Abstract
Polychlorinated biphenyls (PCBs) are toxic, persistent, and bioaccumulative chemicals which, because of their lipophilic properties, are abundant in human breast milk. Breastfed infants are therefore at risk of being exposed to considerable amounts of PCBs. The commonly used exposure estimations, based solely on breast milk PCB levels and duration of breastfeeding, may lead to exposure misclassification. To improve assessments of exposure to PCBs, we determined PCB 153 serum concentration, as a model substance for PCBs, at the critical time of weaning for each child in 305 breastfed infants from 5 single time point concentration measurements spread over 7 years and data on duration of breastfeeding, using an earlier developed model of the system type. We approximated the dependence of PCB 153 serum concentration, Ctbf, adjusted to cord serum concentration, C0, on nursing period, by a polynomial function Ctbf/C0=0.596+0.278t−0.0047t2 which reliably predicts exposure to PCB 153 of breastfed infants, important for assessment of dose-outcome relationships. Adjustment of current serum concentrations to cord serum concentration improved validity of exposure assessment.
Keywords: breastfeeding, PCB exposure assessment, PCB 153 serum concentration, polynomial function, toxicokinetics
1. Introduction
Large quantities of toxic polychlorinated biphenyls (PCBs) have been released into the environment as a result of PCB production and widespread use. Because of their high lipophilicity, PCBs bioaccumulate in the fatty tissues of organisms. For example, human milk with its 3–5 % fat content (Jenness, 1979) is an important carrier of these compounds and lactational transfer represents one of the principal routes of PCB exposure to developing mammals, including humans. The concentration time course of organochlorines in blood during nursing is peaking at the time of weaning and therefore many authors consider duration of breastfeeding a measure of exposure. Higher perinatal exposures to organochlorines, such as PCBs, have been associated with poorer cognitive development (Shonkoff et al., 2009; Shonkoff and Garner, 2012) and deficits of immune system function (Grandjean et al., 2008). Therefore assessment of accompanying toxicokinetic processes is essential for understanding relevant dose-effect relationships.
A measure of exposure of infants to lipophilic organochlorines through breastfeeding is the amount of milk taken up into the body. Therefore the duration of breastfeeding is a strong predictor of body burden of organochlorines (Hardell et al., 2010; Lackmann et al., 2004; Patandin et al., 1997; Patandin et al., 1999; Kreuzer et al., 1997; Barr et al., 2003; Lanting et al., 1998; Chao et al., 2004; Tohyama et al., 2011). A more accurate assessment of exposure is a product of the concentration of the compound in milk and the rate of consumption of breast milk (Patandin et al., 1999; Bergkvist et al., 2010; Arcus-Arth et al., 2005; Koopman-Esseboom et al., 1996; Ulaszewska et al., 2011) or alternatively a product of the breast milk levels and the number of weeks of breastfeeding (Pan et al., 2009; Pan et al., 2010; Weisglas-Kuperus et al., 2004). All these approaches are accompanied by many uncertainties. Known is imprecision of reporting duration of breastfeeding by the mother and also data on exclusive breastfeeding may imprecisely reflect the situation (Aarts et al., 2000). We have recently shown that estimations based solely on breast milk PCB levels and duration of breastfeeding may lead to exposure misclassification (Verner et al., 2013).
In 2002–2003 we started long term observation of children living in an area polluted by PCBs and information on lactational exposure to PCBs of each child of our cohort was essential for description of the respective concentration-effect relationships (Hertz-Picciotto et al., 2003). We had two options. The first was to apply a system model (Trnovec et al., 2011) using data on time series serum concentration measurements and duration of breastfeeding. The second was based on PBPK simulations using individual physiologic parameters, duration of breastfeeding, and levels of PCBs measured in maternal blood at delivery, cord blood, or breast milk (Verner et al., 2013). In the currently used former option we describe dependence of the concentration of PCB 153 in serum of 305 breastfed infants on length of breastfeeding. Although we determined the concentrations of 15 PCB congeners in serum specimens, we focused our analyses on PCB-153 for two reasons: 1) PCB-153 is highly correlated with total PCB concentration in this cohort (Jusko et al., 2010), and 2) it was detectable in the vast majority of child specimens (Kocan et al., 2004).
2. Material and methods
We included into the study altogether 305 infants from Michalovce district in eastern Slovakia (Hertz-Picciotto et al., 2003). This is a subgroup from originally 1082 children for which data for all 5 measurement times (cord blood at birth, 6, 16, 45 and 72 months later) were available. We have shown that for a slightly different subgroup maternal PCB-153 concentrations were highly similar between participants in the subgroup study and the entire cohort at birth (p=0.99) (Jusko et al., 2014). The Michalovce area was polluted by PCBs which were produced at the chemical facility Chemko Strážske. The extent of pollution can be illustrated by comparing the median serum concentration for the same time period in 1009 adult subjects for congener PCB 153 of 578 ng/g of lipid in the Michalovce district (Petrik et al., 2006) with the median serum concentration in 415 nonpregnant women for congener PCB 153 of 8.3 ng/g of lipid in USA (Woodruff et al., 2011). The characteristics of infants and mothers participating in the study were described earlier (Jusko et al., 2012; Jusko et al., 2011; Jusko et al., 2010; Park et al., 2010; Sonneborn et al., 2008a, 2008b). The study protocol was approved by Institutional Review Boards at the University of California, Davis and the Slovak Medical University. Mothers gave informed consent and were enrolled at the time they came to the hospital for delivery. The protocol excluded (1) mothers with more than four previous births, (2) mothers less than 18 years of age, (3) mothers who had resided fewer than 5 years in their district, and (4) mothers with a major illness during pregnancy.
Following birth, we also excluded mothers whose infants had severe birth defects. Follow-up occurred at 6, 16, 45and at 72 months. Data on breastfeeding were collected by a qualified nurse during follow-up examinations. Concentrations of 15 PCB congeners (IUPAC No. 28, 52, 101, 123, 118, 114, 153, 105, 138, 167, 156, 157, 180, 170 and 189) in the umbilical cord and child serum were determined as described elsewhere (Kocan et al., 2004; Conka et al., 2005). All analytical measurements were carried out at the National Reference Centre for Dioxins and Related Compounds (Department of Toxic Organic Pollutants, Slovak Medical University) which has been certified by the Slovak National Accreditation Service (ISO/IEC 17 025:2005, certification No. S-111) and regularly participates in interlaboratory studies and proficiency tests on dioxins and PCBs in food and feed. Control charts were plotted for QC samples, blanks, and verification calibration standards to check accuracy, precision, and reliability of the analytical process. Only samples with PCB concentrations ≥LOD were taken into account. We report lipid adjusted concentrations. We estimated total serum lipids using the enzymatic summation method (Akins et al., 1989). We fitted a concentration-time function (Trnovec et al., 2011) to serum PCB 153 concentration data of each child and calculated PCB 153 serum concentration at time of weaning. The serum concentration values at the time of weaning for each particular child were approximated by polynomial function of degree 2 of the form y = ax2 + bx + c. Statistical calculations were carried out with statistics program SPSS 16, Softonic International S.L.
3. Results
We have used data on PCB 153 serum concentration measured in time intervals 0, 6, 16, 45 and 72 months after delivery (Table 1) for determination of the PCB 153 serum concentration at the time of weaning.
Table 1.
Data on concentration of PCB 153 in blood serum of infants in ng/g serum lipids at various time intervals after birth and on equivalents to duration of exclusive breastfeeding in months.
| Time after birth (Months) |
Concentration of PCB 153 |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Minimal value |
Median | Maximal value |
||
| Child serum | 0 | 185.65 | 170.5 | 14.1 | 137.45 | 1373.7 |
| 6 | 228.21 | 312.54 | 1.69 | 141.17 | 2640.76 | |
| 16 | 268.86 | 402.75 | 0.52 | 137.91 | 3024.45 | |
| 45 | 227.71 | 325.63 | 3.2 | 116.49 | 2749.41 | |
| 72 | 93.84 | 133.56 | 4.4 | 48.36 | 1011.62 | |
| Equivalent to duration of exclusive breastfeeding | 10.27 | 11.13 | 0 | 5 | 46 | |
We present the descriptive statistics on parameters of the function approximating the concentration time course of each child in Table 2.
Table 2.
Parameter values of the model applied to postnatal PCB 153 serum concentration values in 305 infants of our cohort. Cbf,∞ is a hypothetical limit value of PCB 153 concentration increase for t and time of breastfeeding, tbf, converging to infinity and Cf,∞ is a hypothetical limit value of PCB 153 concentration increase for t converging to infinity and tbf converging to zero and MT is mean time, months, of PCB 153 in the body of infants. Area under the curve from time zero to 72 months, AUC, is in ng.months.(g lipids)−1. C0 is concentration in cord blood serum.
| Mean | SD | Minimal value |
Median | Maximal value | |
|---|---|---|---|---|---|
| MT | 66.52 | 193.51 | 0.003 | 5.19 | 2330 |
| AUC | 15150.7 | 20994.9 | 824 | 8660 | 151000 |
| Cbf,∞ | 1543.74 | 4929.44 | 0.5 | 395.72 | 75000 |
| Cbf,∞/C0 | 10.37 | 25.7 | 0.008 | 2.74 | 265.63 |
| Cf,∞ | 1009.26 | 3984.32 | 3.18 | 136.93 | 62000 |
| Cf,∞/C0 | 7.02 | 20.49 | 0.05 | 1.03 | 214.45 |
| Cf,∞/C0,∞ | 0.58 | 1.59 | 0.03 | 0.42 | 26.82 |
| Ct/C0 | 2.41 | 1.8 | 0.02 | 1.82 | 9.65 |
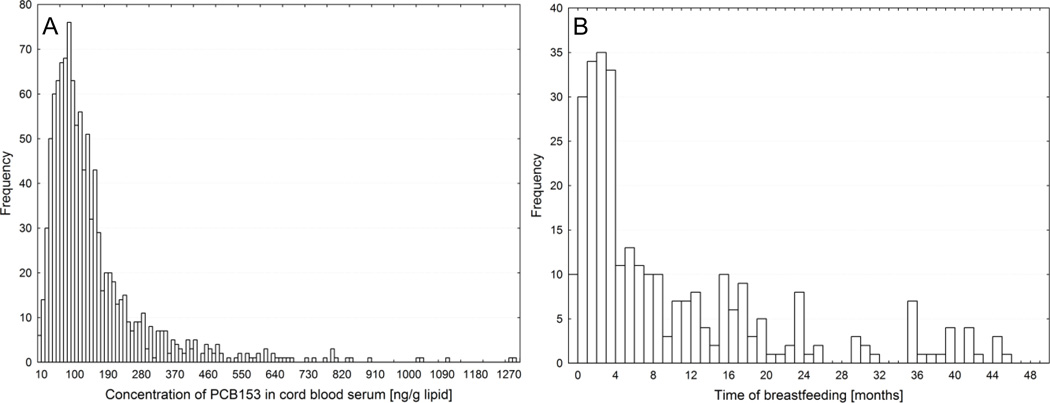
In Fig. 1A we show the over 3 orders of magnitude variability of PCB 153 cord serum concentration. It is reflecting variation of PCB 153 in maternal blood and rate of placental transfer of PCB. In order to eliminate the projection of this variability to postnatal period we have adjusted the current postnatal serum concentration values of infants to cord serum PCB 153 concentration as Ctbf/C0. In Fig. 1B we present a frequency diagram of time of breastfeeding.
Fig. 1.
Frequency diagram of PCB 153 concentration, ng/g lipid, in cord blood serum, C0, of 1082 children of which are 305 children of the current cohort a subgroup (A). Frequency diagram of duration of breastfeeding, tbf, of mothers of the current cohort (B).
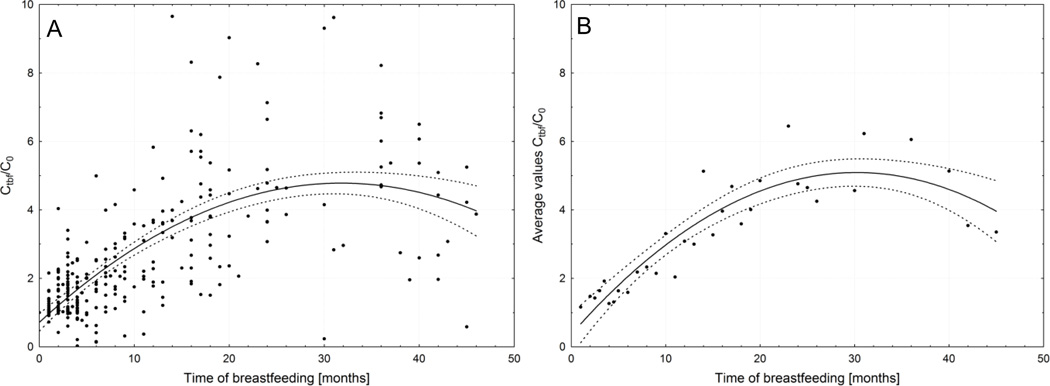
We show the resulting serum concentration values at the time of weaning for each particular child in Fig. 2A. We have examined approximation of this relationship by several functions and obtained optimal fit for polynomial approximation of degree 2. Replacing Ctbf/C0 values by arithmetic means calculated for every month (while at least 2 values were available) markedly improved the fit (Fig. 2B).
Fig. 2.
Computed serum concentrations of PCB 153 adjusted to cord serum concentrations, Ctbf/C0, at the time of weaning, tbf. Each point represents a child and the curve is the best fit of a polynomial function of degree 2 (Ctbf/C0=0.596+0.278t−0.0047t2) and its 95% confidence interval (A). Averaged data (Ctbf/C0=0.156+0.334t−0.0059t2) (B).
When calculating the values of the model parameters by iterative Monte-Carlo simulation procedure, we observed that the model was very sensitive to improbable data reported by mothers on duration of breastfeeding. The model assumes continuous uptake of PCB at a constant rate and an instantaneous stop of uptake, i.e. abrupt finishing of breastfeeding. In real life however the rate of breastfeeding diminishes gradually. Abraham (2002) obviously was aware of this fact when modeling exposure and introduced the "equivalent to duration of exclusive breastfeeding" in his study. The model also does not account for reuptakes of PCB 153, however breast milk scarcely can be a source of reuptakes. These can occur with normal food, but in infancy may be rare. If the child is living in an area highly polluted by PCBs and after weaning is fed by food produced locally (Sonneborn et al., 2008a, 2008b), reuptakes at adolescent age can occur, as we have shown previously (Wimmerová et al., 2011). A slight modification of the weaning time usually improved the fit. Consequently we consider our tbf values equivalents to duration of exclusive breastfeeding as introduced earlier by Abraham (2002).
4. Discussion
We are characterizing the relationship between PCB 153 serum level of infants and duration of breastfeeding as a continuous nonlinear function. It is based on serial sampling in 305 infants. For each 5 concentration measurements spaced in time were available and information on length of lactation. The function for averaged data in Fig. 2B reliably predicts burden of PCB 153 of breastfed infants at time of weaning which is important for dose-outcome relationships.
The concentration time course, Ct, in each child starts from C0 which largely varies as we have shown in Fig. 1A. By adjustment of current concentrations, Ctbf, to cord blood serum concentration, C0, all individual time courses start from a value of 1, as for time zero Ct=C0. Such adjustment eliminates dependence of the infant’s body burden of PCB 153 on a variable represented by maternal body burden which depends on mother’s age, changes in body composition and exposures early in life (Lignell et al., 2011). Similar presentation as in Fig. 2 can be found in literature (Tohyama et al., 2011; Abraham, 2002), however without adjustment to C0. Adjustment to C0 and fit by a polynomial instead a linear function improved the outcome.
Curves in Fig. 2 show that Ctbf/C0 peaked at extremely long nursing periods at values between 4 and 5 which is in agreement with 4.5 published for children at 42 months post partum (Lanting et al., 1998). The flattening and downward trend may result from approaching equilibrium between intake and elimination and distribution of PCBs into an increasing pool of lipids as child grows.
To predict the absolute PCB 153 concentration Ctbf from Ctbf/C0 values we need to know C0. An advantage for obtaining C0 is however that when expressed on a lipid basis, maternal plasma, cord plasma, and milk concentrations of PCBs are strongly intercorrelated, indicating that PCB concentration in any of these biologic media is a good indicator of C0 (Ayotte et al., 2003). We confirmed that conversion of PCB data from one matrix to another is feasible (Govarts et al., 2012). An advantage of our model is that it predicts infant’s PCB concentration over whole nursing period contrary to published single time point predictions (Ayotte et al., 2003).
Conclusion
Our aim was to improve assessment of exposure to PCBs resulting from breastfeeding and to upgrade the prediction of exposure based on known time of nursing. In a cohort of 305 children we determined PCB 153 serum concentration at the time of weaning and described this relationship with a mathematical function enabling reliable prediction of PCB exposure in infants for scientific purposes or risk analysis.
Highlights.
Breastfed infants are at risk of exposure to considerable amounts of PCBs.
Determination of perinatal exposures to PCB 153 is essential for risk assessment.
We determined PCB 153 serum concentration at time weaning of breastfed infants.
We reliably predicted exposure to PCB 153 of breastfed infants.
Acknowledgements
This project has been supported by the European Commission through the 7FP project OBELIX (No. 227391), the Competence Center for SMART Technologies for Electronics and Informatics Systems and Services, ITMS 26240220072, funded by the Research & Development Operational Programme from the ERDF and Scientific Grant Agency VEGA (Bratislava, Slovakia), grant 1/0120/12, Ministry of Health, Slovak Republic through projects 2007/07-SZU-03, 2012/41-SZU-5 and 2012/47-SZU-11, Slovak Research and Development Agency through projects APVV-0571-12 and APVV-0444-11 and the project "Center of Excellence of Environmental Health", ITMS No. 26240120033, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund. This work also received support from U.S. National Institutes of Health grants R01 CA096582, R03 TW007152, K12 ES019852, and P30 ES001247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kinga Lancz, Email: kinga.lancz@szu.sk.
Irva Hertz-Picciotto, Email: ihp@ucdavis.edu.
Todd A. Jusko, Email: todd_jusko@urmc.rochester.edu.
Ľubica Murínová, Email: lubica.palkovicova@szu.sk.
Soňa Wimmerová, Email: sona.wimmerova@szu.sk.
Eva Šovčíková, Email: eva.sovcikova@szu.sk.
Ladislav Dedík, Email: ladislav.dedik@stuba.sk.
Maximilián Strémy, Email: maximilian.stremy@stuba.sk.
Beata Drobná, Email: beata.drobna@szu.sk.
Dana Farkašová, Email: rektor@szu.sk.
Tomáš Trnovec, Email: tomas.trnovec@szu.sk.
References
- Aarts C, Kylberg E, Hörnell A, Hofvander Y, Gebre-Medhin M, Greiner T. How exclusive is exclusive breastfeeding? A comparison of data since birth with current status data. Int J Epidemiol. 2000;29:1041–1046. doi: 10.1093/ije/29.6.1041. [DOI] [PubMed] [Google Scholar]
- Abraham K. Venia legendi for pediatrics. Germany: Humboldt-Universität zu Berlin; 2002. Exposition gegenüber Dioxinen und verwandten Substanzen–ein Risiko für Säuglinge? http://edoc.hu-berlin.de/habilitationen/abraham-klaus-2003-01-28/HTML/front.html. [Google Scholar]
- Akins JR, Waldrep KJT, Bernert JR. The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Arcus-Arth A, Krowech G, Zeise L. Breast milk and lipid intake distributions for assessing cumulative exposure and risk. J Expo Anal Environ Epidemiol. 2005;15:357–365. doi: 10.1038/sj.jea.7500412. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E Inuit Cohort Study. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit Cohort Study. Environ Health Perspect. 2003;111:1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Weihe P, Needham LL, Davis MD, Roman W, Hurtz D, et al. PCBs and organochlorine pesticide concentrations in a Faroe Island 14-year old cohort: Measurement using new methodology and evaluation of correlations and patterns. Organohalogen Comp. 2003;63:385–388. [Google Scholar]
- Bergkvist C, Lignell S, Sand S, Aune M, Persson M, Håkansson H, et al. A probabilistic approach for estimating infant exposure to environmental pollutants in human breast milk. J Environ Monit. 2010;12:1029–1036. doi: 10.1039/b914504d. [DOI] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee CC, Yu HY, Lu YK, Päpke O. Level of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls (PCDD/Fs, PCBs) in human milk and the input to infant body burden. Food Chem Toxicol. 2004;42:1299–1308. doi: 10.1016/j.fct.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Conka K, Drobna B, Kocan A, Petrık J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J Chromatogr. 2005;1084:33–38. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect. 2012;120:162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Bellinger D, Bergman A, et al. The Faroes statement: Human health effects of developmental exposure to chemicals in our environment. Basic Chemical Pharmacology and Toxicology. 2008;102:73–75. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Hardell E, Carlberg M, Nordström M, van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci Total Environ. 2010;408:4412–4419. doi: 10.1016/j.scitotenv.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kocan A, Charles MJ, Ciznar P, Langer P, et al. PCBs and early childhood development in Slovakia: Study design and background. Fresen Environ Bull. 2003;12:208–214. [Google Scholar]
- Jenness R. The composition of human milk. Semin Perinatol. 1979;3:225–239. [PubMed] [Google Scholar]
- Jusko TA, De Roos AJ, Schwartz SM, Lawrence BP, Palkovicova L, Nemessanyi T, et al. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ Res. 2010;110:388–395. doi: 10.1016/j.envres.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, De Roos AJ, Schwartz SM, Lawrence BP, Palkovicova L, Nemessanyi T, et al. Maternal and early postnatal polychlorinated biphenyl exposure in relation to total serum immunoglobulin concentrations in 6-month-old infants. J Immunotoxicol. 2011;8:95–100. doi: 10.3109/1547691X.2010.549096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Sisto R, Iosif AM, Moleti A, Wimmerová S, Lancz K, et al. Prenatal and Postnatal Serum PCB Concentrations and Cochlear Function in Children at 45 Months of Age. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307473. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Sonneborn D, Palkovicova L, Kocan A, Drobna B, Trnovec T, et al. Pre- and postnatal polychlorinated biphenyl concentrations and longitudinal measures of thymus volume in infants. Environ Health Perspect. 2012;120:595–600. doi: 10.1289/ehp.1104229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan A, Drobna B, Petrik J, Jursa S, Chovancova J, Conka K, et al. Human exposure to PCBs and some other persistent organochlorines in eastern Slovakia as a consequence of former PCB production. Organohalogen Comp. 2004;66:3490–3497. [Google Scholar]
- Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, van der Paauw CG, Tuinstra LG, et al. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants' mental and psychomotor development. Pediatrics. 1996;97:700–706. [PubMed] [Google Scholar]
- Kreuzer PE, Csanády GA, Baur C, Kessler W, Päpke O, Greim H, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch Toxicol. 1997;71:383–400. doi: 10.1007/s002040050402. [DOI] [PubMed] [Google Scholar]
- Lackmann GM, Schaller KH, Angerer J. Organochlorine compounds in breast-fed vs. bottle-fed infants: preliminary results at six weeks of age. Sci Total Environ. 2004;329:289–293. doi: 10.1016/j.scitotenv.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Lanting CI, Fidler V, Huisman M, Boersma ER. Determinants of polychlorinated biphenyl levels in plasma from 42-month-old children. Arch Environ Contam Toxicol. 1998;35:135–139. doi: 10.1007/s002449900360. [DOI] [PubMed] [Google Scholar]
- Lignell S, Aune M, Darnerud PO, Soeria-Atmadja D, Hanberg A, Larsson S, et al. Large variation in breast milk levels of organohalogenated compounds is dependent on mother's age, changes in body composition and exposures early in life. J Environ Monit. 2011;13:1607–1616. doi: 10.1039/c1em10151j. [DOI] [PubMed] [Google Scholar]
- Pan IJ, Daniels JL, Goldman BD, Herring AH, Siega-Riz AM, Rogan WJ. Lactational exposure to polychlorinated biphenyls, dichlorodiphenyltrichloroethane, and dichlorodiphenyldichloroethylene and infant neurodevelopment: an analysis of the pregnancy, infection, and nutrition babies study. Environ Health Perspect. 2009;117:488–494. doi: 10.1289/ehp.0800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan IJ, Daniels JL, Herring AH, Rogan WJ, Siega-Riz AM, Goldman BD, et al. Lactational exposure to polychlorinated biphenyls, dichlorodiphenyltrichloroethane, and dichlorodiphenyldichloroethylene and infant growth: an analysis of the Pregnancy, Infection, and Nutrition Babies Study. Paediatr Perinat Epidemiol. 2010;24:262–271. doi: 10.1111/j.1365-3016.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, Weisglas-Kuperus N, de Ridder MA, Koopman-Esseboom C, van Staveren WA, van der Paauw CG, et al. Plasma polychlorinated biphenyl levels in Dutch preschool children either breast-fed or formula-fed during infancy. Am J Public Health. 1997;87:1711–1714. doi: 10.2105/ajph.87.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, Dagnelie PC, Mulder PG, Op de Coul E, van der Veen JE, Weisglas-Kuperus N, et al. Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: A comparison between breast-feeding, toddler, and long-term exposure. Environ Health Perspect. 1999;107:45–51. doi: 10.1289/ehp.9910745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J. Serum PCBs and organochlorine pesticides in Slovakia: age, gender, and residence as determinants of organochlorine concentrations. Chemosphere. 2006;65:410–418. doi: 10.1016/j.chemosphere.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, et al. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Environ Epidemiol. 2008a;18:581–587. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, et al. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birthweight. Paediatr Perinat Epidemiol. 2008b;22:202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Tohyama C, Uchiyama I, Hoshi S, Hijiya M, Miyata H, Nagai M, et al. Polychlorinated dioxins, furans, and biphenyls in blood of children and adults living in a dioxin-contaminated area in Tokyo. Environ Health Prev Med. 2011;16:6–15. doi: 10.1007/s12199-010-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnovec T, Dedík L, Jusko TA, Lancz K, Palkovičová L, Kočan A, et al. Assessment of exposure to PCB 153 from breast feeding and normal food intake in individual children using a system approach model. Chemosphere. 2011;85:1687–1693. doi: 10.1016/j.chemosphere.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaszewska MM, Zuccato E, Davoli E. PCDD/Fs and dioxin-like PCBs in human milk and estimation of infants' daily intake: a review. Chemosphere. 2011;83:774–782. doi: 10.1016/j.chemosphere.2011.02.066. [DOI] [PubMed] [Google Scholar]
- Verner MA, Sonneborn D, Lancz K, Muckle M, Ayotte P, Dewailly É, et al. Toxicokinetic Modeling of Persistent Organic Pollutant Levels in Blood from Birth to 45 Months of Age in Longitudinal Birth Cohort Studies. Environ Health Perspect. 2013;121:131–137. doi: 10.1289/ehp.1205552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Vreugdenhil HJ, Mulder PG. Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children. Toxicol Lett. 2004;149:281–285. doi: 10.1016/j.toxlet.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Wimmerová S, Lancz K, Tihányi J, Sovčíková E, Kočan A, Drobná B, et al. Half-lives of serum PCB congener concentrations in environmentally exposed early adolescents. Chemosphere. 2011;82:687–691. doi: 10.1016/j.chemosphere.2010.10.099. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]