Abstract
The effect of serial in vitro subculturing on three pathogenic strains of Saprolegnia parasitica was investigated. The isolates were passed through Atlantic salmon, Salmo salar L. parr, and then re-isolated as single spore colonies. All strains caused infection. The isolate obtained from diseased fish served as a virulent reference culture and was designated ‘AP’ (‘activated through passage’). Successive subculturing was made by obtaining an inoculum from AP to produce the 2nd subculture and then passaged to the 3rd subculture (from the 2nd), until the 15th passage was obtained. Spores used to produce storage cultures were collected at passages 5, 10 and 15. The different passages of each strain were used to artificially infect Atlantic salmon parr. Morphological characterization of growth patterns was performed to observe differences occurring due to serial in vitro subculturing. Two of the strains declined in virulence after 15 successive in vitro subcultures, whereas one did not. This study is the first to investigate attenuation of virulence in Saprolegnia and whether or not isolates of S. parasitica should be passed through the fish host prior to challenge experiments. It reveals that some strains degenerate more rapidly than others when subjected to successive in vitro subculturing on glucose–yeast extract.
Keywords: attenuation, in vitro passaging, Saprolegnia
Introduction
Saprolegnia parasitica (Saprolegniaceae, Oomycota) is the causative agent of saprolegniasis, a well-known and increasing problem in aquaculture of salmonids and other fish species worldwide (Beakes, Wood & Burr 1994; Van West 2006). Scientific efforts related to S. parasitica and other Saprolegnia spp. have increased after malachite green, an efficient remedy, was banned from using in aquaculture in the European Union in 2000 (Sudova et al. 2007) because the general public may become exposed to malachite green through the consumption of treated fish. In the United States, the use of malachite green is not approved by the Food and Drug Administration (Hernando et al. 2006). Emphasis has been put on finding new ways to control the disease. In particular, a number of fungicides have been tested (Barnes, Sayler & Cordes 2001; Kashiwagi et al. 2002, 2003). Efficacy testing of drugs and control regimes rely upon virulent strains of the infective agent being available for testing, particularly when the focus is on in vivo experiments and infection rates. Loss of virulence, a well-known phenomenon observed in fungi and bacteria maintained in media and viruses grown in cell culture (Druelle et al. 2008; Almaguer-Chávez et al. 2011; Ansari & Butt 2011), can be a confounding factor in research studies. Various terms have been used to describe this phenomenon, including phenotypic degeneration, degeneration, phenotypic instability, phenotypic deterioration, dual phenomenon, saltation and attenuation (Kawakami 1960; Nagaichi 1973; Butt 2002; Ibrahim, Butt & Jenkinson 2002). Potential loss of virulence is highly relevant to the maintenance of strains in general and in the production of strains for comparative bioassays (Brownbridge, Costa & Jaronski 2001). Although attenuation causes problems in various situations, from production of biocontrol agents to testing of vaccine or drug efficacy, it has hitherto not been reported in oomycetes, even those of high economic importance such as Pythium, Phytophthora and S. parasitica. Therefore, this study is the first known to document the stability and/or instability of S. parasitica strains in continuous in vitro cultivation. The aim of this study was to explore whether in vitro subculturing impacts on the virulence of different strains of S. parasitica, assayed by infection rates in challenge experiments in Atlantic salmon.
Materials and methods
Saprolegnia strains and culture procedures
Three virulent strains of S. parasitica were included in this study, all originating from different salmonid species of fish. Table 1 summarizes their identities and sources. All three strains have previously been shown to be highly pathogenic to Atlantic salmon, Salmo salar L. All S. parasitica isolates had been stored in the culture collection of the Norwegian Veterinary Institute using standard culture and storage conditions (Stueland, Hatai & Skaar 2005), but to prevent any bias between strains at the start of in vitro culturing, all isolates were passed through Atlantic salmon (Salmo salar L.) parr and then re-isolated as single spore colonies. This principle is concordant with what has been used for entomopathogenic fungi (Fargues et al. 1997; Vidal, Lacey & Fargues 1997). All isolates VIO 2736, VIO 5337 and VIO 5708 produced >90% infection rate after challenge, and the isolate obtained from diseased fish served as a virulent reference culture and was referred to as the primary isolate and designated ‘AP’ (‘activated through passage’). Successive subculturing was carried out by obtaining an inoculum from AP to produce the 2nd subculture and then passaged to the 3rd subculture (from the 2nd), until the 15th passage was obtained. This was achieved by inducing asexual spore reproduction in Glucose-Yeast (GY) agar and broth as described by Stueland et al. (2005). Spores used to produce storage cultures were collected at passages 5, 10 and 15. The cultures were stored at 4 °C in sterilized aquarium water (SAW) until use and no longer than 1 week for any of the passages. From the cultures obtained at the given intervals, we produced challenge inocula (spore suspensions) according to Stueland et al., for the challenge experiments. Inoculum concentrations were assessed by counting the spores in a Bürker-türk haemocytometer (BT, Brand, Wertheim, Germany), and final exposure doses were adjusted by dilution, directly into the fish tanks.
Table 1.
Saprolegnia parasitica isolates included in the present study. All isolates were originally isolated from salmonids
| Strain | NVI strain collection numbera | GenBank accession number | Host or source of origin | Geographical location |
|---|---|---|---|---|
| A | VIO 2736 | KC994645 | Infected Atlantic salmon (Salmo salar L.) parr | Scotland |
| B | VIO 5337 | KC994646 | Infected rainbow trout (Oncorhynchus mykiss) | Norway |
| C | VIO 5708 | KC994647 | Infected spawned sea trout (Salmo trutta L.) | Norway |
Refers to the number in the strain collection of the Norwegian Veterinary Institute, Oslo.
Fish and experimental conditions
For the challenge experiments, this study used 315 Atlantic salmon parr of AquaGen strain with an average weight of 30 g (at start of the experiment). The fish were acclimatized for 1 week in a 1.0-m fibreglass flow-through tank before we moved them to 10-L challenge tanks at the time of the challenge. Water flow rate of 0.8 L kg min− and temperature of 11 °C ± 1 were maintained throughout the study. The fish were fed a commercial pellet salmon feed (Biomar, 2 mm) during the study. The groups of fish were identified by the strain number of the Saprolegnia strain and then the passage number in upper case, AP, 5P and 15P. Thus, 2736AP represented the primary passage of strain VIO 2736, 53375P the 5th passage of VIO 5337 and so on.
Experimental challenge procedure
Prior to onset, the experiment was approved by the Norwegian Animal Research Authority and conducted in the Wet laboratory at the Norwegian Veterinary Institute/Norwegian School of Veterinary Science, Oslo, Norway.
The fish were stocked in groups of 7 or 8 per tank, in quadruple tanks per challenge inoculum. Two negative, non-challenged control tanks (n = 7 or 8) were also included. The latter groups were subjected to ‘ami-momi’ treatment, but not exposed to zoospores. The challenge was performed as follows. Water flow was turned off in all of the experimental tanks. The fish received ‘ami-momi’ treatment as described by Hatai & Hoshiai (1993) and later slightly modified by Stueland et al. (2005). After ami-momi treatment, they were transferred back to the tanks, followed by the addition of challenge inoculum to obtain an equal spore concentration of 1.0 × 104 spores L−1 for all tanks and isolates. The exposure lasted for 24 h before water flow was resumed, and during the exposure period, the water was aerated to keep an oxygen saturation of 80–90% in all tanks. Water quality parameters were checked every day in each tank.
Assessment of infection
After the challenge, the fish were maintained and monitored daily for signs of clinical infection, including tufts of cotton-wool-like growth on the surface of the fish, focal areas of haemorrhage, necrosis and ulceration, lethargy and loss of equilibrium over a period of 11–14 days. All fish that showed disease signs were promptly removed and killed in benzocaine. Saprolegniasis was confirmed by stereomicroscopic (Olympus) inspection, and the fish were photographed. The number of fish showing gross lesions of saprolegniasis was recorded.
In addition to macroscopic inspection, documentation of Saprolegnia infection was carried out by collecting a sample from the skin/muscle tissues from one infected fish from each tank and passage and testing for the presence of Saprolegnia hyphae (Stueland et al. 2005). In brief, the infected area (as observed macroscopically and by stereomicroscopy) was disinfected with 70% alcohol (cotton wool), and a small piece of skin and superficial muscle tissue were carefully excised. The sample was placed on GY agar supplemented with 200 mg L− chloramphenicol and incubated at 21 ± 1 °C for 2–5 days. Growing mycelia were identified according to Seymour (1970) and Willoughby (1985).
Rechallenge with the passage 15 isolates
We also performed a final challenge experiment, in which we compared infection rates between passage 15 isolates (533715P, 273615P and 570815P), the primary passage isolates (AP) and an isolate obtained from diseased fish infected with the passage 15 isolates. The isolate obtained from these fish were termed ‘5337AP15P’, ‘2736AP15P’ and ‘5708AP15P’.
Preparation of histopathology sections
Skin and muscle samples (<1 cm3), including the leading edge of the lesion and the surrounding tissue, were collected for histopathological examination. Other tissues included samples of gills, liver, heart, spleen and posterior kidney. All samples were immediately fixed in 10% buffered formalin. Histopathology sections were prepared by routine laboratory procedures and stained with haematoxylin and eosin. Special stains periodic acid-Schiff (PAS) and Grocott's modification of Gomori's methenamine silver (GMS) stain were employed to further demonstrate the presence of hyphae.
Testing for growth rate on solid agar
Using a 5-mm cork borer, an agar block colonized by Saprolegnia hyphae from each passage was put in the centre of a 90-mm Petri dish containing GY agar and incubated at 21 ± 1 °C for 72 h, according to Stueland et al. (2005). Radial growth was measured every 24 h. Hyphae would be regarded as showing radial growth (>40 mm) when they reached the edge of the Petri dish. The test was performed in triplicate per generation per strain.
Morphological investigations of Saprolegnia isolates
Two millilitres of encysted zoospore suspension (equivalent to about 5 × 105 zoospores) from each passage under comparison was pipetted under aseptic conditions onto Petri dishes with sterilized aquarium water (SAW) and six sterilized hemp seeds (Ali 2004). This was done in triplicate per passage. Thereafter, the Petri dishes were incubated at 21 °C for 2 weeks. Morphological characteristics of the developing colonies were evaluated by microscopic examination, starting from the second day and subsequently extended daily during the 2-week period of incubation. The cultures were thoroughly examined for abilities to produce oogonia on hemp seed at different generations (AP, 5, 15), zoospore release patterns, percentage of cysts with indirect germination after incubation in 25% GY broth at 21 ± 1 °C for 3 h, percentage of cysts germinating after incubation of Saprolegnia mycelium in SAW at 21 ± 1 °C for 20 h, formation of sexual reproductive structures and chlamydospores. The approximate total number of sporangia and sporangial releases and oogonia and chlamydospore production per colony (hemp seed) were assessed and scored. According to the scores, they were rated as follows: H = high rate, >9 sporangia per hemp seed, >16 oogonia per seed, >10 chlamydospores per seed; M = moderate rate, 4–9 sporangia per seed, 8–16 oogonia per seed, 5–10 chlamydospores per seed; L = low rate, 2–3 sporangia per seed, 4–7 oogonia per seed, 2–4 chlamydospores per seed; R = rarely present, <2 sporangia per seed, <4 oogonia per seed, <2 chlamydospores per seed; NP = not present at all.
Medium for physiological investigations
Glucose–yeast extract with 200 mg L−1 chloramphenicol was used in solidified form for purification and as an inoculum source of S. parasitica. Also, it was used as a liquid broth for physiological investigations into the oomycete at different passages.
Statistical analyses
Statistical analyses were performed by two-way ANOVA (Tukey's HSD) using the statistical package JMP®7.0.1 (SAS Institute Inc., Cary, NC). Fisher's exact test (two-sided) was used to analyse cumulative percentage infection (CPI) between groups at endpoint. A P-value <0.05 was considered to represent significant differences between groups/treatments.
Results
Challenge experiments
All strains and all passages of all strains resulted in an infection over a period of 11–14 days post-challenge. As fish, for ethical reasons and with consideration of humane endpoints, were collected and killed prior to death, an infection was considered as having been established when macroscopic signs of saprolegniasis were present. The infection was confirmed by stereomicroscopic examination and re-isolation from infected fish. We expressed the outcome of challenge as CPI, and the results are summarized in Table 2 and Figs3.
Table 2.
Cumulative infection rate at the end of challenge with strains VIO 5337, 2736 and 5708 and different passage numbers
| Generation | Morbidity (%) | ||
|---|---|---|---|
| Strain | |||
| VIO 5337 | VIO 2736 | VIO 5708 | |
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| AP | 77.2 ± 11.0a | 23.3 ± 16.5a | 67.4 ± 15.0 |
| 5P | 47.3 ± 16.3 | 6.7 ± 0.0 | 46.9 ± 8.5 |
| 15P | 49.6 ± 14.9 | 3.3 ± 0.0 | 79.3 ± 7.4b |
The control was prepared in duplicate and values of the different strain passages represent four replicates.
Denotes significantly different from other passages of the same strain.
Denotes significantly different from the 5th passage of the same strain; PI, Post-infection.
Figure 3.
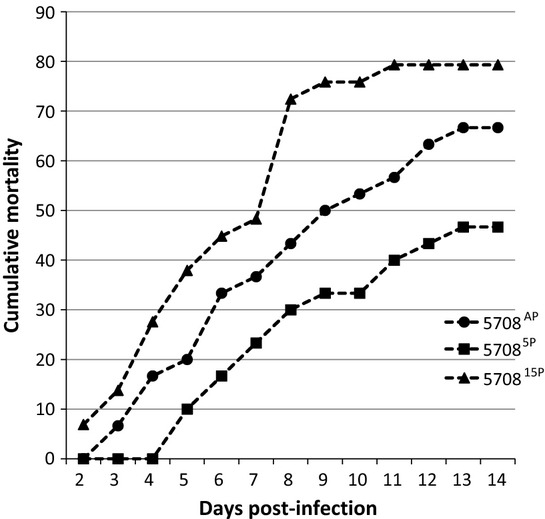
VIO 5708 Percentage of fish showing gross signs of infection over a period of 14 days.
Strain 5337AP produced visible, typical lesions in a few fish within 48 h post-challenge, and the first moribund fish were collected and killed at this time point (48 h post-challenge) (Fig. 1). By day 3, some infected fish were observed in 53375P and 533715P tanks (Fig. 1). The CPI for 5337AP was significantly higher than those in the 53375P and 533715P groups by day 11 (Fig. 1).
Figure 1.
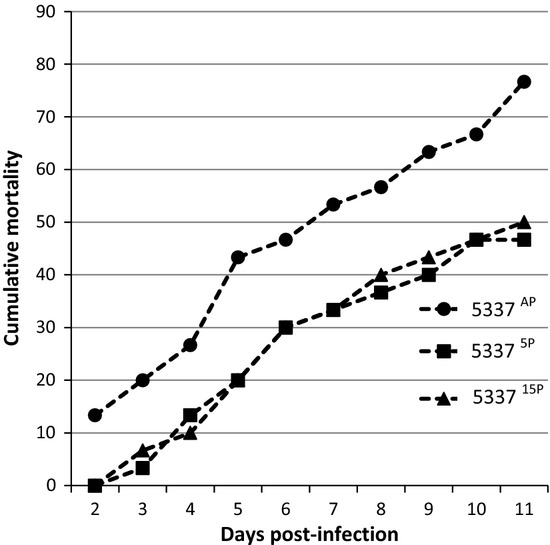
VIO 5337 Percentage of fish showing gross signs of infection over a period of 11 days.
The 2736 strain gave low CPI for all passages tested (Fig. 2), with a CPI of 23.3 by day 14 post-challenge (Fig. 2; Table 2). The first infected fish were observed by day 5 post-infection for the 2736AP-challenged fish (Fig. 2). Passage reduced the virulence of strain 2736, with a CPI at 14 days of 6.7 for 27365P and 3.3 for 273615P. There were significant differences in CPI between AP and 5P/15P challenges (Fig. 2).
Figure 2.
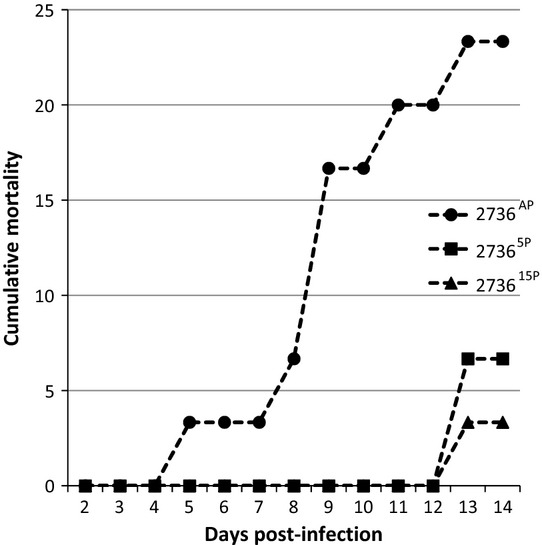
VIO 2736 Percentage of fish showing gross signs of infection over a period of 14 days.
For strain 5708, the infection rate was high, with CPI ending at 67.4, 46.9 and 79.5 for 5708A, 57085P and 570815P, respectively. This shows that passage initially resulted in lower CPI (P = 0.0065), while it increased by 15P. 570815P gave a CPI not significantly different from that of the AP isolate (P = 0.0792) but was significantly more virulent than that of the 5P isolate (P = 0.0001). None of the control groups showed any signs of infection, and there were no fish dying in these tanks (Table 2).
As there was a trend that passage reduces the virulence of the tested strains (5337 and 2736), we were interested in finding out whether the passaged strains (15P) when re-isolated from dead fish would remain with reduced virulence or yield a successful infection. For strain 5337, all three passages (5337AP, 533715P and 5337AP15P) were significantly different from the control at 14 days post-infection. 533715P was also significantly different from the other two. However, there was no difference between 5337AP and 5337AP15P (Fig. 4).
Figure 4.
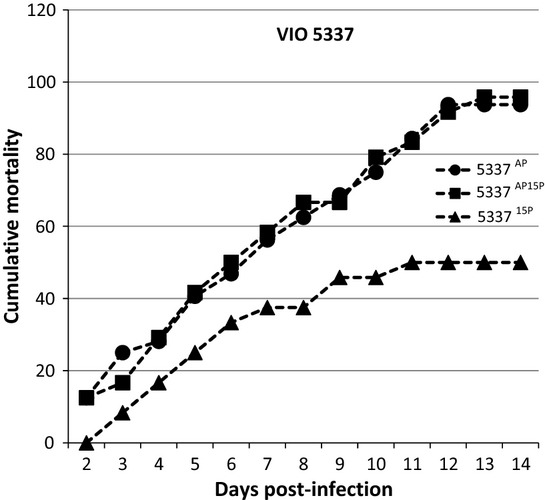
VIO 5337 Percentage of fish showing gross signs of Saprolegniasis over a period of 14 days after rechallenge with the 15th passage isolate.
For strain 2736, all three passages, 2736AP, 273615P and 2736AP15P, were significantly different from the control at 14 days post-infection. 273615P also gave significantly lower CPI compared with the other two passages. There was no difference between 2736AP and 2736AP15P (Fig. 5).
Figure 5.
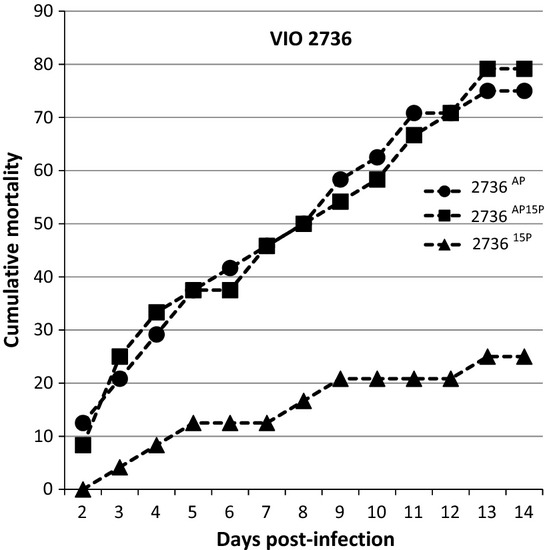
VIO 2736 Percentage of fish showing gross signs of Saprolegniasis over a period of 14 days after rechallenge with the 15th passage isolate.
Strain 5708 was also included despite the fact that we did not observe a loss of virulence after passage. While we found that all passages resulted in significantly higher mortality than the controls 14 days post-infection, again there were no significant differences (statistically) between 5708AP, 570815P and 5708AP15P (Fig. 6).
Figure 6.
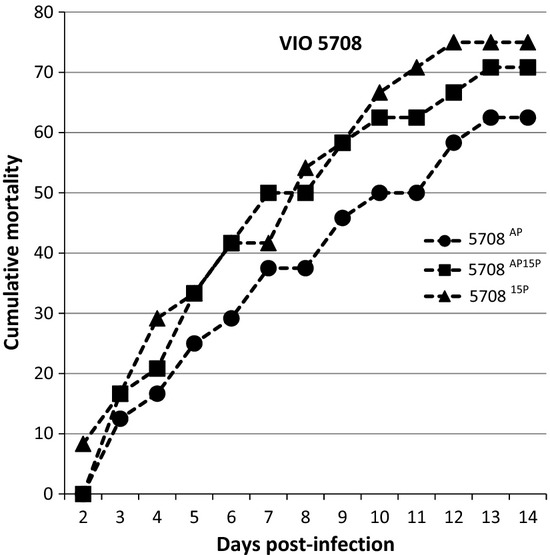
VIO 5708 Percentage of fish showing gross signs of Saprolegniasis over a period of 14 days after rechallenge with the 15th passage isolate.
Gross and histopathology
Infection was confirmed by typical gross changes where signs varied from cotton-wool-like tufts on the fins, head, integument and gills (Fig. 7), to focal areas of haemorrhage, necrosis and ulceration. Clinical signs included lethargy and loss of equilibrium in cases where the fish was seen swimming in circles almost above the water surface.
Figure 7.
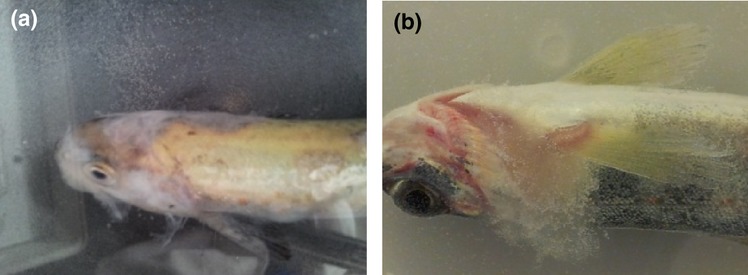
Gross lesions on the infected fish (a) an Atlantic salmon parr that got infected 3 days after being challenged with 5337AP. Note the cotton wool-like mycelial growth around the head. (b) 27365P primarily affecting the gills.
By histopathology, a wide array of findings was evident in tissues infected with S. parasitica strains of different passages. One skin/muscle section from a fish infected with 5337AP showed necrosis of the epidermal layers extending into the dermis. There was very little inflammatory reaction present, and only a few neutrophils and lymphocytes were found in infected areas. Oedema and mild haemorrhage were also observed, with wipeout of the epidermis (Fig. 8a). The kidney interstitium of the same fish (5337AP) exhibited severe karyorrhexis and oedema (Fig. 8b). Mild pathological changes were detected in tissues collected from fish infected with 533715, characterized by moderate congestion of the liver (Fig. 8c) and mild degeneration on secondary lamellae of the gills (Fig. 8d). GMS staining did not show the Saprolegnia hyphae well, and the same was true for PAS, which showed very poorly defined hyphae (not shown).
Figure 8.
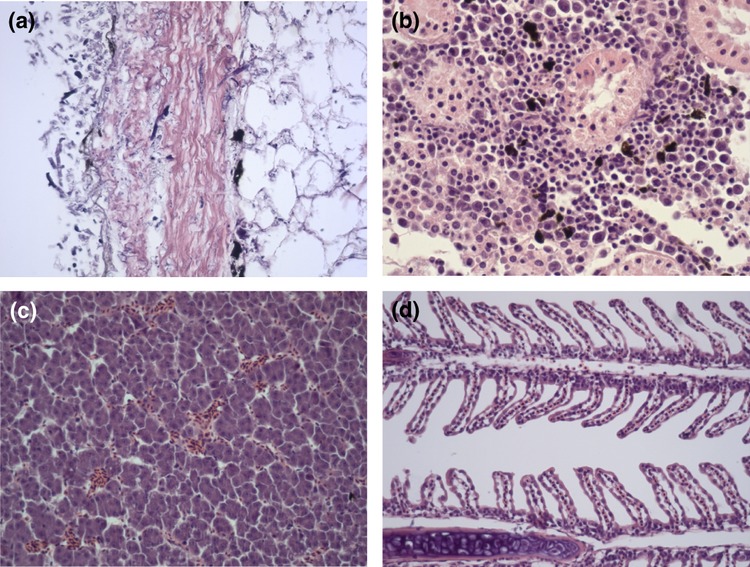
Histopathology of tissues from infected fish. (a) Skin, oedema and mild haemorrhage, with complete wipeout of the epidermis; (b) kidney, severe karyorrhexis and oedema; (c) liver, moderate congestion; and (d) gills, mild degeneration of secondary lamella.
Morphological characteristics of the S. parasitica
The impact of passage on the virulence of the strains included motivated us to examine in more detail the morphological characteristics of the different S. parasitica strains at different passages (summarized in Table 3).
Table 3.
Morphological characteristics of the S. parasitica strains at different passages
| Morphological features | Saprolegnia parasitica strain/passage (VIO) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5337AP | 53375P | 533715P | 2736AP | 27365P | 273615P | 5708AP | 57085P | 570815P | |
| Growth rate on agar (mm) | 30.0 | 30.0 | 30.0 | 28 | 28 | 28 | 29 | 29 | 29 |
| Sporangia formation | 25 | 25 | 25 | 20 | 20 | 18 | 23 | 23 | 23 |
| Sporangia release | 25 | 25 | 25 | 20 | 12 | 10 | 23 | 23 | 23 |
| Oogonia | NP | NP | NP | NP | R | NP | R | R | R |
| Chlamydospore formation | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 |
H: High rate; more than 9 sporangia per hemp seed, > 16 oogonia per seed, >10 chlamydospores per seed. M: Moderate rate; 4-9 sporangia per seed, 8-16 oogonia per seed, 5-10 chlamydospores per seed. L: Low rate; 2-3 sporangia per seed, 4-7 oogonia per seed, 2-4 chlamydospores per seed. R: Rarely present; less than 2 sporangia per seed, less than 4 oogonia per seed, less than 2 chlamydospores per seed. NP: Not present at all.
While passage resulted in significant reduction in virulence for strain 5337, examination for sexual reproductive structures (oogonia and antheridia) of isolates by day 14 post-challenge did not show production of oogonia or antheridia. The samples from this time point were incubated for an extra 2 weeks in the laboratory, and still no sexual reproductive structures were observed for any of the cultures. For strains 2736 and 5708, oogonia were observed at very low rates (Fig. 9), but these findings did not associate with virulence/mortality figures.
Figure 9.

Oogonia observed in strains 2736 and 5708.
The impact of in vitro passage on virulence for strains 5337 and 5708 was different (Figs1 & 3), but we found a high rate of sporangia production by the different passages of both strains. The most striking finding was that 273615P, which was the most attenuated by passage, also showed the lowest rates of sporangial release and proliferation.
On solid GY, no significant differences were observed in growth rates of the cultures of different passages, and the diameters of the vegetative colonies were in principle the same at all passages for all three strains, <35 mm.
Finally, production of enlarged chlamydospores was abundant in all the cultures at the different passages (Fig. 10) and no differences were seen.

Chlamydospores of 5337AP at: (a) X4, (b) X10 and (c) X20 in a 4-week-old culture. Note the absence of reproductive structures.
Discussion
The present study demonstrates that successive in vitro subculturing may have varying effects on different strains of S. parasitica. In this study, the pattern of change in virulence was strain dependent. Fifteen successive in vitro subcultures resulted in reduced virulence for strains 5337AP and 2736AP. Noteworthy among the results in this study was the fact that the strains that showed a loss of virulence due to successive in vitro subculturing were the ones that regained their virulence following passage through Atlantic salmon parr.
Strain 5708 produced the highest all-round morbidity and did not show a decline in virulence after 15 in vitro subcultures. A passage of this particular strain through the susceptible fish host did not cause any increment in its morbidity rate. So far, there is no single documented research report on attenuation of virulence in oomycetes, including those pathogenic to plants, but if reports from researchers working with other types of organisms are anything to go by, ours is not an isolated finding (Hayden, Bidochka & Khachatourians 1992; Brownbridge et al. 2001; Vandenberg & Cantone 2004; Shah, Wang & Butt 2007; Nahar et al. 2008). No obvious differences were found in the severity of lesions caused by the different passages of all three strains. 273615 had an endpoint CPI of only 3%. We may attribute this to the fact that sporangia were very rarely observed in cultures of this isolate.
On the whole, our study shows that the ability to maintain virulence in vivo can vary between S. parasitica strains, and so not all isolates of S. parasitica need to be passed through susceptible fish prior to challenge experiments. Based on our findings, it would be advisable to be cautious and preserve several isolates of the same strain of S. parasitica on hemp seed. Until now, the sole possibility of overcoming attenuation in many different types of organisms is with a periodical passage through the target host (Ito et al. 2001; Ansari & Butt 2011), but this method is time-consuming and there is a risk of contamination (Butt & Goettel 2000). It may also raise questions of ethics and animal welfare.
The fact that the special stains were not able to demonstrate fungal hyphae in histopathology sections may be due to the fact that there is very little chitin in Saprolegnia (Yanong 2003). Most studies have indicated that in Saprolegnia infections, there is very little or no inflammatory response in the integument (Pickering & Richards 1980; Pickering & Willoughby 1982; Xu & Rogers 1991; Bly et al. 1992; Lopez-Doriga 1995) or inner infections (Bruno & Stamps 1987; Alvarez et al. 1988, 1995). These reports are consistent with our findings in the present study. The lack of inflammatory response seems to be related to immunosuppressive factors, because immune-competent catfish experimentally infected with Saprolegnia were able to produce inflammatory processes (Bly et al. 1994).
On hemp seed cultures, none of the isolates belonging to the strain VIO 5337 produced oogonia at 21 °C, but some of the passages of the other two strains (VIO 2736 and VIO 5708) did, albeit at very low rates of <4 per seed. In this particular study, there was no correlation between oogonia formation and the ability of an isolate to cause morbidity.
Production of enlarged S. parasitica chlamydospores was abundant all-round. The fact that chlamydospores were observed at high rates might not have much significance as far as pathogenicity is concerned. Yuasa & Hatai (1995) reported that highly pathogenic strains developed abundant chains of chlamydospores, but this was not confirmed by Fregeneda, Fernandez Diez & Aller Gancedo (2001). In the present study, the diameters of vegetative colonies of all the different generations of the three Saprolegnia strains in question were less than 35 mm after 72 h. This is in accordance with findings by Stueland et al. (2005) in which only S. diclina and S. ferax, but not S. parasitica strains, attained diameters above 40 mm over a similar time point and at a similar water temperature, 21 ± 1 °C.
Acknowledgments
This work has been funded by the European Commission through the European Union Marie Curie Initial Training Network project SAPRO (238550).
References
- Ali EH. Morphological and biochemical alterations of oomycete fish pathogen Saprolegnia parasitica as affected by salinity, ascorbic acid and their synergistic action. Mycopathologia. 2004;159:231–243. doi: 10.1007/s11046-004-6670-z. [DOI] [PubMed] [Google Scholar]
- Almaguer-Chávez JA, Welsh O, Lozano-Garza HG, Said-Fernández S, Romero-Díaz VJ, Ocampo-Candiani J. Vera-Cabrera L. Decrease of virulence for BALB/c mice produced by continuous sub-culturing of Nocardia brasiliensis. BMC Infectious Diseases. 2011;11:290–296. doi: 10.1186/1471-2334-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez F, Razquin B, Villena A, Lopez Fierro P. Zapata A. Alterations in the peripheral lymphoid organs and differential leukocyte counts in Saprolegnia-infected brown trout, Salmo trutta fario. Veterinary Immunology and Immunopathology. 1988;18:181–193. doi: 10.1016/0165-2427(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Alvarez F, Villena A, Zapata A. Razquin B. Histopathology of the thymus in Saprolegnia-infected wild brown trout, Salmo trutta L. Veterinary Immunology and Immunopathology. 1995;47:163–172. doi: 10.1016/0165-2427(94)05384-5. [DOI] [PubMed] [Google Scholar]
- Ansari MA. Butt TM. Effects of successive subculturing on stability, virulence, conidial yield, germination and shelf-life of entomopathogenic fungi. Journal of Applied Microbiology. 2011;110:1460–1469. doi: 10.1111/j.1365-2672.2011.04994.x. [DOI] [PubMed] [Google Scholar]
- Barnes ME, Sayler WA. Cordes RJ. Use of formalin treatments during incubation of eyed eggs of brown trout. North American Journal of Aquaculture. 2001;63:333–337. [Google Scholar]
- Beakes GW, Wood SE. Burr AW. Features which characterize Saprolegnia isolates from salmonid fish lesions-a review. In: Mueller GJ, editor; Salmon Saprolegniasis. Portland: Bonneville Power Administration; 1994. pp. 33–66. [Google Scholar]
- Bly JE, Lawson LA, Dale DJ, Szalai AJ, Durborow RM. Clem LW. Winter saprolegniosis in channel catfish. Diseases of Aquatic Organisms. 1992;13:155–164. [Google Scholar]
- Bly JE, Lawson LA, Abdel-Aziz ES. Clem LW. Channel catfish, Ictalurus punctatus, immunity to Saprolegnia sp. Journal of Applied Aquaculture. 1994;3:35–50. [Google Scholar]
- Brownbridge M, Costa S. Jaronski ST. Effects of in vitro passage of Beauveria bassiana on virulence to Bemisia argentifolii. Journal of Invertebrate Pathology. 2001;77:280–283. doi: 10.1006/jipa.2001.5020. [DOI] [PubMed] [Google Scholar]
- Bruno DW. Stamps DJ. Saprolegniasis of Atlantic salmon, Salmo salar L., fry. Journal of Fish Diseases. 1987;10:513–517. [Google Scholar]
- Butt TM. Use of entomogenous fungi for the control of insect pests. In: Kempken F, editor. The Mycota XI Agricultural Applications. Berlin, Heidelberg: Springer-Verlag; 2002. pp. 111–134. [Google Scholar]
- Butt TM. Goettel MS, Navon A. Bioassays of entomopathogenic fungi. In: Ascher KRS, editor; Bioassays of Entomopathogenic Microbes and Nematodes. Wallingford, Oxon, UK: CAB International; 2000. pp. 141–195. [Google Scholar]
- Druelle J, Sellin CI, Waku-Kouomou D, Horvat B. Wild FT. Wild type measles virus attenuation independent of type I IFN. Virology Journal. 2008;5:22. doi: 10.1186/1743-422X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargues JF, Ouedraogo A, Goettel MS. Lomer CJ. Effects of temperature, humidity and inoculation method on susceptibility of Schistocerca gregaria to Metarhizium flavoviride. Biocontrol Science and Technology. 1997;7:345–356. [Google Scholar]
- Fregeneda GJM, Fernandez Diez M. Aller Gancedo JM. Experimental pathogenicity in rainbow trout, Oncorhynchus mykiss (Walbaum), of two distinct morphotypes of long-spined Saprolegnia isolates obtained from wild brown trout, Salmo trutta L., and river water. Journal of Fish Diseases. 2001;24:351–359. [Google Scholar]
- Hatai K. Hoshiai GI. Characteristics of two Saprolegnia species isolated from Coho salmon with saprolegniosis. Journal of Aquatic Animal Health. 1993;5:115–118. [Google Scholar]
- Hayden TP, Bidochka MJ. Khachatourians GG. Entomopathogenicity of several fungi toward English grain aphid (Homoptera: Aphididae) and enhancement of virulence with host passage of Paecilomyces farinosus. Journal of Economic Entomology. 1992;85:58–64. [Google Scholar]
- Hernando MD, Mezcua M, Suarez-Barcena JM. Fernandez-Alba AR. Liquid chromatography with time-of-flight mass spectrometry for simultaneous determination of chemotherapeutant residues in salmon. Analytica Chimica Acta. 2006;562:176–184. [Google Scholar]
- Ibrahim L, Butt TM. Jenkinson P. Effect of artificial culture media on germination, growth, virulence and surface properties of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycological Research. 2002;106:705–715. [Google Scholar]
- Ito T, Goto H, Yamamoto E, Tanaka H, Takeuchi M, Kuwayama M, Kawaoka Y. Otsuki K. Generation of a highly pathogenic avian influenza A virus from an avirulent field isolate by passaging in chickens. Journal of virology. 2001;75:4439–4443. doi: 10.1128/JVI.75.9.4439-4443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi M, Okumura S, Nakanishi S, Yoshioka M, Ueno R, Hoshiai G. Hatai K. Fungicidal effect of sodium hypochlorite solution on a fish-pathogen Oomycete, Saprolegnia parasitica. Suisanzoshoku. 2002;50:385–386. [Google Scholar]
- Kashiwagi M, Khomvilai C, Okada A, Maekawa Y, Okumura S, Nakanishi S. Yoshioka M. Proceedings of the JSPS-NRCT International Symposium. Thailand: Layong; 2003. Prevention effect of sodium hypochlorite solution on saprolegniasis of salmon eggs; pp. 224–231. In: and Kaseart University, Bangkok. [Google Scholar]
- Kawakami K. On the changes of characteristics of the silkworm muscardines through successive cultures. Bulletin Sericulture Experimental Station Japan. 1960;16:83–99. [Google Scholar]
- Lopez-Doriga MV. Patologia ultraestructural del tegumento de truchas,Salmo trutta L. Infectadas por Saprolegnia. Asturias: Universidad d e Oviedo; 1995. [Google Scholar]
- Nagaichi BB. Verticillium species pathogenic on aphids. Indian Phytopathology. 1973;26:163–165. [Google Scholar]
- Nahar PB, Kulkarani SA, Kulye MS, Chavan SB, Kulkarani G, Rajendran A, Yadav PD. Shouche Y. Effect of repeated in-vitro sub-culturing on the virulence of Metarhizium anisopliae against Helocoverpa armigera (Lepidoptera Noctuidae) Biocontrol Science and Technology. 2008;18:337–355. [Google Scholar]
- Pickering AD. Richards RH. Factors influencing the structure, function and biota of the salmonid epidermis. Proceedings of the Royal Society of Edinburgh Section B: Biology. 1980;79:93–104. [Google Scholar]
- Pickering AD. Willoughby LG. Saprolegnia infections of salmonid fish. In: Roberts RJ, editor; Microbial Diseases of Fish. London: Academic Press; 1982. pp. 271–297. [Google Scholar]
- Seymour RL. The Genus Saprolegnia. Nova Hedwigia. 1970;19:1–124. [Google Scholar]
- Shah FA, Wang CS. Butt TM. Repeated in-vitro sub-culturing alters spore surface properties and virulence of Metarhizium anisopliae. FEMS Microbiology Letters. 2007;251:259–266. doi: 10.1111/j.1574-6968.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- Stueland S, Hatai K. Skaar I. Morphological and physiological characteristics of Saprolegnia spp. strains pathogenic to Atlantic salmon, Salmo salar L. Journal of Fish Diseases. 2005;28:445–453. doi: 10.1111/j.1365-2761.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- Sudova E, Machova J, Svobodova Z. Vesely T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Journal Veterinarni Medicina. 2007;52:527–539. [Google Scholar]
- Van West P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist. 2006;20:99–104. [Google Scholar]
- Vandenberg JD. Cantone FA. Effect of serial transfer of three strains of Paecilomyces fumosoroseus on growth in vitro, virulence, and host specificity. Journal of invertebrate pathology. 2004;85:40–45. doi: 10.1016/j.jip.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Vidal C, Lacey LA. Fargues J. Pathogenicity of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against Bemisia argentifolii (Homoptera: Aleyrodidae) with a description of a bioassay method. Journal of Economic Entomology. 1997;90:765–772. [Google Scholar]
- Willoughby LG. Rapid preliminary screening of Saprolegnia on fish. Journal of Fish Diseases. 1985;8:473–476. [Google Scholar]
- Xu D. Rogers WA. Electron microscopy of infection by Saprolegnia spp. in channel catfish. Journal of Aquatic Animal Health. 1991;3:63–69. [Google Scholar]
- Yanong RPE. Fungal diseases of fish. In: Michael PJ, editor. Fungal Diseases. Philadelphia, PA: Veterinary Clinics of North America, Exotic Animal Practice 6. WB Saunders Co.; 2003. pp. 377–400. [DOI] [PubMed] [Google Scholar]
- Yuasa K. Hatai K. Relationship between pathogenicity of Saprolegnia spp. isolates to rainbow trout and their biological characteristics. Fish Pathology. 1995;30:101–106. [Google Scholar]


