Abstract
We have developed a hypoxia-inducible gene therapy approach for the expression of the mature form of human endonuclease G to facilitate cell death in hypoxic regions of the tumor. The chimeric therapeutic gene is placed under the control of a hypoxia response element based promoter and contains a translocation motif linked in frame to an oxygen-dependent degradation domain and the endonuclease G gene. Transient expression of the chimeric therapeutic gene in breast and prostate cancer cell lines resulted in efficient cell death under hypoxia-mimetic conditions. Stable MDA-MB-435 cells expressing the chimeric therapeutic gene under 1% O2 showed an increase in stable HIF-1α protein levels and synthesis of the endonuclease G protein in a time-dependent manner. In normoxic conditions, these stable transgenic cells exhibited no change in growth rate, invasion and motility when compared to parental cells. Moreover, xenografts generated using the transgenic cells exhibited highly significant suppression of tumor growth in a preclinical cancer model compared to the parental cell line. Thus, the hypoxia-modulated endonuclease G expression has the potential to be used as a gene-based-therapy system to kill malignant cells within hypoxic regions of tumors.
Keywords: hypoxia response element, oxygen-dependent degradation, human endonuclease G, cancer gene therapy
Introduction
Targeting hypoxic tumor cells with therapeutic agents has been considered an important and possibly cancer-specific therapy.1,2 In this respect, the use of bioreductive alkylating drugs has shown promise due to the enhanced reducing potential of the intracellular compartment of hypoxic cells. A more recent strategy has been the use of hypoxia-inducible gene therapies where the expression of a cytotoxic gene is modulated by a hypoxia response element (HRE)-based promoter.3–8 To obtain a tight control of expression in low oxygen concentrations, an additional oxygen-regulated component has been tested in some applications. Thus, the oxygen-dependent degradation domain (ODD) from the hypoxia-inducible factor 1α (HIF-1α) protein has been fused in-frame to proteins aimed at tumor cell killing.9,10 Under normoxic conditions, the ODD is hydroxylated at two proline residues (amino-acid residues 402 and 564 with respect to the full length HIF-1α protein), which generates a von Hippel-Lindau factor binding site.11,12 The von Hippel-Lindau factor is a component of an ubiquitin ligase complex and proteins bound to the von Hippel-Lindau factor are targeted for ubiquitin-mediated degradation by proteosomes.11–13 As O2 is a substrate for the proline hydroxylation reaction, hydroxylation is minimal at low O2 concentrations, thereby increasing the stability of ODD containing proteins. Thus, the use of HRE promoter to synthesize an ODD containing protein increases the activity of the protein only under hypoxic conditions.
To test the efficacy of a hypoxia-induced gene therapy system, we have generated a chimeric protein construct with human endonuclease G, which is capable of digesting both RNA and DNA.14 Human endonuclease G is normally compartmentalized in the mitochondria and following its release into the cytoplasm, it can function in apoptotic processes. 15–19 The expression of this novel protein construct has been placed under the control of a conditional promoter, which consists of five HREs in tandem upstream of a minimal cytomegalovirus promoter.20 In addition, we fused the coding sequence of the mature hEndoG (without the mitochondrial localization signal) to the coding sequence of the ODD domain of HIF-1α. This modification was introduced to tighten the conditional regulation of the toxic gene in hypoxic conditions. A translocation motif (TLM)21 has also been placed at the N-terminus, to potentially provide bystander-killing effects in therapeutic settings. Transient expression of TLM-ODD-hEndoG (henceforth referred to as hEndoG) in MDA-MB-435 (henceforth referred to as 435) and PC-3 cells indicated that this protein could conditionally kill cells. Generation of xenografts using stable MDA-MB-435-5HRE-TLM-ODD-hEndoG cells (henceforth referred to as 435-hEndoG) demonstrated highly significant suppression of tumor growth in a SCID (severe combined immunodeficiency) mouse model system indicating that the presence of hypoxic regions within the tumor induced hEndoG expression results in cell death. These series of experiments provide a proof of principle of the utility of the chimeric hEndoG construct and its applicability in ablating hypoxic regions in tumors. This in combination with adjuvant therapy may increase the efficacy of chemotherapeutic agents in the treatment of cancers.
Material and methods
Cloning of the chimeric hEndoG toxic gene under HRE promoter
The DNA sequence that encodes the N-terminus of the chimeric protein (Figure 1a) was amplified by PCR from a synthetic template encoding the TLM sequence in frame with the linker sequence: 5′-TTTATGGGGCCCTTAT CGTCAATCTTCTCGAGGATTGGGGACCCCGGGCCATACCGGCAGCACCGGCAGCGGCAGCTCCGGCACCGCCTCCTCCGAGGACAACACCGGTAAA-3′, using sense primer 5′-TTTATGGGGCCCTTATCG-3′ and anti-sense primer 5′-TTTACCGGTGTTGTCCT C-3′. An Xma I site engineered between the TLM and linker sequences and an Age I site at the 3′-end of the template are underlined. The PCR product was purified and subcloned into the pCR2.1 TA cloning vector (Invitrogen, Carlsbad, CA) generating pCR2.1-TLM-L. The ODD sequence was PCR amplified from linearized pCEP4-HIF-1α vector (a gift from Dr Paul van Diest, University Medical Center Utrecht, The Netherlands) with sense primer 5′-ACCGGTACCAAAGTTGAAT CAGAAGATACAAGTAGC-3′ and anti-sense primer 5′-CATATGGGCAGTGGTAGTGGTGGCATTAG-3′. The Age I and Nde I sites are underlined in the sense and anti-sense primers, respectively. The PCR product was gel purified and subcloned into pCR2.1. A linker sequence with 5′-Nde I and 3′-Nhe I sites was PCR amplified using linearized pCR2.1-TLM-L as template with sense primer 5′-CATATGGGACATACCGGCAGCAC-3′ and anti-sense primer 5′-GCTAGCGTTGTCCTCGGAGGAG G-3′. The purified product was subcloned into pCR2.1. The mature form of the human endonuclease G coding sequence was PCR amplified from a human breast epithelial cells cDNA library (Clontech, CA). PCR reaction was carried out using sense primer 5′-GCTAGCGCAGCCGAGTTGCCCCCTGT-3′ and anti-sense primer 5′-TCTAGAACCCTCACTTACTGCCCGCCGT-3′). The Nhe I and Xba I site are underlined in the sense and anti-sense primer, respectively. The purified product was subcloned into pCR2.1. The full length coding sequence of TLM-ODD-hEndoG was assembled by sequentially cloning each of the pCR2.1 generated pieces (pCR2.1-ODD, pCR2.1-L and pCR2.1-hEndoG) into pCR2.1-TLM-L by using the appropriate restriction digests. TLM-ODD-hEndoG was then cloned into a variant of the mammalian expression vector pEF-1α-myc/his (Invitrogen) where the EF-1α promoter had been deleted (ΔEF-1α) and replaced by a HRE-based promoter giving p(ΔEF-1α)5HRE-TLM-ODD-hEndoG.
Figure 1.
Ilustration of the chimeric hEndoG toxic protein and verification of its killing function during transient expression in tumor cells, (a) A linear representation of the chimeric hEndoG construct is depicted. The N-terminus of the fusion protein contains a translocation motif (TLM) that is fused in-frame with a linker (L) that is predicted to be a random coil. An exploded view of this portion of the protein is shown beneath the illustration and provides the amino acid and nucleotide sequence for the novel N-terminus. The underlined nucleotide sequences indicate Xma I and Age I restriction sites. The Xma I site demarcates the junction between the TLM and L. The Age I site provided an in-frame fusion site with the oxygen-dependent degradation domain (ODD). The two proline residues designated 402 and 564 are the positions that are hydroxylated in the native HIF-1α protein. The ODD is followed by another L sequence, which is connected with the mature form of hEndoG. The 435 (b–e), PC-3 (f–i) and MCF-7 (j–m) cells were transiently transfected with the p(ΔEF-1α)-5HRE-TLM-ODD-hEndoG plus pEF-1α tdTomato and grown without CoCl2 (b, c, f, g, j and k) or in the presence of CoCl2 (d, e, h, i, I and m). Most transfected cells were undergoing death, have detached from the substratum, and are thus are seen as floating.
Transient expression of hEndoG in MCF-7, PC-3 and 435 cells and in vitro killing assay
Cells were seeded onto six-well plates at 3 × 105 per well and transfected using MIRUS Trans-IT transfection kit (Mirus Bio Corporation, Madison, WI) according to the manufacturer’s protocol with 1 µg of p(ΔEF-1α)5HRE-TLM-ODD-hEndoG and 250 ng of pEF-1α-tdTomato per well. Expression of tdTomato provided a red fluorescent marker of transfected cells.22 Approximately 24 h later the media was changed and after another 24 h CoCl2 was added (200 µm) to half of the wells. The time course of induced death was followed microscopically.
Generation of stable 435 clones
Transfections were done with the MIRUS Trans-IT transfection kit. Briefly, 3 × 105 435 cells, seeded 24 h prior to transfection in six-well plates, were transfected with 2 µg of plasmid DNA. Following transfections the plates were incubated overnight (16–18 h) and the media changed. Twenty-four hours later the cells were split and plated onto 100 mm dishes at 500, 1000, 2500 and 5000 cells per dish. After 24 h, fresh media supplemented with G418 at a selection concentration of 500 µg ml−1 was added. Approximately 4–5 weeks later, single colonies were expanded, split into duplicates and one of each set was subjected to CoCl2 treatment 2–3 days later. Transgenic clones exhibiting substantial amounts of death at 48–72 h in CoCl2 were selected for further expansion and characterization.
Motility and invasion assay
Matrigel (100 µl; 7–8 mg protein per milliliter) in serum-free medium was added to each well of a Transwell Corning Costar plate and dried overnight in a tissue culture hood. The following day, 2.5 × 104 cells in serum-free medium were pipetted onto the matrigel and complete medium was added to the bottom trough. Following incubation, the transmembrane filter was stained with crystal violet and the number of cells counted. Motility assays were performed similar to the invasion assay, except that matrigel was omitted. All experiments were performed in triplicates.
Wound healing assay
Monolayers of 435 and 435-hEndoG cells grown in 100 mm dishes were scratched or cleared along a diameter of the plate with a sterile pipette tip. Subsequently, the plates were rinsed once with phosphate buffered saline (PBS) and fresh media containing 10% fetal bovine serum was added. Cell migration from the wound/scratch edge was photographed every 24 h using a Nikon TS100 inverted microscope equipped with a CoolSNAP ES camera (Roper Scientific/Photometries, Tucson, AZ). All experiments were performed in triplicates.
Growth rate assays
The 435 and 435-hEndoG (1 × 105 cells) were plated in six-well plates. Twelve hours after seeding, a set of wells of each cell line was washed in PBS, dislodged using 1 % trypsin-EDTA and counted using a hemocytometer. The average number of cells at 12 h (n = 2) was considered as baseline. Duplicates (24 h) and triplicates (48 and 72 h) were harvested, counted and the growth rate curves estimated.
Hypoxic chamber incubations
The 435 and 435-hEndoG cells were seeded at 4.5 × 105 per 60 mm dish in duplicate. Twenty-four hours later the plates were transferred to modular incubators (Billups-Rothenberg, Inc.) and flushed at 3 psi for 3 min with a gas mixture consisting of 1% O2, 94% N2 and 5% CO2. A modular chamber was used for each time point (4, 12, 24 and 48 h). Hypoxic chambers were transferred to a standard 37 °C incubator along with normoxic duplicate plates. Total cellular proteins were extracted at the end of each time point by quickly washing the cells with ice cold PBS and lysing with 100mM Tris, 12% glycerol, 2% SDS pH6.7 buffer solution plus 1:200 dilution of protease inhibitor cocktail (Sigma, St Louis, MO).
SDS-PAGE and immunoblots
Seventy micrograms of total cellular protein was separated on 10% SDS-PAGE (SDS-polyacrylamide gel electrophoresis) and transferred to Hybond-C nitrocellulose (Amersham-GE Healthcare, Piscataway, NJ) membrane. Blots were blocked 1 h in 5 % skim milk TBST and then incubated with 1:3000 dilution of anti-HIF-1α (ODD epitope specific; Chemicon International Inc., Temecula, CA) in 5% BSA-TBST overnight at 4°C. Anti-36B4 was scored as a loading control using in house rabbit anti-36B4 antibody at 1:2000. Blots were then incubated for 1 h at room temperature with 1:2500 dilution of horseradish peroxidase conjugated goat anti-mouse or anti-rabbit (GE Healthcare/Amersham, Piscataway, NJ). Chemiluminescence was generated with the SuperSignal West Pico Chemiluminescent substrate (Pierce, Rockford, IL) and detected on autoradiography film.
Generation of orthotopic xenografts
The SCID mice were maintained and animal experiments were done under NIH and institutional guidelines established for the Animal Core Facility at Johns Hopkins University. 435 or 435-hEndoG cells (4 × 106) in sterile PBS (100 µl) were injected into the second thoracic mammary fat pads of 8-week-old anesthetized mice. Tumor growth was measured weekly and volumes (mm3) calculated as volume = 0.524 × d1 × d2 × d3; where d1, d2 and d3 were the diameters of each of the three axes of the ellipsoidal tumors.
Fluorescent microscopy
Fluorescent photomicrographs were captured on a Nikon TS100 inverted microscope and processed with Image Pro Plus 5.1 (MediaCybernetics, Silver Spring, MD) software.
Statistics
Statistical data analysis was done using the Excel spreadsheet software. A one-tailed students t-test23 provided P-values live 435 versus 435-hEndoG cell populations after CoCl2 treatment and the mean tumor volumes of xenografts.
Results
Inducible expression of hEndoG promotes cell death in cancer cells
A linear representation of the novel therapeutic chimeric protein, hEndoG, is depicted in Figure 1a. The N-terminus has a short24 TLM from the herpes simplex virus, which is fused in-frame to a linker (L). The TLM is 14 amino acids long whereas the linker is 19 amino acids long with a high content of G, S and T. The linker is adapted from a linker-spacer sequence used in the construction of tdTomato.25 Similar sequences have been shown to fold into random flexible conformations that function well as linkers.26,27 To determine if TLM-ODD-hEndoG could function as an inducible killing protein, 435 (Figures 1b–e), PC-3 (Figures 1f–i) and MCF-7 (Figures 1j–m) cells were transiently transfected with the mammalian expression vector p(ΔEF-1α)5HRE-hEndoG and pEF-1α-tdTomato as a marker for transfection efficiency.21,24 Non-transfected parental cell lines served as controls (data not shown). The phase contrast and fluorescence photomicrographs were obtained 72 h after the addition of CoCl2, which stabilizes HIF-1α. In the case of 435 and PC-3 cells, CoCl2 treatment induced cell death as indicated by the floating, rounded up cells (Figures 1d and h). In contrast, the non-treated transfected cells (Figures 1b and f) showed no cell death. Very little toxic effects of CoCl2 were observed in non-transfected cells. In the case of MCF-7 cells, we noticed significant leakiness of the expression of hEndoG even in normoxic conditions (Figures 1j–m). These experiments indicate that our chimeric hEndoG protein, when induced by CoCl2, is functional and can bring about cell death in different cancer cell lines.
Characterizations of stable clones of 435 expressing hEndoG under hypoxic conditions
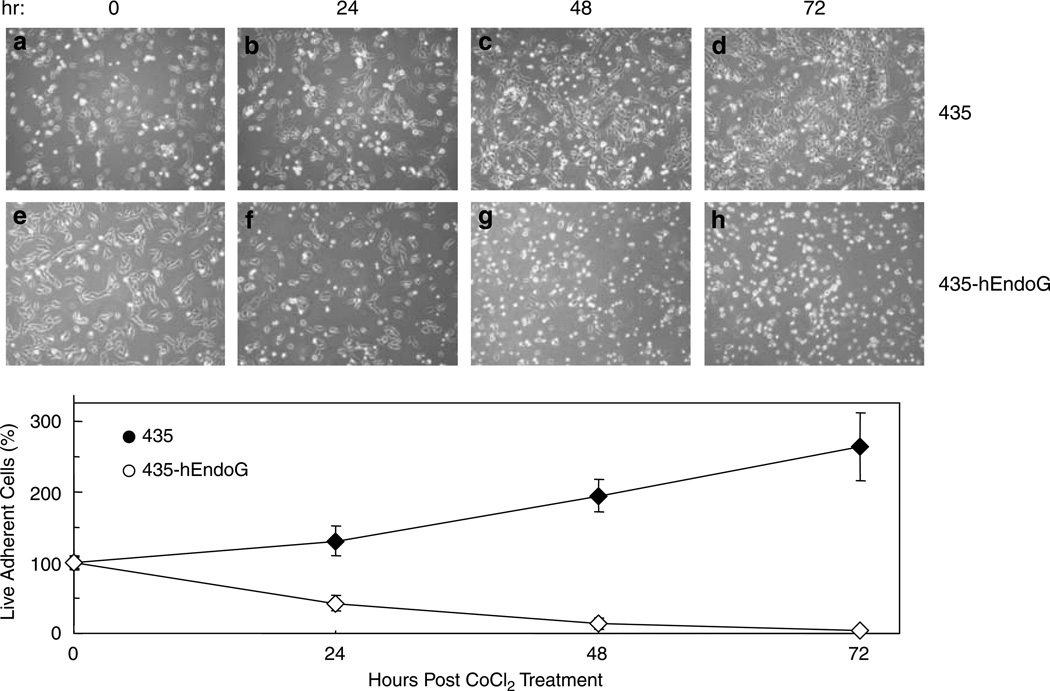
Based on our preliminary results (Figure 1), we generated stable clones of 435 containing hEndoG expression cassette under the regulation of HRE. To determine the longitudinal course of cell death, after CoCl2 addition, phase contrast photomicrographs were obtained at 0, 24, 48 and 72 h. Figure 2 shows a representative experiment with native 435 (Figures 2a–d) and 435-hEndoG cells (Figures 2e–h). Adherent live cell counts were obtained for each time point during different experiments and average cell numbers determined. The zero time points have been scored as 100%. The numbers of live adherent cells at each time point were normalized to these zero time points and the resulting average percentages are shown in the graph of the Figure 2. Thus, substantial death was observed within 24 h of CoCl2 treatment in the transgenic cells and only about 42% of the cells remained adherent at this time. The numbers of dead/dying transgenic cells were greatly increased after 48 h with only an average of 14% cells remaining adherent and cells were nearly completely ablated by 72 h (3.5% adherent). This was in stark contrast to the native cells, which did exhibit some cell death and growth arrest in the presence of CoCl2. Nevertheless, at 72 h native 435 cells increased their numbers by greater-than 2.5 times, that is, a 263 % increase in adherent cells, whereas nearly complete death was observed in the transgenic cell line at this time point.
Figure 2.
Induction of hEndoG in stable 435-hEndoG cells promotes cell death. Representative phase contrast photomicrographs are shown of parental 435 cells (a–d) and a stable clone of 435-hEndoG cells (e–h) obtained post CoCl2 treatment (0, 24, 48 and 72 h). Adherent live cell counts were obtained for each time point during different experiments and average cell numbers determined. The average live cell counts at each time point were normalized to zero time points (100%) as shown in plots of these resulting average percentages (±s.d.; n = 8). The difference between the means at the 24, 48 and 72 h time points was significant as determined by a one-tailed t-test (P-value <6 × 10−7 for each case). Three independent clones were analyzed.
Evaluating the growth potential of 435-hEndoG cells following CoCl2 treatment
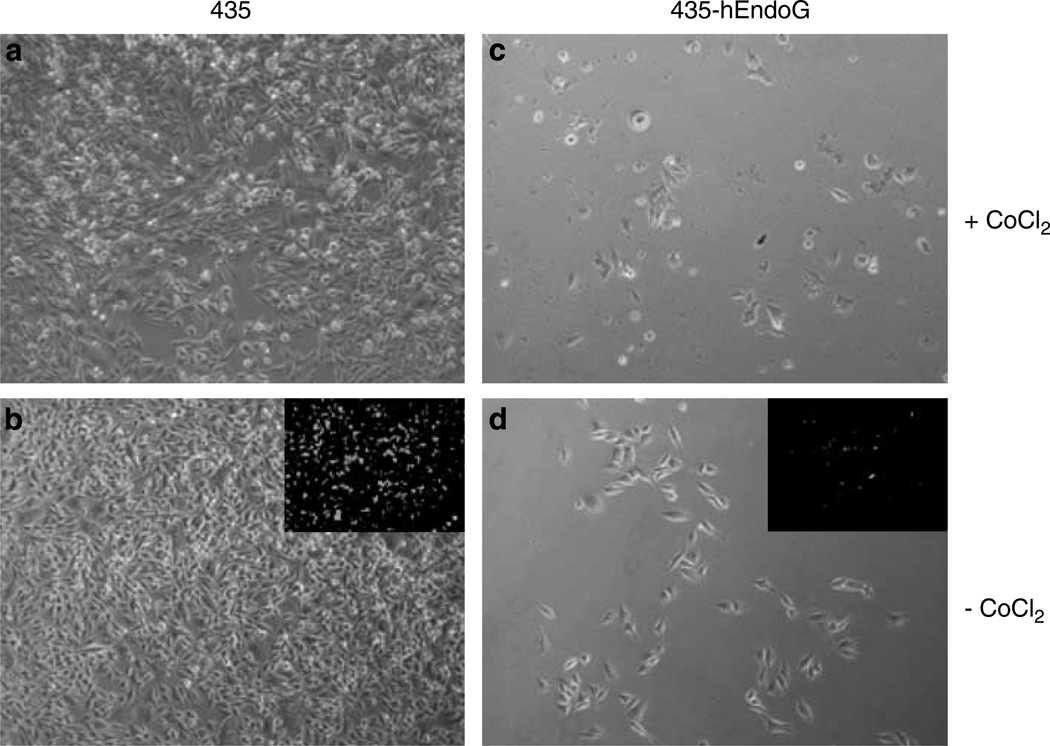
Having established that induced expression of hEndoG brings about cell death, we tested the viability and proliferation capacity of 435-hEndoG cells after CoCl2 treatment. Following treatment of the parental and transgenic cells with CoCl2 for 72 h (Figures 3a and c), the cells were cultured for an additional 5 days in the absence of CoCl2 (Figures 3b and d) and stained with Calcein-AM (insets) to determine cell viability. Although from the Calcein-AM staining all remaining cells appeared to be viable, the transgenic cells exhibited decreased grow rate as seen from the sparse cell density compared to the growth of the parental cells. Thus, under these conditions, it appears that expression of hEndoG induced significant cell damage, which the cells were unable to recover from under normal growth conditions. This supports our hypothesis regarding the toxic effects of hEndoG on cell growth and proliferation.
Figure 3.
Viability of 435 and 435-hEndoG cells following CoCl2 treatment. The 435 (a and b) and 435-hEndoG (c and d) cells were treated with CoCl2 for 72 h. Following CoCl2 incubation, the cells were cultured for 5 days in normal media conditions (b and d) and stained with Calcein-AM (insets) to determine cell viability. As shown, 435-hEndoG cells were unable to completely recover as is evident from the sparse cell density as compared to the 435 cells.
In vitro characterization of 435-hEndoG cells
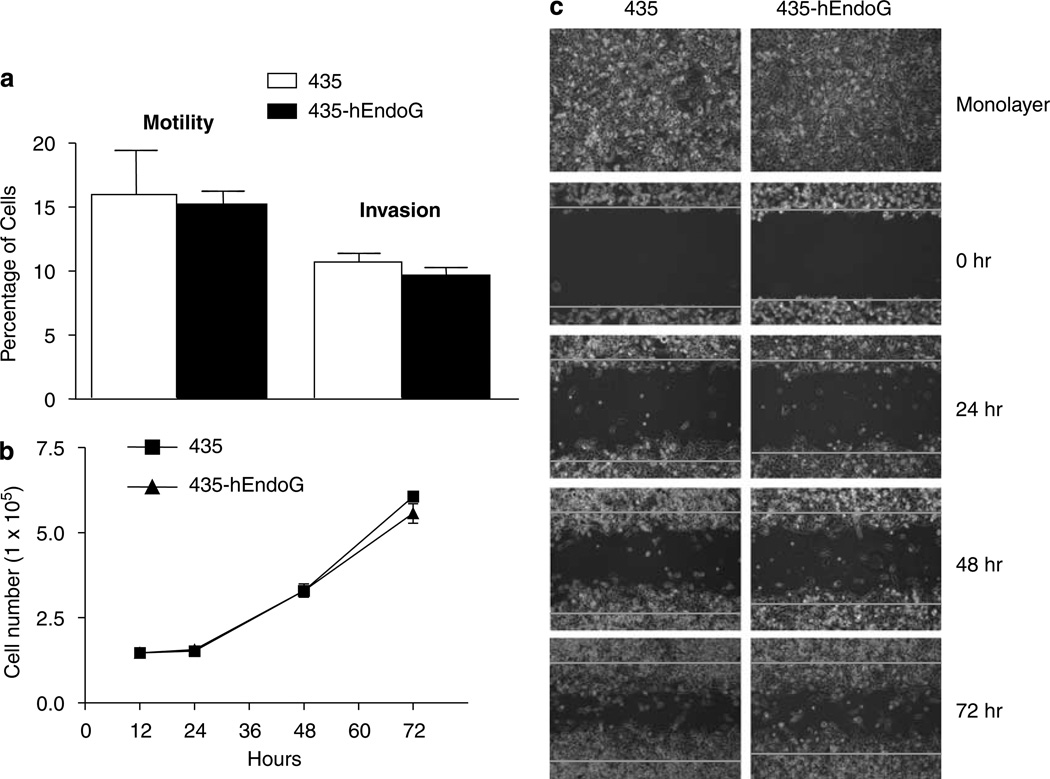
Following the generation of the stable cell lines, we next wanted to eliminate the possibility that integration of the transgene has not phenotypically or genotypically compromised or altered the transgenic cell line. Thus, we characterized 435-hEndoG cells in vitro with respect to growth rate, motility, invasion and wound healing. As shown in Figure 4a, there was little or no difference in either motility or invasive properties of the transgenic cell line as compared to the parental cell line. The same was also true for the growth rate (Figure 4b). In addition, the longitudinal tracking (0, 24, 48 and 72 h) of cell migration and proliferation into the cleared area of the wound healing assay showed no substantial difference (Figure 4c). Thus, the stable integration of the transgene in 435 cells did not affect the motility, invasiveness, growth rate and migration in in vitro conditions.
Figure 4.
In vitro characterization of 435-hEndoG. (a) Bar graphs showing percentage of cells exhibiting motility across a transwell membrane or invasion through Matrigel for 435 (white bars) and 435-hEndoG (black bars). It is seen that the both cell lines had very similar motilities and invasiveness in these assays (n = 3±s.d.). (b) Growth curves (n = 2 for 12 & 24 h and n = 3 for 48 and 72 h ±s.d.) for 435 (filled squares) and 435-hEndoG (filled triangles) shows that these cell lines have identical growth rates, (c) Longitudinal tracking (0, 24, 48 and 72 h) of migration into and filling of a ‘wound’ in a wound healing assay by 435 or 435-hEndoG cells. The top photographs shows that initially both cell lines were nearly confluent. Within each time point photograph, red lines demarcate the boundaries of the introduced wound.
Next, we followed the time course of hEndoG expression, with immunoblots using a monoclonal HIF-1α-ODD antibody, under normoxic (20% O2) and moderately hypoxic (1% O2) conditions. Whole cell protein extracts from 435 and 435-hEndoG cells maintained either under normoxic condition or cultured in moderate hypoxia were prepared at 4, 12, 24 and 48 h post hypoxia. As shown in Figure 5a, hEndoG expression is induced in 435-hEndoG cells following exposure to 1% O2 for 4h and persisted up to 12 h. In a similar manner, 435 and 435-hEndoG cells were cultured either under normal media conditions or in the presence of CoCl2 and genomic DNA was extracted from the cells at 24, 48, 72 and 96 h after CoCl2 treatment. Electrophoresis of genomic DNA on a 0.6% agarose gel revealed that less highly polymerized DNA remained in the wells loaded with DNA prepared from 435-hEndoG cells than in wells loaded with DNA from the parental cells (Figure 5b). This is particularly apparent at DNA isolated at the 72 and 96 h time points. Also, at these later time points, DNA fragmentation as a result of hEndoG overexpression was observable as a smear pattern. In contrast, little or no digested DNA from parental cells was seen. Thus, these results indicate that hEndoG protein is expressed during the first several hours of moderate hypoxia and that during CoCl2 treatment such expression results in degradation of genomic DNA.
Figure 5.
Kinetics of expression of hEndoG and its affect on genomic DNA. (a) The 435 and 435-hEndoG cells were maintained under normoxic condition (20% O2) or cultured in moderate hypoxia (1% O2). Cells were harvested at the indicated time points and whole cell protein extracts prepared. The membrane was scored for HIF-1α and hEndoG with monoclonal anti-HIF-1α-ODD. The ribosomal protein 36B4 was used as a loading control and scored for with an in house generated polyclonal anti-36B4. (b) Following addition of CoCl2, genomic DNA was extracted at 12, 24, 48, 72 and 96 h as well as from untreated control cells. Following electrophoresis in a 0.6% agarose gel, the DNA smear pattern was predominant in 435-hEndoG lanes as compared to the 435 lanes.
435-hEndoG generated tumors show greatly reduced growth rate
Our goal in these studies was to demonstrate that hEndoG expression could hinder tumor growth by eliminating cells that are capable of sustained growth at low oxygen concentrations, that is, hypoxic cells. Thus, we used a mammary fat pad xenograft model in SCID mice to test the tumor growth capabilities of 435-hEndoG cells relative to parental cells. Following injection into the second thoracic mammary fat pads of SCID mice, the tumor volumes were measured (Figure 6). It is seen that at 6 weeks after inoculation the 435-hEndoG derived tumors have a greatly decreased growth in comparison to the 435 tumors. Individual and combined tumor growth rate curves are presented beneath the photographs for each tumor. The combined means are for mice 1–4 except at week 7 where averages of tumor growth on mice 2 and 4 are presented. These growth curves unambiguously demonstrate that throughout the course of the experiment 435-hEndoG tumors grew at a highly significant slower rate in comparison to the growth rate of native 435 derived tumors. Thus, at the end of 6 weeks the mean tumor size of 435-hEndoG was 37 ± 4 versus 135 ± 24mm3 (P-value = 0.0005) for 435 for mice 1–4 derived tumors.
Figure 6.
Stable 435-hEndoG cells exhibit greatly attenuated growth as xenografts. The same animals (1–5) are shown in both photographs, that is, 435 cells were injected into the second mammary fat pad on the left side (ventral side up) whereas 435-hEndoG cells were injected into the contra-lateral mammary fat pad. All mice received 4 × 106 cells per mammary fat pad and photographs were taken 6 weeks later. In all cases, tumors are encircled in yellow, which shows that tumors generated from 435 cells are visibly much larger than tumors generated from 435-hEndoG cells. Growth curves for 435 (filled squares) and 435-hEndoG cells (open squares) generated tumors for each mouse (1–5) are presented beneath the photographs. The far right-hand graph presents mean tumor sizes (error bars are ± s.d.) for mice 1–4 except at week 7 where averages of tumor growth on mice 2 and 4 are presented. One-tailed t-test P-values are: P = 0.0009, P = 0.001, P = 0.01 and P = 0.0005, for weeks 3, 4, 5 and 6, respectively. Neither cell line produced a tumor of substantial size on mouse 5.
Histological comparison of 435 and 435-hEndoG derived tumor
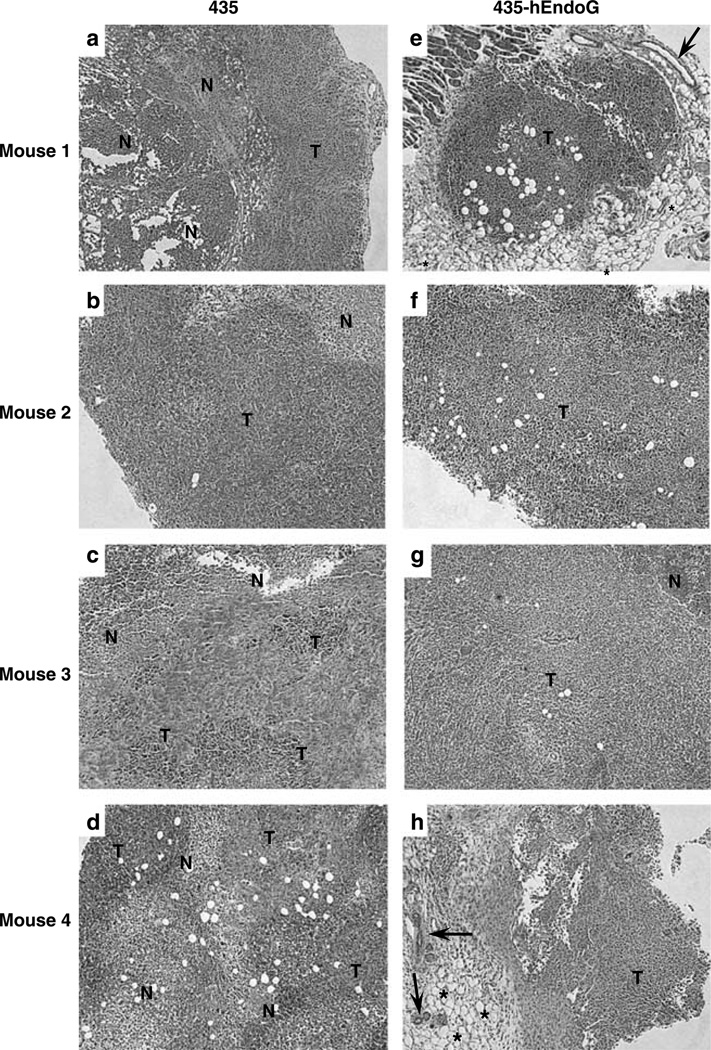
To evaluate the tumor architecture of the generated xenografts, formalin-fixed sections were photographed following hematoxylin and eosin staining. All four 435 tumors show necrotic (N) core surrounded by tumor (T) cells (Figures 7a–d). In contrast, necrotic regions are completely (Figures 7e–h) or largely absent (Figure 7g) from the much smaller 435-hEndoG tumors indicating the possible ablation of hypoxic regions during tumor growth and progression.
Figure 7.
Histological comparison of 435 and 435-hEndoG tumor sections reveals differences in composition. Formalin-fixed 5µm sections of 435 (a–d) and 435-hEndoG (e–h) tumors were hematoxylin and eosin stained. All four 435 tumors are shown to have a necrotic (N) core that is surrounded by tumor (T) cells. In contrast, necrotic regions are completely (e, f and h) or largely (g) absent from the much smaller 435-hEndoG tumors. Arrows in e and h point to residual ductal structures whereas asterisks in these images indicate adipocytic tissue.
Discussion
It has been recognized for sometime that hypoxia has a negative impact on effective cancer therapy regimes. In 1933, Crabtree and Cramer28 reported that tumor slices placed under anaerobic conditions were resistant to radium irradiation as subsequent transplantation of these irradiated slices into mice resulted in tumor growth that matched the non-irradiated controls. These findings allowed them to hypothesize as to why cancers growing in regions of poorly vascularized bone and in anemic patients are resistant to radiation therapy. Subsequently, a large body of evidence has been accrued that establishes hypoxic regions within tumors as being populated by cells that are chemo- and radio-resistant.1,2,29,30
Hence, the design of therapeutic agents that specifically target hypoxic tumor regions has been considered an important cancer therapy. Strategies include radiosensitizers and bioreductive drugs, which are developed on the basis that hypoxic cells have increased reductive potential.1,2 Molecular characterization of the pathways that are specific to hypoxic cells11–13 has lead to the development of gene therapies6–10,31 such as those described here. It is known that stabilization of the α-subunits of the HIF transcription factors, namely HIF-1α and HIF-2α, results in the gene repertoire that is exhibited by cells in response to hypoxia.12 The number of genes reported to be directly regulated by HIF-1α and HIF-2α increases every year.12 In both cases, the gene products are known to provide cells with the means of surviving the periods of low oxygen. This enhanced survival likely promotes malignant transformation and generates the characteristic chemo-and radio-resistance properties that hinder therapeutic intervention.2 The modulation of gene expression by HIF-1α and HIF-2α is mediated by their binding to HREs within the promoter and the 3′ UTR regions of target genes.3,12 The use of synthetic variants of this element, usually as a tandem oligomer, fused to a minimal promoter has now been extensively reported for hypoxia-inducible transgene expression.3,8 For instance, reporter genes such as luciferase31 and green fluorescence protein20 as well as therapeutic genes such as p53 tumor suppressor6 and herpes simplex thymidine kinase5 genes have been targeted for expression in hypoxic regions of tumors by using HRE-based expression systems. An additional means of modulating transgene expression is based on extensive molecular characterization of HIF-α subunits, which has provided maps of domains that are essential for oxygen-dependent stability and efficient transcriptional activity.32,33 Thus, it is now possible to utilize this information for tighter conditional regulation of therapeutic gene strategies, which have the goal of generating chimeric toxic proteins with decreased stability under normoxic conditions and conversely increased stability during hypoxia.10
We have previously shown that an HRE-based promoter could be used in conjunction with green fluorescent protein to aid in the mapping of hypoxic regions of solid tumors.20 We have now built on that technology by incorporating the HIF-1α ODD into our version of toxic gene therapy, which is aimed at eliminating hypoxic regions of solid tumors. We have chosen a well-characterized naturally occurring human endonuclease as the toxic protein so as to have decreased toxicity and immune response complications in translation of this technology to the clinic. hEndoG has been shown to digest both single strand RNA as well as DNA and functions during apoptosis.14–19 It is normally sequestered between the inner and outer membranes of the mitochondria and is released with other apoptotic factors during apoptosis. In addition, we have incorporated a TLM21 into the N-terminus of our chimeric protein to provide a possible bystander capability that can be tested in future applications.
The experiments presented here demonstrate that incubation of transiently transfected cells with CoCl2, which stabilizes HIF-1α, induced stable expression of hEndoG resulting in cell killing within a 72-h period. Similar results were obtained using stable clones of 435 containing hEndoG under the control of HRE. Incorporation of stable expression of hEndoG into 435 cells was not detrimental to the normal cellular functions as determined by motility, invasion and growth rate assays. In addition, following release from CoCl2, hEndoG expressing cells remained compromised as they were unable to recover, indicating that the cytotoxic effects of hEndoG is potent and can have long lasting affects. Most importantly, 435-hEndoG-generated xenografts exhibited a greatly reduced growth rate, which provides very strong evidence to the therapeutic effects of hEndoG.
One advantage of our approach is that hEndoG is inherently cytotoxic and requires no additional administrations of prodrug as do those suicide gene therapy approaches that are based on herpes simplex virus thymidine kinase or bacterial cytosine deaminase expression. Therapies that require subsequent injections of prodrug are complicated by a number of factors such as dilution affects, the adequate biodistribution of prodrug throughout the tumor and the pharmacokinetics, that is, optimal administration timing.34–36 An additional advantage is that hEndoG will likely not elicit an immune response as it is a naturally occurring human protein.
Thus, the chimeric hEndoG expression system has the potential to be developed as a gene-based-therapy system to ablate hypoxic regions in patient tumors. Our future studies will concentrate on the development of hEndoG into a robust adjuvant cancer therapy that will be formulated using a viral vector or liposomal-based delivery system.
Acknowledgements
This work was supported by NIH Grant P50 CA103175.
References
- 1.Sartorelli AC. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Res. 1988;48:775–778. [PubMed] [Google Scholar]
- 2.Ahn GO, Brown M. Targeting tumors with hypoxia-activated cytotoxins. Front Biosci. 2007;12:3483–3501. doi: 10.2741/2329. [DOI] [PubMed] [Google Scholar]
- 3.Boast K, Binley K, Iqball S, Price T, Spearman H, Kingsman S, et al. Characterization of physiologically regulated vectors for the treatment of ischemic disease. Hum Gene Ther. 1999;10:2197–2208. doi: 10.1089/10430349950017185. [DOI] [PubMed] [Google Scholar]
- 4.Binley K, Iqball S, Kingsman A, Kingsman S, Naylor S. An adenoviral vector regulated by hypoxia for the treatment of ischaemic disease and cancer. Gene Ther. 1999;6:1721–1727. doi: 10.1038/sj.gt.3301001. [DOI] [PubMed] [Google Scholar]
- 5.Koshikawa N, Takenaga K, Tagawa M, Sakiyama S. Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res. 2000;60:2936–2941. [PubMed] [Google Scholar]
- 6.Zhao HC, Zhang Q, Yang Y, Lu MQ, Li H, Xu C, et al. p53-expressing conditionally replicative adenovirus CNHK500-p53 against hepatocellular carcinoma in vitro. World J Gastroenterol. 2007;13:683–691. doi: 10.3748/wjg.v13.i5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Harada H, Ogura M, Shibata T, Hiraoka M. Adenovirus-mediated hypoxia-targeting cytosine deaminase gene therapy enhances radiotherapy in tumour xenografts. Br J Cancer. 2007;96:1871–1878. doi: 10.1038/sj.bjc.6603812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong CH, Lee YM, Choi KS, Seong YR, Kim YJ, Im DS, et al. Hypoxia-responsive element-mediated soluble Tie2 vector exhibits an anti-angiogenic activity in vitro under hypoxic condition. Int J Oncol. 2005;26:211–216. [PubMed] [Google Scholar]
- 9.Harada H, Kizaka-Kondoh S, Hiraoka M. Mechanism of hypoxia-specific cytotoxicity of procaspase-3 fused with a VHL-mediated protein destruction motif of HIF-1alpha containing Pro564. FEBS Lett. 2006;580:5718–5722. doi: 10.1016/j.febslet.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Koshikawa N, Takenaga K. Hypoxia-regulated expression of attenuated diphtheria toxin A fused with hypoxia-inducible factor-1alpha oxygen-dependent degradation domain preferentially induces apoptosis of hypoxic cells in solid tumor. Cancer Res. 2005;65:11622–11630. doi: 10.1158/0008-5472.CAN-05-0111. [DOI] [PubMed] [Google Scholar]
- 11.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- 12.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 13.Tan M, Gu Q, He H, Pamarthy D, Semenza GL, Sun Y. SAG/ROC2/RBX2 is a HIF-1 target gene that promotes HIF-la ubiquitination and degradation. Oncogene. 2008;27:1404–1411. doi: 10.1038/sj.onc.1210780. [DOI] [PubMed] [Google Scholar]
- 14.Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz-Carrillo A, et al. Structural and functional characterization of mitochondrial EndoG, a sugar non-specific nuclease which plays an important role during apoptosis. J Mol Biol. 2004;338:217–228. doi: 10.1016/j.jmb.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 15.van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, Gevaert K, et al. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 2001;8:1136–1142. doi: 10.1038/sj.cdd.4400944. [DOI] [PubMed] [Google Scholar]
- 16.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 17.Basnakian AG, Apostolov EO, Yin X, Abiri SO, Stewart AG, Singh AB, et al. Endonuclease G promotes cell death of non-invasive human breast cancer cells. Exp Cell Res. 2006;312:4139–4149. doi: 10.1016/j.yexcr.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida A, Pommier Y, Ueda T. Endonuclease activation and chromosomal DNA fragmentation during apoptosis in leukemia cells. Int J Hematol. 2006;84:31–37. doi: 10.1007/BF03342699. [DOI] [PubMed] [Google Scholar]
- 19.Hamada M, Sumi T, Iwai S, Nakazawa M, Yura Y. Induction of endonuclease G-mediated apopotosis in human oral squamous cell carcinoma cells by protein kinase C inhibitor safingol. Apoptosis. 2006;11:47–56. doi: 10.1007/s10495-005-3348-z. [DOI] [PubMed] [Google Scholar]
- 20.Raman V, Artemov D, Pathak AP, Winnard PT, Jr, McNutt S, Yudina A, et al. Characterizing vascular parameters in hypoxic regions: a combined magnetic resonance and optical imaging study of a human prostate cancer model. Cancer Res. 2006;66:9929–9936. doi: 10.1158/0008-5472.CAN-06-0886. [DOI] [PubMed] [Google Scholar]
- 21.Hillemann A, Brandenburg B, Schmidt U, Roos M, Smirnow I, Lemken ML, et al. Protein transduction with bacterial cytosine deaminase fused to the TLM intercellular transport motif induces profound chemosensitivity to 5-fluorocytosine in human hepatoma cells. J Hepatol. 2005;43:442–450. doi: 10.1016/j.jhep.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Winnard PT, Jr, Kluth JB, Raman V. Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia. 2006;8:796–806. doi: 10.1593/neo.06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havlicek LL, Crain RD. Practical Statistics for the Physical Sciences. Washington, DC: American Chemical Society; 1988. [Google Scholar]
- 24.Oess S, Hildt E. Novel cell permeable motif derived from the PreS2-domain of hepatitis-B virus surface antigens. Gene Ther. 2000;7:750–758. doi: 10.1038/sj.gt.3301154. [DOI] [PubMed] [Google Scholar]
- 25.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange, yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 26.Robinson CR, Sauer RT. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci USA. 1998;95:5929–5934. doi: 10.1073/pnas.95.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai R, Wriggers W, Nishikawa Y, Nagamune T, Fujisawa T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins. 2004;57:829–838. doi: 10.1002/prot.20244. [DOI] [PubMed] [Google Scholar]
- 28.Crabtree HG, Cramer W. The action of radium on cancer cells II. – Some factors determining susceptibility of cancer cells to radium. Proc Roy Soc Lon B. 1933;113:238–250. [Google Scholar]
- 29.Gray LH. Radiobiologic basis of oxygen as a modifying factor in radiation therapy. Am J Roentgen, Rad Therapy, Nuc Med. 1961;85:803–815. [PubMed] [Google Scholar]
- 30.Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metast Rev. 1987;5:313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- 31.Cowen RL, Williams KJ, Chinje EC, Jaffar M, Sheppard FCD, Telfer BA, et al. Hypoxia targeted gene therapy to increase the efficacy of tirapazamine as an adjuvant to radiotherapy: reversing tumor radioresistance and effecting cure. Cancer Res. 2004;64:1396–1402. doi: 10.1158/0008-5472.can-03-2698. [DOI] [PubMed] [Google Scholar]
- 32.Harada H, Kizaka-Kondoh S, Itasaka S, Shibuya K, Morinibu A, Shinomiya K, et al. The combination of hypoxia-response enhancers and an oxygen-dependent proteolytic motif enables real-time imaging of absolute HIF-1 activity in tumor xenografts. Biochem Biophys Res Commun. 2007;360:791–796. doi: 10.1016/j.bbrc.2007.06.149. [DOI] [PubMed] [Google Scholar]
- 33.Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002;62:2013–2018. [PubMed] [Google Scholar]
- 34.Burgos JS, Rosol M, Moats RA, Khankaldyyan V, Kohn DB, Nelson MD, Jr, et al. Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. BioTechniques. 2003;34:1184–1188. doi: 10.2144/03346st01. [DOI] [PubMed] [Google Scholar]
- 35.Ignowski JM, Schaffer DV. Kinetic analysis and modeling firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotech Bioeng. 2004;86:827–834. doi: 10.1002/bit.20059. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Winnard PT, Jr, Takagi T, Artemov D, Bhujwalla ZM. Multimodal image-guided enzyme/prodrug cancer therapy. J Am Chem Soc. 2007;128:15072–15073. doi: 10.1021/ja066199i. [DOI] [PubMed] [Google Scholar]