Abstract
Objectives
The soluble receptor for advanced glycation end products (sRAGE) has been implicated in the development of diabetes-related vascular complications, but the variability of concentrations of sRAGE in the blood is unknown. The objective of this study was to characterize within-person three-year variability of plasma levels of sRAGE.
Methods
We measured sRAGE in plasma samples from 179 men and women in the community-based Atherosclerosis Risk in Communities (ARIC) Study at two time points, three years apart. We calculated correlation coefficients and the within-person coefficient of variation (CVw) to characterize variability in sRAGE. We compared these estimates to total cholesterol and white blood cell count (WBC) in the same participants.
Results
Mean sRAGE concentrations at the two time points (mean time between measurements = 2.9 years) were 1096.2 pg/mL and 990.2 pg/mL, respectively (mean difference = −106.0 pg/mL, p-value < 0.001). The Pearson’s correlation was 0.78 (Spearman’s, 0.73). The intra-class correlation coefficient was 0.76 and the CVw was 26.6%. Compared to sRAGE, Pearson’s and Spearman’s correlations for total cholesterol (0.76 and 0.77) and white blood cell count (0.61 and 0.72) were similar, although CVw for both were lower (8.7% for cholesterol, 15.6% for WBC). Less than 4% of participants’ values changed substantially (50% or greater) over the three-year interval.
Conclusions
We observed that sRAGE concentrations remained relatively stable over three years. Our findings suggest that a single measure of circulating sRAGE tracks well in a community-based population and could be a useful measure in clinical and epidemiologic studies of long-term risk.
Keywords: sRAGE, Advanced glycation end products, reliability and validity, biological markers
INTRODUCTION
Advanced glycation end products (AGEs) are a group of compounds hypothesized to contribute to the pathogenesis of vascular disease, particularly in diabetic populations(1, 2). The receptor for advanced glycation end products (RAGE), found on endothelial and inflammatory cell surfaces, binds to circulating AGEs in the body, activating a pro-inflammatory protein cascade that contributes to oxidative stress, vascular inflammation, atherosclerosis, and neurological disease(3, 4). The truncated, soluble form of RAGE (sRAGE) is believed to counteract the damaging effects of cellular RAGE, acting as a “sponge” for AGEs without propagating deleterious cellular signaling. Given the association between AGEs and sRAGE with long-term outcomes in individuals without and with diabetes, there is growing interest in measuring and studying sRAGE in clinical and epidemiologic studies (5).
Samples obtained at a single time point are often the basis of large research studies, yet the degree to which concentrations of sRAGE track in individuals over time is unknown; understanding temporal changes in sRAGE concentrations has important implications for the use of single blood measurements in research and the interpretation of sRAGE concentrations as a risk factor for clinical outcomes. Thus, the purpose of this study was to quantify the three-year within-person variability of sRAGE in a community-based study of U.S. adults.
MATERIALS AND METHODS
Population
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based cohort study of 15,792 adults from four U.S. communities aged 45–64 years at enrollment during 1987–1989(6). Participants completed three follow-up examinations, spaced approximately three years apart; a fourth follow-up exam is ongoing (2011–2013). Details regarding the participant selection process and data collection methods have been previously published (6). As part of two ancillary studies, sRAGE was available for a small subsample of ARIC participants. All participants were free of diabetes (based on previous diagnosis, medication use, or measured fasting glucose ≥ 126 mg/dL) at baseline and had an glomerular filtration rate >60 mL/min/1.73 m2. sRAGE was measured in EDTA plasma samples collected at the first two ARIC visits (approximately three years apart) and stored at −70°C until thawed for analysis. The present study included the subset of 179 participants with sRAGE measured at both examinations; individuals with prevalent diabetes and chronic kidney disease were excluded. An institutional review board at each study site approved all study procedures and participants provided written informed consent.
Lab Measures
sRAGE was measured by enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN) in stored plasma samples at the Baylor College of Medicine in accordance with the manufacturer’s instructions; samples were stored at −70°C until analysis. The detection limit of the assay was 25 pg/mL. The intra-assay coefficients of variation based on 20 separate measurements were 6.2%, 4.8% and 6.1% at mean sRAGE concentrations of 546, 1527, and 3117 pg/mL, respectively. The inter-assay coefficients of variations based on 40 repeated measurements were 8.2%, 8.2% and 6.7% at mean concentrations of 519, 1449 and 2890 pg/mL, respectively. The stability and reliability of sRAGE measurement in stored samples has been documented previously, where it was documented a mean recovery of greater than 98% after three freeze-thaw cycles (7). All samples for visit 1 and visit 2 were performed in the same laboratory at Baylor College of Medicine; a total of 31 independent runs were performed for visit 1 data and 72 independent runs were performed for visit 2 data.
Lipids were measured using enzymatic methods and white blood cell count was determined using automated particle counters within twenty-four hours of venipuncture as part of the original ARIC protocols (6). Participant demographic information was obtained using standard procedures describe previously (6).
Analyses
To characterize the three-year within-person variability in sRAGE, we calculated the within-person coefficient of variation (CVw) as we asll the Pearson’s, Spearman’s, and intra-class correlation (ICC) coefficients to compare the measurements at the two time points. The ICC was calculated as:
where represented the between-subject variability and represented variability due to random measurement error.
Scatterplots allowed for visual inspection of the concordance between the two measurements. For comparison, we also calculated indices of within-person variability for total cholesterol and white blood cell count, two common clinical tests that were also measured in these same participants. All analyses were performed using Stata, Version 12.1 (College Station, TX: StataCorp LP).
RESULTS
The mean age of participants at the first visit was 55 years, 65% were female, and 62% were white (Supplemental Table 1). Approximately 6% of participants had prevalent coronary heart disease. The mean time between visits was 2.9 years (range: 2.6–4.0 years). sRAGE values ranged from 193.1–2059.0 pg/mL, with a mean at visit 1 of 1096.2 pg/mL (standard deviation: 625.8 pg/mL) and at visit 2 of 990.2 (standard deviation: 559.0 pg/mL) (Table). The mean change from visit 1 to visit 2 was −106.0 pg/mL (standard deviation: 395.4 pg/mL; p < 0.001).
Table.
Summary statistics comparing plasma sRAGE (pg/mL) at two time points approximately three years apart (n=179).
| Visit 1 (1987 to 1989) | Visit 2 (1990 to 1992) | Difference (Visit 2 - Visit 1) | |
|---|---|---|---|
|
|
|
||
| Mean (SD) | 1096.2 (625.8) | 990.2 (559.0) | −106.0 (395.4)* |
| Range | 225.9 to 4286.7 | 193.1 to 4059.0 | −1961.5 to 869.6 |
| Median [IQR] | 950.7 [690.8, 1311.2] | 867.3 [598.0, 1199.1] | −96.1 [−286.6, 124.1] |
SD = standard deviation. IQR = interquartile range.
p-for-difference=0.0004.
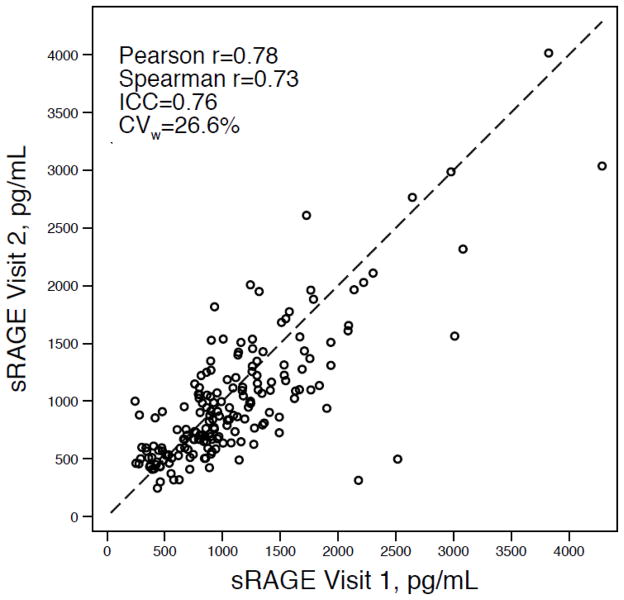
The Pearson correlation between the two sRAGE measurements was 0.78, the Spearman correlation was 0.73, and the ICC was 0.76. The CVw was 26.6% (95% CI: 22.5 – 30.2%) (Figure). Fifty-eight percent of participants (n=103) had values that changed less than 25% over the three-year period; 25% (n=44) had values that changed less than 10%. Three percent of participants’ values changed 50% or greater from visit 1 to visit 2. In same participants, the Pearson’s and Spearman’s correlations for total cholesterol were 0.76 and 0.77, respectively. The ICC for total cholesterol was 0.76 and the CVw was 8.7%. For white blood cell count, the Pearson’s correlation was 0.61 and the Spearman’s correlation was 0.77; the ICC was 0.76. The CVw for white blood cell count was 15.6%. For both total cholesterol and white blood cell count, the change in mean values over the three-year period was not significant (p-values > 0.05).
Figure.
Scatterplot of plasma sRAGE measured at two time points approximately three years apart, n=179.
DISCUSSION
sRAGE has been implicated in the development of diabetic vascular disease (3) and may be a useful biomarker for identifying individuals at increased risk for disease. Recent investigations have reported an inverse association of circulating concentrations of sRAGE with clinical outcomes including coronary heart disease and mortality in both diabetic and non-diabetic populations (5, 8).
The use of plasma biomarkers in clinical practice and research studies depends on their reliability. Our results demonstrate that sRAGE tracks over time and estimates of the within-person variability in sRAGE were comparable to other commonly used biomarkers. For example, in the Framingham Heart Study where two measures of glycated hemoglobin—a measure of chronic hyperglycemia—were obtained four to six years apart, the ICC was 0.59 (9). In our study, we found that the correlation coefficients for sRAGE were similar to those for total cholesterol and white blood cell count, but the coefficient of variation for sRAGE was somewhat higher than these other biomarkers. We also observed a small but significant overall decline in sRAGE, which may reflect age- or disease -related declines. Additionally, this decline could be partially explained by changes in factors such as lifestyle behaviors or medication use. However, we cannot rule out the possibility that this decline reflects laboratory drift or a batch effect (since samples from the two time points were measured at different times), though all lab methods remained consistent across visits.
Over the three-year time period between measurements, while we observed an overall decline in mean sRAGE concentration, concentrations of sRAGE within individuals were in most cases stable. Our results support the notion that sRAGE reflects chronic disease processes and that a single measurement of sRAGE can successfully rank individuals in the population for calculating measures of association in clinical and epidemiologic studies. As our measurements of sRAGE were spaced approximately three years apart, we cannot generalize our findings to longer-term within-person variations in sRAGE or characterize potential acute fluctuations. Our study is strengthened by the rigorous collection and storage protocols of the ARIC Study and the use of identical methods and materials at the same laboratory for the two sRAGE measurements.
Low circulating sRAGE may be a useful biomarker of vascular damage in individuals with hyperglycemia (1–3, 10). While further data are needed regarding the association of sRAGE with clinical outcomes, our data suggest that a single measure of circulating sRAGE may be sufficient for characterizing risk in the general population.
Supplementary Material
Highlights.
The soluble receptor for advanced glycation end products (sRAGE) has been implicated in the development of diabetes-related vascular complications.
The within-person variability of concentrations of sRAGE in the blood is unknown.
We characterized within-person three-year variability of plasma levels of sRAGE.
sRAGE concentrations remained relatively stable over three years.
A single measure of circulating sRAGE tracks well in a community-based population and could be a useful measure in clinical and epidemiologic studies of long-term risk.
Acknowledgments
This research was supported by NIH/NIDDK grant R01 DK076770 and a grant from the American Heart Association to Dr. Selvin, as well as NIH/NIDDK grant R01 DK056918 to Dr. Pankow. Dr. Bower was supported by NIH/NHLBI T32HL007024 Cardiovascular Epidemiology Training Grant. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 2.Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones. 2005;4(1):28–37. doi: 10.14310/horm.2002.11140. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. Journal of internal medicine. 2002;251(2):87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 4.Thornalley PJ. Dietary AGEs and ALEs and risk to human health by their interaction with the receptor for advanced glycation endproducts (RAGE)--an introduction. Molecular nutrition & food research. 2007;51(9):1107–10. doi: 10.1002/mnfr.200700017. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Halushka M, Rawlings A, Hoogeveen RC, Ballantyne CM, Coresh J, et al. sRAGE and risk of diabetes, cardiovascular disease and death. Diabetes. 2013 doi: 10.2337/db12-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The ARIC Investigators. The Atherosclerosis Risk on Communities (ARIC) Study: design and objectives. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 7.Wittwer C, Lehner J, Fersching D, Siegele B, Stoetzer OJ, Holdenrieder S. Methodological and Preanalytical Evaluation of a RAGE Immunoassay. Anticancer Research. 2012;32(5):2075–8. [PubMed] [Google Scholar]
- 8.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, et al. Plasma Levels of Soluble Receptor for Advanced Glycation End Products and Coronary Artery Disease in Nondiabetic Men. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(5):1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 9.Meigs JB, Nathan DM, Cupples LA, Wilson PWF, Singer DE. Tracking of glycated hemoglobin in the original cohort of the Framingham Heart Study. Journal of clinical epidemiology. 1996;49(4):411–7. doi: 10.1016/0895-4356(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.