF, Wenger JR. Cholecystokinin produces conditioned place-aversions, not place-preferences, in food-deprived rats: evidence against involvement in satiety. Life Sci. 1983;32:2087–2093. doi: 10.1016/0024-3205(83)90096-6. [DOI] [PubMed] [Google Scholar]
- 4.Colpaert FC, Koek W. Empirical evidence that the state dependence and drug discrimination paradigms can generate different outcomes. Psychopharmacology. 1995;12:272–279. doi: 10.1007/BF02311174. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa Y, Iwasaki T. Ethanol-induced state-dependent learning is mediated by 5-hydroxytryptamine-3 receptors but not by N-methyl-D-aspartate receptor complex. Brain Res. 1996;706:227–232. doi: 10.1016/0006-8993(95)01040-8. [DOI] [PubMed] [Google Scholar]
- 6.Slot LB, Colpaert FC. Opiate states of memory: receptor mechanisms. The Journal of neuroscience. 1999;19(23):10520–10529. doi: 10.1523/JNEUROSCI.19-23-10520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slot LA, Colpaert FC. Recall rendered dependent on an opiate state. Behavioral neuroscience. 1999;113(2):337. doi: 10.1037//0735-7044.113.2.337. [DOI] [PubMed] [Google Scholar]
- 8.Slot BL, Koek W, Colpaert FC. Ethanol state dependence involving a lever press response requirement in rats. Behavioural pharmacology. 1999;10(2):229–233. doi: 10.1097/00008877-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Zarrindast MR, Rezayof A. Morphine state-dependent learning: sensitization and interactions with dopamine receptors. Eur J Pharmacol. 2004;497(2):197–204. doi: 10.1016/j.ejphar.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 10.Swanson JM, Kinsbourne M. Stimulant-related state-dependent learning in hyperactive children. Science. 1976;192(4246):1354–1357. doi: 10.1126/science.1273596. [DOI] [PubMed] [Google Scholar]
- 11.Sanday L, Zanin KA, Patti CL, Fernandes-Santos L, Oliveira LC, Longo BM, Frussa-Filho R. Role of state-dependent learning in the cognitive effects of caffeine in mice. Int J Neuropsychopharmacol. 2013;16(7):1547–1557. doi: 10.1017/S1461145712001551. [DOI] [PubMed] [Google Scholar]
- 12.Romieu P, Lucas M, Maurice T. Sigma1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology. 2005;31(7):1431–43. doi: 10.1038/sj.npp.1300885. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat in the presence of stimuli previously paired with systemic injections of morphine. Pharmacology Biochemistry and Behavior. 1990;35:367–372. doi: 10.1016/0091-3057(90)90171-d. [DOI] [PubMed] [Google Scholar]
- 14.Fiorino DF, Phillips AG. Facilitation of Sexual Behavior and Enhanced Dopamine Efflux in the Nucleus Accumbens of Male Rats after D-Amphetamine-Induced Behavioral Sensitization. J Neuroscience. 1999a;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology. 1999b;142:200–208. doi: 10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- 16.Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behavioural Brain Research. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 17.Levens N, Akins CK. Chronic cocaine pretreatment facilitates Pavlovian sexual conditioning in male Japanese quail. Pharmacol Biochem Behav. 2004;79(3):451–457. doi: 10.1016/j.pbb.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Domjan M, Lyons R, North NC, Bruell J. Sexual Pavlovian conditioned approach behavior in male Japanese quail (Coturnix japonica) J Comp Psychol. 1986;100(4):413–421. [PubMed] [Google Scholar]
- 19.Akins CK, Bolin BL, Gill KE, Reinhardt EK. Unpublished manuscript. University of Kentucky; Lexington, KY: Cocaine pre-exposure enhances sexual conditioning and increases resistance to extinction in male Japanese quail. [Google Scholar]
- 20.Troisi JR, 2nd, Akins C. The discriminative stimulus effects of cocaine in a pavlovian sexual approach paradigm in male Japanese quail. Exp Clin Psychopharmacol. 2004;12(4):237–242. doi: 10.1037/1064-1297.12.4.237. [DOI] [PubMed] [Google Scholar]
- 21.Spear NE, Riccio DC. Memory: Phenomena and principles. Allyn & Bacon; 1994. [Google Scholar]
- 22.Colpaert FC. A method for quantifying state-dependency with chlordiazepoxide in rats. Psychopharmacology (Berl) 1986;90:144–146. doi: 10.1007/BF00172887. [DOI] [PubMed] [Google Scholar]
- 23.Koek W. Drug induced state-dependent learning: review of an operant procedure in rats. Behavioural Pharmacology. 2011;22:430–440. doi: 10.1097/FBP.0b013e328348ed3b. [DOI] [PubMed] [Google Scholar]
- 24.Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21(3):261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 25.Schein A, Berdahl BJ, Low M, Borek E. Deficiency of the DNA of Micrococcus radiodurans in methyladenine and methylcytosine. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis. 1972;272(3):481–485. doi: 10.1016/0005-2787(72)90400-5. [DOI] [PubMed] [Google Scholar]
- 26.Geary EH, Akins CK. Cocaine sensitization in male quail: temporal, conditioning, and dose-dependent characteristics. Physiol Behav. 2007;90(5):818–824. doi: 10.1016/j.physbeh.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan JA. An experimental study of conflict and fear: an analysis of behavior of young chicks toward a mealworm. I. The behavior of chicks which do not eat the mealworm. Behavior. 1965;25(1):45–97. doi: 10.1163/156853965x00110. [DOI] [PubMed] [Google Scholar]
- 28.Jones RB. Open-field responses of domestic chicks in the presence or absence of familiar cues. Behav Processes. 1977;2(4):315–323. doi: 10.1016/0376-6357(77)90002-X. [DOI] [PubMed] [Google Scholar]
- 29.Jones RB. Repeated exposure of the domestic chick to a novel environment: effects on behavioural responses. Behav Processes. 1977;21:163–173. doi: 10.1016/0376-6357(77)90018-3. [DOI] [PubMed] [Google Scholar]
- 30.Kolar AF, Brown BS, Weddington WW, Ball JC. A treatment crisis: cocaine use by clients in methadone maintenance programs. Journal of substance abuse treatment. 1990;7(2):101–107. doi: 10.1016/0740-5472(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 31.Condelli WS, Fairbank JA, Dennis ML, Rachal J. Cocaine use by clients in methadone programs: significance, scope, and behavioral interventions. Journal of Substance Abuse Treatment. 1991;8(4):203–212. doi: 10.1016/0740-5472(91)90040-h. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman JA, Klein H, Eber M, Crosby H. Frequency and intensity of crack use as predictors of women's involvement in HIV-related sexual risk behaviors. Drug and alcohol dependence. 2000;58(3):227–236. doi: 10.1016/s0376-8716(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 33.Overton DA. Historical context of state dependent learning and discriminative drug effects. Behavioural pharmacology. 1991;2(4-5):253–264. [PubMed] [Google Scholar]


