Abstract
Chronic alcohol consumption results in bone loss through increased bone resorption and decreased bone formation. These effects can be reversed by estradiol (E2) supplementation. Soy diets are suggested to have protective effects on bone loss in men and women, as a result of the presence of soy protein-associated phytoestrogens such as genistein (GEN). In this study, male mice were pair-fed (PF) a control diet, an ethanol (EtOH) diet or EtOH diet supplemented with 250 mg/kg of GEN for 8 weeks to test if GEN protects against bone loss associated with chronic drinking. Interestingly, alcohol consumption reduced cortical area and thickness, and trabecular bone volume in both EtOH and EtOH/GEN groups when compared to the corresponding PF and PF/GEN controls, p<0.05. However, in the trabecular bone compartment, we observed a significant increase in overall trabecular bone density in the PF/GEN group compared to the PF controls. Bone loss in the EtOH-treated mice was associated with inhibition of osteoblastogenesis as indicated by decreased alkaline phosphatase staining in ex vivo bone marrow cultures, p<0.05. GEN supplementation improved osteoblastogenesis in the EtOH/GEN cultures compared to the EtOH group, p<0.05. Vertebral expression of bone formation markers, osteocalcin, and runt-related transcription factor 2 (Runx2), were also significantly up-regulated in the PF/GEN and EtOH/GEN groups compared to the PF and EtOH-treated groups. GEN supplementation also increased expression of receptor activator of nuclear factor κ-B ligand (RANKL) in the PF/GEN, an increase that persisted in the EtOH/GEN-treated animals (p<0.05) and increased basal hydrogen peroxide production and RANKL mRNA expression in primary bone marrow cultures in vitro, p<0.05. These findings suggest GEN supplementation increases overall bone remodeling, and in the context of chronic alcohol consumption, does not protect against the oxidative stress associated EtOH-mediated bone resorption.
Keywords: Alcohol, bone loss, genistein, RANKL
Introduction
Osteoporosis increases fracture risk and is a major cause of morbidity and mortality in the United States, costing the health care system billions of dollars every year1. There are many hormones that regulate skeletal growth; however, the most important systemic hormones that maintain normal bone remodeling are estrogens2. Estrogen deficiency leads to an increase in the rate of bone resorption that surmounts bone formation, resulting in a decrease of bone mass3. Although osteoporosis is more common in females, the rate of age-related cortical and trabecular bone loss in men is almost identical to that of women2. Initially, male bone loss studies have focused on androgens and their role in bone metabolism. However, interest in the role of estrogens in male bone metabolism was sparked by the report of a mutated estrogen receptor gene in men with severe osteopenia4. Additionally, other studies have shown that bone loss in men is also due to estrogen deficiency2,5.
Increasing dietary soy intake is a promising, natural approach to treating and/or preventing bone loss related to estrogen deficiency. It has been reported that osteoporosis related fracture injury is significantly lower in Southern and Eastern Asian countries, and a possible explanation for this may be their high consumption of soy products6. Genistein (GEN) is a soy isoflavones that is structurally similar to 17-β-estradiol (E2) and have been shown to be estrogenic under certain physiological conditions7. For example, in a 24-month study, Marini et al demonstrated that osteopenic post-menopausal women who were give daily genistein experienced increased bone mineral density at the anteroposterior lumbar and the femoral neck8. Moreover, in older men, Newton et al. has demonstrated a net gain in both spinal and hip bone mineral density following daily supplementation of daily isoflavones, 45.6 mg GEN, 31.7 mg daidzein, for 12-months9. Despite the evident beneficial effects, the mechanisms underlying GEN’s action on the skeleton remain unclear.
Estrogen affects bone through both osteoblast and osteoclast activities; the actions are mediated through the estrogen receptor isoforms, α and β, that are present in men and women, and the effects of estrogen on bone differ in the stages of osteoblast differentiation10,11. Estrogen also suppresses osteoclast differentiation by inhibiting receptor activator of nuclear factor k-B ligand (RANKL)-RANK signaling through up regulation of osteoprotegerin (OPG) expression in osteoblasts and osteocytes11. Clinically, GEN has been shown to prevent bone loss and induce a net gain of bone mass in estrogen deficient post-menopausal women by decreasing bone resorption and increased bone formation, which have been attributed to GEN’s estrogenic properties8. However, GEN also has additional biological effects that are independent of estrogen. For example, GEN is a potent tyrosine kinase inhibitor, and has been show to stimulates bone cell proliferation independent of estrogen receptor signaling12,13.
Chronic and binge alcohol consumption in early adulthood is known to produce bone loss as a result of suppression of osteoblastogenesis and induction of osteoclast differentiation and activation14-17. As a result, chronic drinkers have an increased risk for age-related osteoporotic fractures18,19. Previously, we have demonstrated that estradiol supplementation during chronic ethanol (EtOH) feeding, completely reverses EtOH-mediated bone loss through suppression of RANKL-stimulated osteoclastogenesis and activation20,21. Since GEN has been suggested to have estrogenic effects, we hypothesized that GEN supplementation during EtOH feeding will also prevent alcohol-mediated bone loss by promoting osteoblastogenesis and decreasing osteoclastogenesis and activity.
Methods
Animals and experimental design
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Mice were housed in an Association Assessment and Accreditation of Laboratory Animals Care approved animal facility. Six-week-old C57BL/6 male mice (Jackson Laboratories, Bar Harbor, ME) were randomly assigned to 4 weight-matched groups (n=10/group), a 28% EtOH Lieber-DeCarli liquid diet (EtOH) and a corresponding pair-fed group (PF), a 28% EtOH Lieber-DeCarli diet supplemented with GEN (EtOH/GEN), and corresponding PF/GEN control group. The supplemented diets contained 250 mg/kg of the soy-isoflavone, GEN(Indofine Chemical Company, Inc, Hillsborough, NJ), which is comparable to GEN concentrations found in diets containing soy protein isolate as the sole source of protein used previously to stimulate bone growth in rats compared to animals fed casein-based diets22,44. In rodents, equol, a daidzein metabolite, is the major serum isoflavone circulating after soy protein isolate diets23. However, equol production in humans is variable because of inter-individual differences in gut microflora metabolism24. Therefore, in this experimental design we chose to focus solely on GEN effects and not the combination of GEN and daidzein supplementation. For the EtOH groups, EtOH was added to the Lieber-DeCarli diet by substituting carbohydrate calories with EtOH calories (Dyets#710260). The EtOH concentration in the diet was increased slowly in a stepwise manner until 28% total calories were reached, which constitutes a final EtOH concentration of 5.0 % (v/v) as previously described16. The PF group received the Lieber-DeCarli control liquid diet (Dyets#710027) and the PF/GEN group received the same control diet supplemented with GEN. Both PF groups were isocalorically fed to their corresponding EtOH group based on the diet consumptions of the previous day. EtOH and PF diets were administered for 60 d. At sacrifice, trunk blood was collected, vertebra were frozen and stored at −80°C, and right tibial bones were formalin fixed for μCT analysis. Femurs were harvested, and bone marrow was used in ex vivo osteoblast cultures. Blood EtOH concentrations were analyzed using an Analox analyzer as previously described16.
Micro computed Tomography (μCT)
Formalin-fixed tibiae were imaged using a μCT 40 (Scanco Medical AG, Bassersdorf, Switzerland) using a 12 μm isotropic voxel size in all dimensions. Fractional bone volume (bone volume/tissue volume; BV/TV) and architectural properties of trabecular bone, Thickness, Number, and Spacing were calculated using previously published methods16. For cortical bone assessment, μCT slices were segmented into bone and marrow regions by applying a visually chosen, fixed threshold for all samples after smoothing the image with a three-dimensional Gaussian low-pass filter (σ = 0.8, support = 1.0) to remove noise, and a fixed threshold (30% of maximal gray scale value). Cross-sectional area, total diameter, cortical thickness, medullary area, periosteal perimeter, and endocortical perimeter were also calculated as described previously16. All μCT analyses were consistent with current guidelines for the assessment of bone microstructure in rodents using micro-computed tomography25.
Ex vivo osteoblast cell cultures
Bone marrow cells were harvested from the left femur of PF, PF/GEN, EtOH and EtOH/GEN treated mice and plated for osteoblast differentiation as previously described26. Briefly, primary bone marrow cells, at a concentration of 2X106 cells, were plated in quadruplicate in 6-well plates, and cultured in osteoblastic media (αMEM supplemented with 10% FBS and 1mM L-ascorbic acid 2-phosphate for 10d, then stained using a leukocyte alkaline phosphatase kit according to manufacturer’s protoco1 (Sigma-Aldrich). In a separate experiment, bone marrow cells were harvested from femurs of WT, 6-week-old male mice to generate in vitro primary bone marrow cultures for osteoblast differentiation. Bone marrow cultures were plated at a concentration of 1X106 cells in 24-well plates, cultured in osteoblastic medium supplemented with a physiologically relevant concentration of GEN (200 nM) for 10d, and stained for alkaline phosphatase. In both experiments, alkaline phosphatase-stained pre-osteoblasts were counted under a microscope at 20X magnification.
Real-time RT PCR analysis
Vertebral bone (L3) taken from PF, EtOH, PF/GEN and EtOH/GEN animals were homogenized in 1 ml of TRI reagent (MRC, Cincinnati OH) using a Precellys homogenizer (Bertin Technologies, Rockville, MD). Total RNA was extracted from vertebral bone using the TRI reagent as previously described27. Gene expression of bone turnover markers was assessed by real time RT-PCR. Bone formation markers included osteocalcin, a well described marker for osteoblast activity16, and Runx2 which is essential transcription factor necessary for osteoblast differentiation and bone formation28. Osteoblasts modulate osteoclast activity through expression of RANKL and its decoy receptor OPG. Thus the ratio of RANKL:OPG is an index of osteoclastic stimuli and activity29. In separate experiments, in vitro primary bone marrow cultures were cultured in osteoblastic medium supplemented with GEN (200 nM) or estradiol (1 nM) at 37°C and 5% CO2 for 10d, at which total RNA was isolated using the RNeasy RNA isolation kit (Qiagen) as per manufacturer’s instructions. All RNA was reverse transcribed using IScript cDNA synthesis (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions, and subsequent real-time PCR analysis was carried out using SYBR green and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Gene expression of bone turnover markers in vertebral bone was quantified using deltaCT method relative to GAPDH and then to PF controls. In cultured cell experiments, gene expression of bone turnover markers was quantified using the deltaCT method relative GAPDH, and then to the appropriate control. Comparisons of the raw CT values did not differ between groups, indicating GAPDH was an appropriate normalizer. Gene specific primers were: osteocalcin F 5’ TTGTGCTGGAGTGGTCTCTATGAC 3’, R 5’CACCCTCTTCCCACA CTGTACA 3’; RANKL F 5’ GGGTTCGACACCTGAATG CT 3’, R 5’ AACTGGTCGGGC AATTCTGG3’Runx2 F 5’ CGGTCTCCTTCCAGGAT GGT 3’, R 5’ GCTTCCGTCAGCGTC AAC A 3’; Osterix F 5’TGCAGCAAATTTGGC GGCTCTA 3’, R 5’ TCCATTGGTGCTTG AGAAGGGA 3’; RANKL F 5’ AACT GGTCGGGCAAT TCTGA 3’, R 5’ GGGTTCGA CACCTGAATGCT 3’; OPG F 5’ AGTCCGTGAAGCAGGAG TG 3’, R 5’ CCATCTGGA CATTTTTTGCAAA 3’ and GAPDH 5’ GTATGACTCGACTCACGGCAAA 3’, R 5’ GGTCTCGCTCCTGGAAGATG 3’
Protein isolation and Western blotting
Total cell lysates were made from L4 vertebra taken from PF-, EtOH-, PF/GEN- and EtOH/GEN-treated mice using ice-cold RIPA buffer plus 1X protease inhibitors (Halt Protease and Phosphatase Inhibitor Cocktail, Thermo Fisher Scientific, Pittsburg, PA) and a Precellys tissue homogenizer (Bertin Corp, Rockville, MD) following standard procedures. Proteins (30 μg) were separated by SDS-polyacrylamide gel electrophoresis using standard methods. Blotted cytosolic proteins were incubated with the anti-RANKL antibody (ab124797, Abcam, Cambridge, MA) or with a polyclonal anti-rabbit antibody recognizing Runx2 (sc-10758, Santa Cruz Biotechnology, Dallas, TX). Primary antibodies were diluted 1:1000 and incubated overnight at 4°C. Secondary antibodies were diluted (1:10,000 to 1:50,000) and incubated at room temperature before chemiluminescense detection using SuperSignal West Pico Chemiluminescense Substrate (Thermo Fisher Scientific, Pittsburg, PA). Protein bands were quantified using a densitometer and band densities were corrected for total protein loaded by staining with 0.1% amido black.
Measuring reactive oxygen species (ROS).
Bone marrow cells were harvested from both femurs of 6-week-old male C57Bl6 mice (n=3) as previously described26. Basal hydrogen peroxide production was measured using the Amplex Red hydrogen peroxide /peroxidase assay (Invitrogen Molecular Probes, Eugene, OR) as per manufacturer’s instructions. Briefly, freshly isolated bone marrow cells were washed twice in Ringer’s solution, and plated (20,000 cells/well) in quadruplicate into wells of a 96-well plate containing 50 μl of Amplex Red reaction buffer (50 μM Amplex red, 0.1 U.ml−1 HRP) with or without 200 nM GEN or 1 nM E2, and incubated at 37°C continuously for 1 hour, at which an absorbance reading (560 nm) was taken. Data is expressed as amount produced (μM), which was corrected for non-specific hydrogen peroxide production by subtracting experimental values from values obtained from control wells not containing cells. Similar results were obtained in a repeat experiment performed in quadruplicate.
Data and Statistical Analysis
Data are presented as means ± SEM. The effect of GEN supplementation, and EtOH and the interaction thereof were determined using two-way ANOVA, followed by Student Newman-Keuls post hoc analysis. Comparisons between two or multiple groups were accomplished by a two-tailed Student’s T-Test. Statistical significance was set at P<0.05. SigmaPlot software package 11.0 (Systat Software, Inc., San Jose, CA) was used to perform all statistical tests.
Results
Study observations
C56Bl/6 male mice received their respective EtOH, PF, EtOH/GEN or PF/GEN liquid diets for 60 days. During this time the daily amount of diet consumed by the each group did not differ (14.8 ml ± 0.21 for EtOH, 14.9 ml ± 0.24 for EtOH/GEN, 15.0± 0.19 for PF, and 14.5± 0.21 for PF/GEN, p=0.326, One-way ANOVA). At sacrifice, weight gain was lower in the GEN-supplemented PF group compared to the PF group, 28.5g ± 0.98 vs. 31.7g ± 0.72, respectively, p<0.05. These findings are not unexpected; the PF diet is high fat diet (35%), and diets supplemented with soy protein isolate or GEN have been shown to be protective against high fat-induced weight gain30, 31. EtOH consumption significantly decreased weigh gain in the EtOH group (26.6g ± 0.97) compared to its PF group, which is a result additional disruptive effects of EtOH on endocrine signaling and white adipose tissue differentiation16. EtOH did not significantly decrease weight gain in the EtOH/GEN group (26.9g ± 1.7) compared to its PF/GEN control, p= 0.308, One-Way ANOVA. The mean blood EtOH concentrations were 85.68±33.8 mg/dL (range 3.6-162.8) and 45.00±36.1 mg/dL (range 2.9-189.2) for the EtOH group and the EtOH/GEN group, respectively and did not statistically differ from each other (p=0.434, Student’s T-test). These values are comparable concentrations corresponding to the legal limit for operating a motor vehicle, 80 mg/dL, in the United States.
EtOH-mediated bone loss is not prevented with GEN supplementation
Following 60 d of chronic EtOH feeding, cortical and trabecular bone was assessed by μCT, in tibia harvested from EtOH and PF and EtOH/GEN and PF/GEN groups. EtOH treatment significantly decreased bone volume (Figure 1). In EtOH-treated mice a significant reduction in %BV/TV and Trabecular Number was observed. These observations were supported by an increase in Trabecular Spacing in comparison to the PF controls, p<0.05 (Table 1). Cortical geometry was also affected by EtOH treatment, and cross-sectional area, total diameter, and thickness were significantly reduced in the EtOH group compared to the PF controls, p<0.05. Interestingly, with GEN supplementation we observed significant increases in %BV/TV, Trabecular Number, and Thickness, and a corresponding decrease in spacing were observed in the PF/GEN mice when compared to just the PF controls (Table 1). However, GEN supplementation had no effect on EtOH-mediated trabecular bone loss. In the cortical bone compartment, GEN supplementation had no beneficial effect on the cortical cross-sectional area, total diameter, or cortical thickness compared to PF controls, p<0.05 and did not mitigate the EtOH-mediated cortical bone loss in comparison to the EtOH-treated mice (Table 1).
Figure 1.
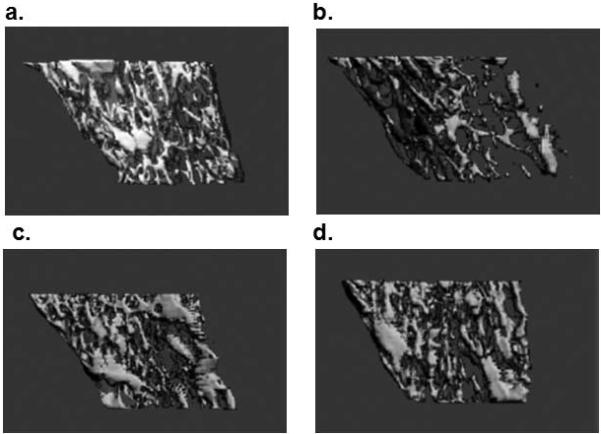
Lateral 3D reconstructions from mCT analyses for a representative bone from each group, bone chosen by best match to mean trabecular thickness of each group (a) PF, (b) EtOH, (c) Pair-Fed/GEN, and (d) EtOH/GEN.
Table 1.
μCT analysis of tibial bone in PF, EtOH, PF/GEN and EtOH/GEN treated male mice.
| PF | EtOH | PF/GEN | EtOH/GEN | |
|---|---|---|---|---|
| Tb. Bone parameters | ||||
|
| ||||
| BV/TV, % | 6.52 (0.01)a | 4.93 (0.01)b | 9.09 (0.004)c | 9.09 (0.004)c |
| Tb.Number, 1/mm | 2.60 (0.21)a | 1.82 (0.18)b | 2.86 (0.11)c | 2.07 (0.20)b |
| Tb.Spacing, mm | 0.37 (0.03)a | 0.55 (0.06)b | 0.32 (0.01)c | 0.48 (0.05)b |
| Tb.Thickness, mm | 0.029(0.001)a | 0.029(0.001)a | 0.030(0.001)c | 0.030 (0.001)b |
|
| ||||
| Ct bone parameters | ||||
|
| ||||
| Cross-sectional Area mm2 | 0.150 (0.001)a | 0.140 (0.003)b | 0.145 (0.003)a | 0.135 (0.004)b |
| Total Diameter, mm | 0.101 (0.001)a | 0.093 (0.002)b | 0.098 (0.001)a | 0.091(0.002)b |
| Cortical Thickness, mm | 0.188 (0.002)a | 0.173 (0.003)b | 0.185 (0.001)a | 0.176 (0.002)b |
| Medullary Area, mm2 | 0.110 (0.003)a | 0.123 (0.008)b | 0.101 (0.001)a | 0.110 (0.005)b |
| Periosteal Perimeter, mm | 0.976 (0.01)a | 0.925 (0.02)a | 0.976 (0.02)a | 0.936 (0.02)a |
| Endocortical Perimeter, mm | 0.617 (0.009)a | 0.644 (0.02)a | 0.575 (0.004)a | 0.601 (0.022)a |
N= 10 mice/group; Values are mean ± St.Err. Trabecular bone (Tb). Statistical differences between treatment groups were determined by two-way ANOVA followed by Student-Newman Keuls post-hoc analysis. Values with different letter subscripts are significant from each other (p<0.05).
GEN supplementation enhances EtOH-mediated induction of RANKL
We and others have shown that chronic EtOH consumption promotes bone loss through the up-regulation of RANKL-stimulated osteoclastogenesis20, 26,32-33. As expected, EtOH treatment alone increased bone resorption, as indicated by an 8-fold increase in RANKL mRNA expression compared to the PF control (relative fold, 8 ± 3.3 vs. 1 ± 0.34, respectively). GEN supplementation significantly increased RANKL expression in the PF/GEN group, relative fold 23.3 ± 1.2, when compared to the EtOH and PF controls, and increased RANKL expression further in the EtOH/GEN group, relative fold 33.2 ± 3.6, compared to all other groups, Two-way ANOVA, p<0.05. Corresponding to the gene expression, in Figure 2a, we also observed increases in RANKL protein expression in the PF/GEN and EtOH/GEN groups compared to the PF and EtOH-treated mice, p<0.05. In contrast, OPG mRNA expression was reduced in the GEN supplemented groups compared to PF and EtOH controls, relative fold, 1.0 ± 0.14 for PF, 1.81 ± 0.19 for EtOH, 0.51 ± 0.14 for PF/GEN and 0.28 ± 0.11, Two-way ANOVA,p<0.05. As a result, we see a significant increase in the RANKL:OPG ratio, (Figure 2b).
Figure 2.
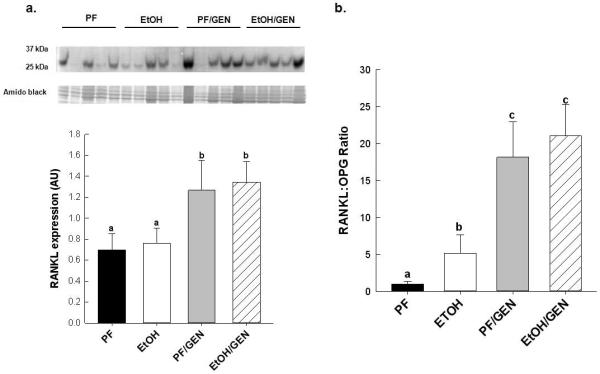
Effects of GEN-supplementation on markers of resorption (a) RANKL:OPG ratio and (b) RANKL protein expression in vertebral bone of PF-, EtOH-, PF/GEN- and EtOH/GEN- treated mice (n=5/group). Gene expression of RANKL and OPG were determined by real-time PCR as described in the Materials and Methods. RANKL protein expression was assessed by Western blot as described in the Materials and Methods. Data is expressed as mean ± SEM. Statistical significance was determined by a two-tailed Student’s T-test. Values with different letters are significantly different from each other (P < 0.05).
GEN supplementation promotes osteoblastogenesis and differentiation
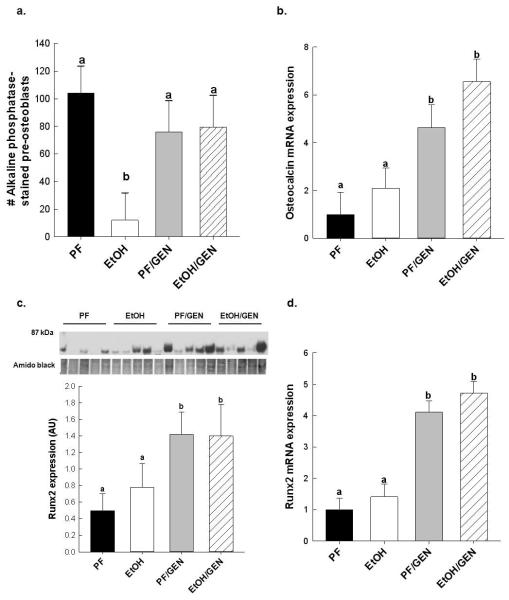
EtOH-mediated bone loss is also associated with decreased bone formation15,32. In Figure 3a, chronic EtOH consumption inhibited osteoblastogenesis as measured by the reduction of alkaline phosphatase stained-pre osteoblasts in primary bone marrow cells taken from EtOH-treated femurs and cultured ex vivo, as compared to cultures from PF femurs, p<0.05. In contrast, GEN supplementation prevented the EtOH-mediated loss of pre-osteoblasts in primary bone marrow cultures taken from EtOH/GEN-treated femurs, p<0.05. Consistent with these findings, GEN supplementation significantly increased mRNA expression of osteocalcin, a well-established marker for osteoblast activity, in vertebral bone compared to PF and EtOH-treated groups (Figure 3b). In Figure 3c,d, mRNA and protein expression of Runx2, is also up regulated in the PF/GEN and EtOH/GEN groups compared to PF and EtOH-treatment alone, p<0.05.
Figure 3.
Effects of GEN-supplementation on osteoblastogenesis (a) alkaline phosphatase staining of primary bone marrow cells from PF-, EtOH-, PF/GEN-, and EtOH/GEN-treated femurs (n=4/group), plated in quadruplicate, and cultured in osteogenic media for 10 days. Gene expression analysis of bone formation markers (b) osteocalcin and (c) Runx2 mRNA protein and (d) Runx2 mRNA expression in vertebral bone (n=5/group). Gene expression was determined by real-time RT-PCR as described in the Materials and Methods. Runx2 protein expression was assessed and quantified as described in the Materials and Methods. Data are expressed as mean ± SEM. Statistical significance was determined by two-tailed Student’s T-test. Values with different letters are significantly different from each other (p < 0.05).
GEN-treatment increases overall bone turnover in cultured primary bone marrow cells
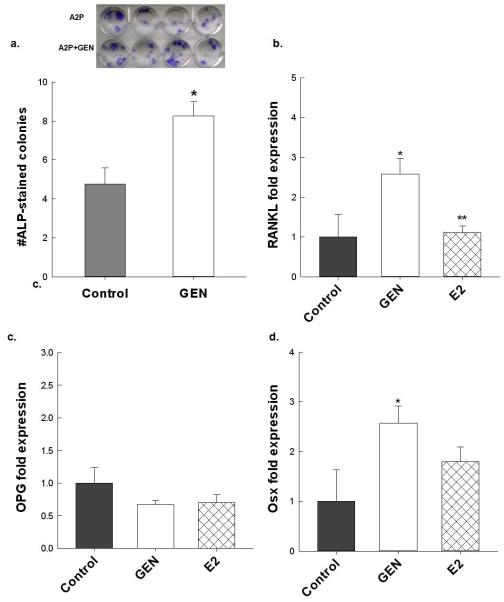
Primary bone marrow cultures were differentiated in vitro using osteogenic media supplemented with GEN (200 nM) or E2 (1nM) for 10d. GEN supplementation significantly increased the number of alkaline phosphatase-stained osteoblasts compared to the control cells (Figure 4a) which corresponded to an increase in Osterix mRNA expression, a down-stream target of Runx2, p<0.05 (Figure 4d). GEN treatment also increased RANKL expression in these cultures, p<0.05, but had no effect on OPG expression compared to control cells (Figure 4b, c). In contrast, treating BMC with a physiologically relevant dose of E2 had no significant effect on Osterix mRNA expression and no measurable impact on RANKL expression when compared to the GEN-treated cells and the control cells. Interestingly, in freshly isolated bone marrow cells, GEN-treatment increased basal production of ROS, as indicated by a 23% increase in hydrogen peroxide production compared to control cells, p<0.05 (Figure 5). Incubation of cells with E2 resulted in a 14% decrease in basal hydrogen peroxide production when compared to the cells without treatment, p<0.05.
Figure 4.
The effects of GEN on markers of osteoblastogenesis, bone marrow cells were isolated from 6wk female C57Bl6 mice (n=3), plated in quadruplicate, and grown in osteogenic media supplemented with GEN for 10d followed by (a) alkaline phosphatase staining. Gene expression of (b) RANKL, (c) Osteoprogetrin (OPG), and (d) osterix in bone marrow cultures grown in osteogenic media supplemented with GEN or E2 for 10 days. For each treatment group, cells were plated in quadruplicate. Data are expressed as mean ± S.E.M. Statistical significance for alkaline phosphastase staining was determined by a two-tailed Student’s T-Test, *p<0.05 control vs. GEN, **p< 0.05 GEN vs. E2.
Figure 5.
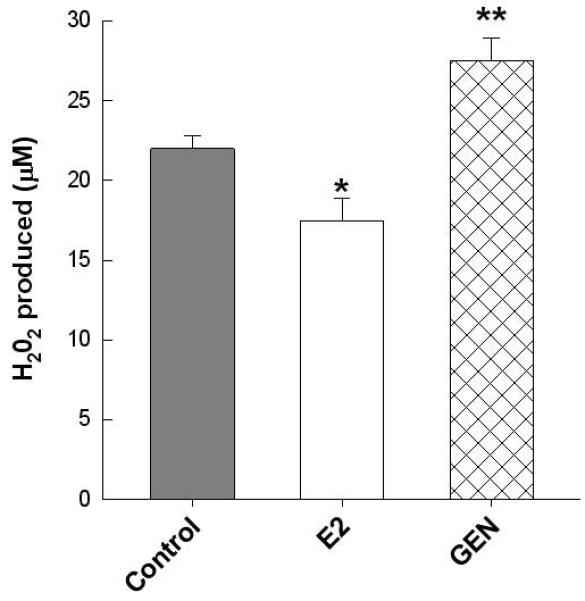
Basal hydrogen peroxide generation was measured in freshly isolated bone marrow cells from 6 wk female C57Bl6 mice (n=3), plated in quadruplicate, and treated with either 200 nM GEN or 1 nM E2 at 37°C for an hour, using the Amplex Red hydrogen peroxide/peroxidase assay kit as described in Materials and Methods. Data expressed as the amount produced (μM). Statistical significance was determined by a two-tailed Student’s T-test for every time point, *p<0.05 control vs. E2, **p<0.05 control vs. GEN.
Discussion
Bone loss is seen in elderly males and females as a result of the loss of estrogen, which protects bone from increases in ROS and oxidative stress34. Chronic alcohol exposure is known to increase ROS signaling in bone, resulting in increased osteoclastogenesis and decreased osteoblastogenesis, thus promoting bone loss. In particular, we have shown in rodents that E2 supplementation prevents EtOH-induced up-regulation of RANKL and subsequent enhancement of osteoclast differentiation and activity through RANKL-RANK signaling between osteoblasts and osteoclast precursors20,27,35. GEN is structurally similar to E2, binds to nuclear estrogen receptors and is estrogenic under certain conditions in vitro and in vivo7. Previously, we have shown that soy diets are protective against high fat diet-and ovariectomy-induced bone loss31,36. In the present study, we tested the hypothesis that GEN supplementation at levels found in soy products would also protect against EtOH-mediated bone resorption in male mice through reduced expression of RANKL. Relatively few studies regarding the effects of estrogens on bone loss in men have been conducted9. Chronic alcohol abusers have a higher risk for osteoporotic fractures compared to non-drinkers37,38. Findings from this study may be used to better understand the biological effects of GEN and to provide additional information regarding use of isoflavone supplements to reduce the risks of osteoporosis in men.
As expected, EtOH alone resulted in bone loss in male mice as a result of decreased osteoblastogenesis and bone formation and increased RANKL-dependent bone resorption20,27,32. Surprisingly, GEN supplementation was not protective against cortical or trabecular bone loss associated with chronic EtOH consumption. Further analysis demonstrated that GEN itself significantly up-regulated RANKL expression, and subsequent osteoclast activity in the PF/GEN and EtOH/GEN groups. In previous reports, we have demonstrated that E2 treatment blocks EtOH-produced ROS, which in turn prevents up-regulation of RANKL which is mediated via NADPH oxidase signaling pathways35. As expected, E2-treatment of freshly isolated primary bone marrow cells decreased basal production of hydrogen peroxide. In contrast to E2, GEN-treatment significantly increased basal ROS production in bone cells. These findings suggest that GEN supplementation may increase bone resorption through ROS signaling. Future studies will include in vitro experiments using co-treatments of GEN with radical scavengers, mitochondrial respiratory chain inhibitors, and NADPH oxidase inhibitors, to determine the mechanism by which GEN increases ROS signaling.
Because bone remodeling rates change throughout development, any overall “beneficial” effect of GEN supplementation on the skeleton may vary with the developmental window of exposure. In addition, multiple studies using pure GEN have reported contradictory effects on bone depending on species, sex, dose, route of administration 39-43. In this study, GEN supplementation did not prevent EtOH-associated bone loss, although there were compartment specific increases in trabecular bone density and architecture in the PF/GEN group. This improvement in bone quality corresponded to increased osteoblast-specific alkaline phosphatase staining, osteocalcin expression, and increased mRNA and protein expression of Runx2 in the PF/GEN group. Our findings suggest that GEN supplementation increases overall bone remodeling, which is consistent with our earlier report showing an overall increase in serum bone formation and bone resorption makers in pre-pubertal female rats receiving a soy protein isolate diet compared to rats receiving a casein-based diet supplemented with E223. These findings also support the idea that bone resorption mechanisms are primarily responsible for EtOH-mediated bone loss.
In conclusion, our findings demonstrate that dietary GEN supplementation does not protect against EtOH-mediated bone loss in male mice. We believe GEN supplementation increases ROS signaling, which subsequently enhances RANKL-mediated osteoclast activity, which overwhelms any beneficial effect of GEN on osteoblastogenesis in the face of EtOH exposure. It is possible that diets containing soy foods or soy isoflavones have beneficial effects on skeletal quality under conditions resulting in other types of bone loss. For example, we have shown that the consumption of soy protein isolate prevented the impairment of bone accrual and improved bone quality in weanling male rats fed high fat diets as a result of improved insulin-signaling in bone cells31. In vitro, both serum from soy protein isolate fed rats and pure GEN stimulate osteoblastogenesis through up-regulation of Runx2 expression in cultured osteoblastic cells31. These results warrant further investigation into the mechanisms underlying soy and GEN’s effects on bone remodeling. Such studies will provide new insights into the potential use of soy products or dietary GEN supplementation to reduce the risks of bone loss in men.
Funding acknowledgements
Information System Grant 6251-51999-007-04S and from the Carl L. Nelson Chair in Orthopedic Creativity, University of Arkansas for Medical Sciences.
Footnotes
The authors of this manuscript do not have any Conflicts of Interest and therefore have nothing to declare.
This work was supported in part by grants from the National Institute of Health (AA0118282) and (CA169389), USDA, Agricultural Service Current
References
- 1.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S, Melton LJ, III, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–74. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 3.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–73. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 4.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Wang XX, Chiba H, Higuchi M, Takasaki M, Ohta A, Ishimi Y. Combined intervention of exercise and genistein prevented androgen deficiency-induced bone loss in mice. J Appl Physiol (1985) 2003;94:335–42. doi: 10.1152/japplphysiol.00498.2002. [DOI] [PubMed] [Google Scholar]
- 6.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2008;62:155–61. doi: 10.1038/sj.ejcn.1602748. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 8.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Adamo EB, Wilson S, Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–47. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 9.Newton KM, LaCroix AZ, Levy L, Li SS, Qu P, Potter JD, Lampe JW. Soy protein and bone mineral density in older men and women: a randomized trial. Maturitas. 2006;55:270–7. doi: 10.1016/j.maturitas.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–26. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 11.Sliwinski L, Folwarczna J, Nowinska B, Cegiela U, Pytlik M, Kaczmarczyk-Sedlak I, Trzeciak H, Trzeciak HI. A comparative study of the effects of genistein, estradiol and raloxifene on the murine skeletal system. Acta Biochim Pol. 2009;56:261–70. [PubMed] [Google Scholar]
- 12.Yoon H-K, Chen K, Baylink DJ, Lau K-HW. Differential effects of two protein tyrosine kinase inhibitors, tyrphostin and genistein, on human bone cell proliferation as compared with differentiation. Calcif Tissue Int. 1998;63:243–249. doi: 10.1007/s002239900521. [DOI] [PubMed] [Google Scholar]
- 13.Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignami F, Riccardi C, Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkatt T-leukemia cells. Leuk Res. 1994;18:431–439. doi: 10.1016/0145-2126(94)90079-5. 1994. [DOI] [PubMed] [Google Scholar]
- 14.Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res. 2004;28:182–91. doi: 10.1097/01.ALC.0000108661.41560.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–90. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- 16.Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, Chen JR, Mason AZ, Badger TM, Ronis MJ. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J Pharmacol Exp Ther. 2012;343:401–12. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wezeman FH, Juknelis D, Himes R, Callaci JJ. Vitamin D and ibandronate prevent cancellous bone loss associated with binge alcohol treatment in male rats. Bone. 2007;41:639–45. doi: 10.1016/j.bone.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark MK, Sowers MF, Dekordi F, Nichols S. Bone mineral density and fractures among alcohol-dependent women in treatment and in recovery. Osteoporos Int. 2003;14:396–403. doi: 10.1007/s00198-003-1387-2. [DOI] [PubMed] [Google Scholar]
- 19.Wuermser LA, Achenbach SJ, Amin S, Khosla S, Melton LJ., III What accounts for rib fractures in older adults? J Osteoporos. 2011;2011:457–591. doi: 10.4061/2011/457591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther. 2006;319:1182–90. doi: 10.1124/jpet.106.109454. [DOI] [PubMed] [Google Scholar]
- 21.Shankar K, Hidestrand M, Haley R, Skinner RA, Hogue W, Jo CH, Simpson P, Lumpkin CK, Jr., Aronson J, Badger TM, Ronis MJ. Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology. 2006;147:166–78. doi: 10.1210/en.2005-0529. [DOI] [PubMed] [Google Scholar]
- 22.Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAr, LXR, and SREBP signaling. J. of Nutr. 2009;139:1431–1438. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Lazarenko O, Wu X, Tong Y, Blackburn M, Gomez-Acevedo H, Shankar K, Badger TM, Ronis MJ, Chen JR. Differential effects of short term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PlosONE. 2012;7(4):e35736. doi: 10.1371/journal.pone.0035736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51:765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 25.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 26.Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009;24:221–30. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJ. Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. J Pharmacol Exp Ther. 2008;324:50–9. doi: 10.1124/jpet.107.130351. [DOI] [PubMed] [Google Scholar]
- 28.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;30:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 29.Thomas GP, Baker SU, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J. Endocrin. 2001;170:451–460. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Nelson-Dooley C, Della-Fera MA, Yang JY, Zhang W, Duan J, Hartzell DL, Hamrick MW, Baile CA. Genistein decreases food intake, body weight and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136:409–411. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 31.Chen JR, Zhang J, Lazarenko OP, Cao JJ, Blackburn ML, Badger TM, Ronis MJJ. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblats. FASEB J. 2013;27:3514–3523. doi: 10.1096/fj.12-226464. [DOI] [PubMed] [Google Scholar]
- 32.Shankar K, Hidestrand M, Liu X, Chen JR, Haley R, Perrien DS, Skinner RA, Lumpkin CK, Jr., Badger TM, Ronis MJ. Chronic ethanol consumption inhibits postlactational anabolic bone rebuilding in female rats. J Bone Miner Res. 2008;23:338–49. doi: 10.1359/jbmr.071023. [DOI] [PubMed] [Google Scholar]
- 33.Alvisa-Negrin J, Gonzalez-Reimers E, Santolaria-Fernandez F, Garcia-Valdecasas-Campelo E, Valls MR, Pelazas-Gonzalez R, Duran-Castellon MC, de Los Angeles Gomez-Rodriguez. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468–75. doi: 10.1093/alcalc/agp038. [DOI] [PubMed] [Google Scholar]
- 34.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM, Ronis MJ. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther. 2011;336:734–42. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Lazarenko O, Badger T, Ronis M, Chen J-R. Persistent effects of a soy diet in early dvelopment on bone in female rats. Experimental Biology. 2013:A233.7. [Google Scholar]
- 37.Pasoto SG, Yoshihara LA, Maeda LC, Bernik MM, Lotufo PA, Bonfa E, Pereira RM. Osteoporotic hip fractures in non-elderly patients: relevance of associated co-morbidities. Rheumatol Int. 2011;32:3149–3153. doi: 10.1007/s00296-011-2154-x. [DOI] [PubMed] [Google Scholar]
- 38.Wuermser LA, Achenbach SJ, Amin S, Khosla S, Melton LJ., III What accounts for rib fractures in older adults? J Osteoporos. 2011:457591. doi: 10.4061/2011/457591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaludjerovic J, Ward WE. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J Nutr. 2010;140:766–72. doi: 10.3945/jn.109.116343. [DOI] [PubMed] [Google Scholar]
- 40.Li YQ, Xing XH, Wang H, Weng XL, Yu SB, Dong GY. Dose-dependent effects of genistein on bone homeostasis in rats' mandibular subchondral bone. Acta Pharmacol Sin. 2012;33:66–74. doi: 10.1038/aps.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner RT, Iwaniec UT, Andrade JE, Branscum AJ, Neese SL, Olson DA, Wagner L, Wang VC, Schantz SL, Helferich WG. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause. 2013;20:677–86. doi: 10.1097/gme.0b013e31827d44df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward WE, Piekarz AV. Effect of prenatal exposure to isoflavones on bone metabolism in mice at adulthood. Pediatr Res. 2007;61:438–43. doi: 10.1203/pdr.0b013e3180332d67. [DOI] [PubMed] [Google Scholar]
- 43.Weaver CM, Alekel DL, Ward WE, Ronis MJ. Flavonoid intake and bone health. J Nutr Gerontol Geriatr. 2012;31:239–53. doi: 10.1080/21551197.2012.698220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JR, Singhal R, Lazarenko OP, Liu X, Hogue WR, Badger TM, Ronis MJ. Short term effects on bone quality associated with consumption of soy protein isolate and other dietary protein sources in rapidly growing female rats. Exp Biol Med. 2008;233:1348–58. doi: 10.3181/0802-RM-63. [DOI] [PubMed] [Google Scholar]