Abstract
Objective
Ovarian granulosa cell tumors tend to respond poorly to chemotherapy. We examined the clinical efficacy of bevacizumab with or without concurrent chemotherapy and evaluated the angiogenic characteristics of these patients' tumors.
Methods
We conducted a retrospective review of all patients seen at our institution from February 2004 to October 2008 who received bevacizumab for ovarian sex cord-stromal tumors. We performed immunohistochemical staining for vascular endothelial growth factor (VEGF) and CD31 when tissue was available; microvessel density was measured based on CD31 staining. Clinical data were abstracted from a chart review.
Results
We identified 8 patients who were treated with bevacizumab; 7 had adult granulosa cell tumors and one had a juvenile granulosa cell tumor. All patients had recurrent disease and had been previously treated with cytotoxic chemotherapy, (median 3.5 regimens; range, 1–6). One patient had a complete clinical response to bevacizumab therapy, 2 patients had a partial response, 2 patients had stable disease, and 3 patients' disease progressed, yielding a response rate of 38% and a clinical benefit rate of 63%. The median progression-free survival was 7.2 months and overall survival was not reached at a median follow-up of 23.6 months after initiating bevacizumab.
VEGF overexpression and microvessel density were associated with poor outcome but sample size was too small to calculate statistical significance.
Conclusions
Anti–VEGF therapy is highly effective in patients with granulosa cell tumors. Based on our observations, a prospective trial has been initiated using single-agent bevacizumab in patients with recurrent ovarian sex cord-stromal tumors.
Introduction
Granulosa cell tumors (GCTs) are rare ovarian neoplasms, accounting for 2%–5% of ovarian malignancies [1-3]. GCTs arise from the sex cord-stromal cells of the ovary and account for a substantial proportion (12-70%) of sex cord-stromal cell tumors (SCSTs) [4]. Based on their histopathologic features, GCTs are subcategorized as “adult” or “juvenile.” Adult GCTs, which represent 95% of GCTs, generally occur in middle-aged and older women, while juvenile GCTs tend to develop before puberty [6, 7].
As with other rare tumors, detailed information on the epidemiology, natural history, molecular characteristics, and optimal treatment of GCTs is limited. As with epithelial ovarian cancer, cytoreductive surgery is the standard initial treatment modality for all patients with GCTs. Postoperative adjuvant chemotherapy has evolved over the past three decades from vincristine, dactinomycin, and cyclophosphamide (VAC) [8] to bleomycin, etoposide, and cisplatin (BEP) [9]. Most recently, a combination of paclitaxel and carboplatin has been shown to be effective and may become the standard of care for adjuvant and postoperative treatment of GCTs [10, 11]. Despite advances in therapy, however, GCTs still tend to recur over long periods, often requiring multiple treatments including surgery, radiotherapy, chemotherapy, and hormonal agents [12, 13]. Unfortunately, all of these approaches have limited efficacy. Therefore, novel therapeutic approaches are needed to improve the outcome of patients with GCTs.
The clinical behavior of patients with SCSTs, which are often large and well vascularized [14], suggest that angiogenesis is important in tumor development and progression. Lymph node metastasis is extremely rare [12, 13], but distant metastasis is common, indicating hematogenous routes of spread. We have shown that increased microvessel density (MVD) and overexpression of vascular endothelial growth factor (VEGF) correlate with the presence of distant metastasis and a shorter disease-specific survival in patients with SCSTs [15]. Together, these observations suggest that antiangiogenic agents may have a role in treating women with SCSTs.
Angiogenesis plays a critical role in tumor development. VEGF [16] is a potent mitogen for vascular endothelial cells, and the cloning of VEGF in 1989 [17] was a milestone in the understanding of tumor angiogenesis [17, 18]. Bevacizumab, a humanized monoclonal antibody to VEGF, was approved for use as an adjuvant therapy in colorectal cancer by the U.S. Food and Drug Administration (FDA) in 2004 [19]. Bevacizumab has been investigated in epithelial ovarian cancer and is well tolerated and active in the second- and third-line treatment of patients with this disease [20, 21], but bevacizumab has not been prospectively studied for GCTs. Therefore, we undertook this study to review the efficacy and adverse effects of bevacizumab, administered alone or in combination with other agents, for the treatment of GCTs. We also evaluated the angiogenic characteristics of GCTs to determine whether markers of angiogenesis can predict clinical response to bevacizumab.
Materials and Methods
Clinical Information
We retrospectively reviewed the medical and pathology records of consecutive patients who were diagnosed with ovarian SCSTs at The University of Texas M. D. Anderson Cancer Center from February 2004 (FDA approval of bevacizumab) through October 2008. Patients were identified through a search of the institution's medical and pathology databases, and complete records were reviewed for patients meeting the inclusion criteria. Although the databases were queried for all SCSTs, all identified patients had GCTs. We collected demographic information; clinical, surgical, chemotherapy, and radiation therapy information; toxicity data; response and survival data; and the dates and nature of follow-up. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 (www.ctep.cancer.gov). This retrospective study was approved by M. D. Anderson's Institutional Review Board, with a waiver of the requirement for informed consent.
The inclusion criteria for this study were as follows: (1) Patients must have had their pathology specimens reviewed at M. D. Anderson and must have had a diagnosis of an ovarian SCST, including adult or juvenile GCT, Sertoli-Leydig cell tumor, sex cord tumor with annular tubules, malignant thecoma, or mixed or unclassified SCST; (2) patients must have been seen at M. D. Anderson on at least one occasion for evaluation and treatment of SCST; and (3) patients must have received bevacizumab with or without chemotherapy for the treatment of SCST. Patients with an additional non-stromal ovarian malignancy were excluded.
Each patient's tumor stage was assessed according to the guidelines of the International Federation of Gynecology and Obstetrics (FIGO) by reviewing operative and pathology reports from her initial surgery. Patients were designated as having measurable or non-measurable disease on the basis of physical examination and radiologic characteristics at the time of treatment with bevacizumab. The endpoints of interest in this study were complete response, partial response, response rate (complete plus partial responses), progression-free survival (PFS), and overall survival (OS). Response was evaluated using Response Evaluation Criteria in Solid Tumors criteria [22]. Complete response was defined as the disappearance of all target lesions; partial response was defined as a 30% decrease in the sum of the longest diameters of target lesions; progressive disease was defined as a 20% increase in the sum of the longest diameters of target lesions; and stable disease was defined as no change or small changes that did not meet the above criteria for response and progression. Radiology reports from the time of treatment were used to confirm these responses. Response rate was defined as patients with complete and partial responses divided by the total number of patients treated. Clinical benefit rate was defined as patients with complete and partial responses plus patients with stable disease, divided by the total number of patients treated. PFS was defined as the time from the first bevacizumab administration to physical or radiologic evidence of disease progression, death as a result of any cause, or last contact, with events defined as progressive disease or death. OS was defined as the time from the first bevacizumab administration to death from any cause. PFS and OS were calculated using the Kaplan-Meier method.
VEGF and CD31 Immunohistochemical Staining
Immunohistochemical staining for VEGF expression was performed on a DAKO autostainer (Carpinteria, CA) with rabbit antihuman VEGF (A-20) monoclonal antibody (dilution 1:10, Santa Cruz Biotechnology, Santa Cruz, CA) on sequentially cut archived clinical human samples. Paraffin-embedded samples were heated at 60°C for 12 hours and deparaffinized with sequential washings of xylene and declining concentrations of ethanol. The samples were then immersed in 3% hydrogen peroxide for 5 minutes and rehydrated in water. Antigen retrieval was performed by using a pretreatment citrate buffer for 45 minutes, cooling the samples for 20 minutes, and adding the primary antibody for 60 minutes. Polymer was added for 30 minutes, and the chromagen 3,3′-diaminobenzidine (DAB, Open Biosystems, Huntsville, AL) was added for 16 minutes. Counterstaining with Mayer hematoxylin was performed for 1 minute, and a coverslip was applied. A gynecologic pathologist who was blinded to the clinical outcome of the patients assessed the percentage of positive cells (distribution) and staining intensity, from which an overall score of VEGF overexpression was derived. Distribution was graded as 1–4, with 0–25% stained cells graded as 1, 26–50% graded as 2, 51–75% graded as 3, and 76–100% graded as 4. Staining intensity was graded as 1–3, with 1 indicating weak intensity, 2 moderate intensity, and 3 strong intensity. The overall score was obtained by multiplying the distribution and intensity scores; the overall scores for all patients were then evaluated to quantitate overexpression, using a method similar to one previously described [23].
Immunohistochemical staining for MVD was performed with lyophilized mouse antihuman CD31 monoclonal antibodies (dilution 1:300, Novocastra Vision Biosystems, Norwell, MA) on sequentially cut archived clinical human samples in a BondMax automated immunostainer (Leica Microsystems, Wetzlar, Germany). Paraffin-embedded samples were heated at 72°C for 30 minutes, deparaffinized with sequential washings of bond dewax solution and declining concentrations of ethanol, and rehydrated with bond wash. Epitope retrieval was performed using Tris-EDTA buffer for 20 minutes, followed by sequential bond washing. Endogenous peroxidases were inhibited by immersion in 3.0% hydrogen peroxide for 5 minutes, followed by sequential washing in bond wash. Polymer enhancer was applied for 8 minutes, and sequential bond washing was performed. Poly-HRP anti-mouse immunoglobulin G was applied to samples for 8 minutes; the samples were then washed using bond wash and deionized water. Visualization was achieved with DAB. DAB enhancer was applied for 5 minutes, and sequential washing in bond wash was performed. Slides were counterstained with Gill No. 3 hematoxylin (Sigma-Aldrich, St. Louis, MO). A vessel was defined as an open lumen with 1 or more CD31-positive cells immediately adjacent to it. Tumor MVD was calculated as the mean of vessel counts recorded at 5 representative locations under 160-power magnification. The investigator performing the density calculations was blinded to the clinical outcomes of patients.
Statistical Analysis
Data were analyzed using SPSS 12.0 for Windows (2003, SPSS, Inc., Chicago, IL). Descriptive statistics were calculated for all patients. Mean, median, range, and percentage were calculated where appropriate. Since the sample size was small, statistical significance was not evaluated, and trends were instead examined.
Results
Patients and Demographics
We identified 8 patients who met all inclusion criteria and were treated with bevacizumab between February 2004 and October 2008. Patient characteristics are summarized in Table 1. The 8 patients had a median age of 38 years (range, 20–53 years), and all could be evaluated for response and toxicity based on the available clinical information. All 8 patients had GCTs (7 adult type, 1 juvenile type). Four patients had disease confined to the pelvis, 2 patients had disease metastatic outside the pelvis, and 2 patients were unstaged. All 8 patients had optimal disease status following initial surgery for their GCT. Only 1 patient, who had stage IIIC adult GCT, received adjuvant chemotherapy during initial treatment (4 cycles of VAC), and 1 patient with stage II disease received adjuvant pelvic radiotherapy.
Table 1. Characteristics of Patients Treated with Bevacizumab.
| Characteristic | No. patients* | % |
|---|---|---|
| Age at diagnosis | ||
| Median | 38 yr | |
| Range | 20–53 yr | |
| Ethnicity | ||
| White | 7 | 88 |
| Hispanic | 1 | 13 |
| Histologic type | ||
| Adult granulosa cell tumor | 7 | 88 |
| Juvenile granulosa cell tumor | 1 | 13 |
| FIGO1 stage at diagnosis | ||
| IA | 1 | 13 |
| IC | 1 | 13 |
| II | 2 | 25 |
| IIIC | 2 | 25 |
| Unstaged | 2 | 25 |
| Disease status at start of bevacizumab therapy | ||
| Measurable disease | 7 | 89 |
| No measurable disease | 1 | 13 |
unless otherwise indicated.
International Federation of Gynecology and Obstetrics
Treatments given for recurrent disease prior to bevacizumab are summarized in Table 2. The median number of episodes of recurrence was 4 (range, 1–7 episodes). All patients had previously been treated for recurrent disease; the median number of prior chemotherapy regimens was 3.5 (range, 1–6 regimens). The chemotherapy regimens administered prior to bevacizumab (and the number of patients receiving each regimen) included platinum/paclitaxel (n = 7); BEP (n = 4); liposomal doxorubicin (n = 3); docetaxel/carboplatin (n = 2); docetaxel (n = 2); ifosfamide/etoposide (n = 1); etoposide (n = 1); gemcitabine (n = 1); topotecan (n = 1); VAC (n = 1); tegafur-uracil (n = 1); cisplatin (n = 1); carboplatin (n = 1); and liposomal doxorubicin/vinorelbine/gemcitabine (n = 1). These numbers reflect the number of regimens given in the patient population, as some patients may have received the same or different regimens on different episodes of recurrence.
Table 2. Treatment for Recurrent Disease Prior to Bevacizumab.
| Patient No. | No. prior recurrences | Sites of recurrence | Prior chemotherapy regimens (response) | Prior hormonal and targeted therapy | Prior RT | No. Prior surgeries |
|---|---|---|---|---|---|---|
| 1 | 7 | Pelvis; Liver | Carboplatin/paclitaxel (CR), BEP (PR), etoposide (P), liposomal doxorubicin (PR), gemcitabine (P), topotecan (P) | Leuprolide acetate, tamoxifen, megestrol acetate, anastrozole | No | 4 |
| 2 | 1 | Abdomen, pelvis | Carboplatin/paclitaxel (PR) | None | Yes | No |
| 3 | 4 | Left ovary, pelvis, abdomen, Liver | Carboplatin/paclitaxel (CR), carboplatin/paclitaxel (PR), docetaxel (S), liposomal doxorubicin (P) | Leuprolide acetate, imatinib mesylate | No | 4 |
| 4 | 4 | Abdomen, pelvis, liver, spleen, lung | Intraperitoneal mitomycin C and VAC (CR), carboplatin/paclitaxel (CR), liposomal doxorubicin (S), tegafur-uracil (P), carboplatin/docetaxel (S) | Leuprolide acetate, anastrozole, megestrol acetate, anastrozole, gefitinib | Yes | 4 |
| 5 | 4 | Abdomen, pelvis, retroperitoneum, liver | BEP (CR), cisplatin (NT), carboplatin (CR) | Anastrozole, leuprolide acetate, erlotinib, tamoxifen | No | 3 |
| 6 | 2 | Pelvis, rectosigmoid | Carboplatin/paclitaxel (CR) | Tamoxifen | Yes | 1 |
| 7 | 6 | Abdomen, pelvis, liver | BEP (CR), carboplatin/docetaxel (PR) | Leuprolide acetate, anastrozole | No | 4 |
| 8 | 1 | Uterus, omentum, pelvis, liver | BEP (P), carboplatin/paclitaxel (P), etoposide/ifosfamide (P), liposomal doxorubicin/vinorelbine/gemcitabine (P) | None | Yes | 1 |
RT: radiotherapy; CR: complete response; BEP: bleomycin/etoposide/cisplatin; PR: partial response; P: progression; S: stable; VAC: vincristine/actinomycin D/cyclophosphamide; NT: did not tolerate chemotherapy
Treatment for recurrent disease prior to bevacizumab also included hormonal therapy in 6 patients and biologic therapy in 3 patients (Table 2). Three patients underwent radiation therapy for recurrent disease. Seven patients underwent debulking surgery for recurrent disease, with a median of 3.5 prior procedures, excluding surgery at time of diagnosis (range, 0–4 prior procedures).
Altogether, the 8 patients received a total of 52 courses of bevacizumab, with a median of 6 courses each (range, 2–12 courses each). Immediately before bevacizumab treatment, 7 patients had measurable disease, and 1 patient had just undergone optimal debulking surgery and had no measurable disease. Three patients received bevacizumab alone and 5 patients received bevacizumab concurrently with cytotoxic chemotherapy. Specific dosages and intervals of bevacizumab administration varied from 5 mg/kg to 15 mg/kg intravenously every 2–4 weeks. Details of treatment with bevacizumab are provided in Table 3.
Table 3. Details of Bevacizumab Treatment and Response.
| Patient No | Site of target lesion(s) | Largest target lesion (cm) | Concurrent chemotherapy regimen | Dosage of bevacizumab | No. cycles of bevacizumab | Response |
|---|---|---|---|---|---|---|
| 1 | Liver | 6.1 | None | 7 mg/kg IV every 2 weeks | 3 | Progression |
| 2 | Abdomen | 18 | Carboplatin/paclitaxel; liposomal doxorubicin | 5 mg/kg IV every 3 weeks | 10 | Stable |
| 3 | Liver | 4.2 | Gemcitabine, then leuprolide acetate; docetaxel | 7.5 mg/kg IV every 2 weeks | 12 | PR |
| 4 | NMD | N/A | Etoposide | 7 mg/kg IV every 2 weeks | 6 | Progression |
| 5 | Colon | 3.2 | Carboplatin/paclitaxel; paclitaxel | 7 mg/kg IV every 4 weeks | 12 | PR |
| 6 | Cul-de-sac | 7.4 | None | 10 mg/kg IV every 2 weeks | 6 | CR |
| 7 | Liver | 5 | Paclitaxel | 5 mg/kg IV every 2 weeks | 2 | Stable |
| 8 | Liver | 12.5 | None | 15 mg/kg IV every 3 weeks | 3 | Progression |
NMD: no measurable disease; IV: intravenously; PR: partial response; CR: complete response
Response and Survival
One patient had a complete clinical response following bevacizumab, 2 had a partial response, 2 had stable disease, and 3 had disease progression, yielding a response rate of 38% and a clinical benefit rate of 63%. Of the 3 patients who received bevacizumab alone, 1 had a complete response and 2 had disease progression. The median PFS was 7.2 months and the overall survival (OS) was not reached at a median follow-up of 23.6 months after starting bevacizumab therapy. Two patients died of recurrent GCT. Details on response and survival information are provided in Table 4.
Table 4. Details of Response and Follow-up for Patients Treated with Bevacizumab.
| Patient No. | Response | Characteristics of response | Patient status |
|---|---|---|---|
| 1 | CR | 7.4 cm vaginal apex mass, resolved after RT/bevacizumab | NED at 8.5 months after start of bevacizumab |
| 2 | PR | Upper abdominal disease and liver mass, stable when bevacizumab was combined with gemcitabine, progression when bevacizumab was combined with leuprolide acetate, and PR when bevacizumab was combined with docetaxel | AWD 25.5 months after start of bevacizumab |
| 3 | PR | PR of abdominopelvic disease with eventual progression | AWD 13.5 months after start of bevacizumab |
| 4 | Stable | Stable disease when combined with paclitaxel and carboplatin or combined with liposomal doxorubicin | AWD 21.6 months after start of bevacizumab |
| 5 | Stable | Mixed response with decrease in liver lesions but increase in peritoneal implant and ascites; did not meet criteria for response or progression | DOD 9.4 months after start of bevacizumab |
| 6 | Progression | Peritoneal implant, lung metastasis, and diaphragmatic lymph node involvement progressed at 3 months | AWD 25.7 months after start of bevacizumab |
| 7 | Progression | Progression in liver and lung | AWD 37.3 months after start of bevacizumab |
| 8 | Progression | Abdominopelvic disease progressed; liver lesion decreased but likely owing to recent liver RT and so considered progression | DOD 28.9 months after start of bevacizumab |
CR: complete response; RT: radiotherapy; PR: partial response; NED: no evidence of disease; AWD: alive with disease; DOD: died of disease
Toxicity
No bevacizumab-related deaths were observed. No patient developed grade 3-4 hematologic toxicity during bevacizumab therapy. Nonhematologic toxic effects were also mild. One patient had a sinus infection and persistent grade 2 peripheral neuropathy after 6 cycles of bevacizumab combined with paclitaxel, and 1 had a partial bowel obstruction. Neither event was considered to be definitely related to bevacizumab. No patient had a bowel perforation.
Immunohistochemistry
Of the 8 patients, 5 had tissue available for immunohistochemical analysis. One of the 5 patients had specimens from 3 separate episodes of recurrence; in this patient, the specimen just prior to the administration of bevacizumab was evaluated in this study. VEGF expression was noted at some level in all samples. A higher VEGF score was associated with progression of disease, a shorter PFS duration and a shorter OS duration, although the sample size was too small to perform statistics. A high MVD was also associated with progression of disease, shorter PFS duration, and, excluding one outlier, a shorter OS duration. VEGF expression and MVD appeared to correlate. These data are presented in Table 5. Figure 1 shows immunohistochemical staining in representative photomicrographs of tissue with low versus high expression of VEGF and MVD.
Table 5. Details of Immunohistochemistry and Patient Outcomes.
| Patient No. | VEGF Score | Average MVD | Response to Bevacizumab | PFS (months) | Status | OS (months) |
|---|---|---|---|---|---|---|
| 3 | 4 | 9.2 | Partial response | 25.5 | AWD | 25.5 |
| 4 | 6 | 12.4 | Progression | 5.5 | AWD | 37.3 |
| 6 | 1 | 9.4 | Complete response | 8.9 | NED | 8.5 |
| 7 | 6 | 10.2 | Stable disease | 1.6 | DOD | 9.4 |
| 8 | 6 | 21 | Progression | 1.7 | DOD | 28.9 |
Figure 1.
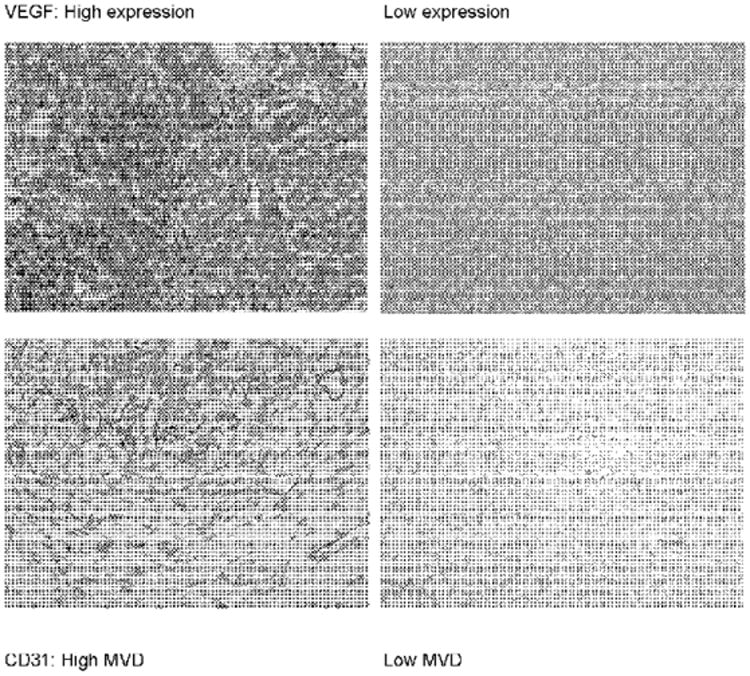
Immunohistochemical peroxidase staining for vascular endothelial growth factor (VEGF) and CD31 in representative granulosa cell tumors. Original magnification of 160 ×. MVD = microvessel density.
Discussion
The key findings of our manuscript are that bevacizumab therapy is effective in patients with ovarian granulosa cell tumors, and that measures of angiogenesis may correlate with clinical outcome in these patients.
Single-agent bevacizumab has been investigated in the treatment of recurrent or advanced epithelial ovarian cancer, with Burger et al. reporting a 21% response rate [20], and Monk et al. reporting a 16% response rate with a 62.5% rate of stable disease [24]. Richardson et al. described 35 patients with recurrent epithelial ovarian cancer who were treated with gemcitabine, platinum, and bevacizumab, yielding a median PFS of 12 months (95% confidence interval, 7–15 months) [25].
Although those studies reported on patients with epithelial ovarian cancer, some intriguing data suggested that anti-angiogenic therapy might also be effective in patients with GCTs. Schmidt et al found that 94% of patients with GCT had VEGF-expressing tumors [14]. Furthermore, our group previously demonstrated that nearly all SCSTs overexpress VEGF, and that VEGF overexpression and increased MVD correlate with poor clinical outcome [15]. Also supporting a role for antiangiogenic therapy in GCTs is the clinical observation that lymphatic metastasis is extremely rare, but hematogenous metastasis is relatively frequent [12, 13]. In addition, a recent study reported a single patient with refractory GCT who experienced symptomatic relief of ascites and stable disease in response to single agent bevacizumab therapy [26]. Our findings in this study are therefore not surprising. As expected, bevacizumab demonstrated activity, with a response rate of 38% and a clinical benefit rate of 63% in our small series of patients with GCT. Additionally, the PFS of 7.2 months seen here after bevacizumab is similar to the PFS of 6.8 months after taxanes and 11.2 months after BEP seen in patients with recurrent measurable disease [10].
The rationale for assessing the clinical benefit rate merits consideration. In 1 study of patients with metastatic colorectal cancer, the continuation of bevacizumab therapy led to a 12-month extension of OS [27]. The authors suggested that maximal clinical benefit would be obtained from the continuation of bevacizumab therapy beyond response or stable disease, and that the benefit might best be determined by measuring complete responses, partial responses, and disease stabilization over a specific period of time [27]. This strategy has recently been adopted in breast cancer patients treated with biologic agents [26]. Therefore, reporting the clinical benefit rate, as we have done here, may be the best way to determine the utility of a biologic agent, rather than focusing on the response rate alone.
It is difficult to determine the relative contribution of bevacizumab to the responses seen in the patients we reviewed, as the concurrent chemotherapy used varied widely. However, 1 patient who had a complete response received single-agent bevacizumab (10 mg/kg intravenously every 2 weeks). Thus, bevacizumab appears to have a demonstrable clinical effect against recurrent GCT. In addition, bevacizumab appears to be well tolerated, consistent with previous studies [20, 27]. The adverse events of neuropathy and partial bowel obstruction observed in our study may not relate to bevacizumab and should not preclude further study of bevacizumab in patients with recurrent GCT.
Our paper has several limitations, including its retrospective nature and small sample size. Also, patients did not receive uniform treatment, with some receiving bevacizumab as a single agent and others receiving bevacizumab in combination with various other agents. Tissue was also not available for immunohistochemistry on each patient, and it was not obtained immediately prior to administration of bevacizumab.
Within these limitations, however, the association of response with immunohistochemical findings supports and explains the potential clinical efficacy of bevacizumab in recurrent GCT. Specifically, MVD was associated with VEGF overexpression, and VEGF overexpression was associated with a shorter PFS. These data suggest that markers for angiogenesis may correlate with poor disease outcome in patients with recurrent GCT. This underscores the role of angiogenesis recurrent GCTs and helps to explain the activity of bevacizumab, albeit in a very small sample size.
One exciting inference of our findings is the potential role for using molecular targets to guide biologic therapies in patients with rare tumors. It may be the case that immunohistochemistry can help predict which patients' tumors will respond to bevacizumab or other biologically targeted therapies. The association between clinical and immunohistochemical data, however, is not yet clear-cut. Further studies will be instructive in identifying molecular biomarkers that might be predictive for response to anti-VEGF therapies.
In summary, our case series demonstrates that bevacizumab has activity in patients with recurrent GCTs. This finding supports further investigation of single-agent bevacizumab therapy for recurrent GCT in a phase II trial. This trial is currently underway through the Gynecologic Oncology Group.
Acknowledgments
This work was supported by a grant from the Houston League of Business and Professional Women, Inc.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjorkholm E, Silfversward C. Prognostic factors in granulosa-cell tumors. Gynecol Oncol. 1981;11:261–74. doi: 10.1016/0090-8258(81)90040-8. [DOI] [PubMed] [Google Scholar]
- 2.Evans AT, 3rd, Gaffey TA, Malkasian GD, Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55:231–8. [PubMed] [Google Scholar]
- 3.Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35:231–41. doi: 10.1002/1097-0142(197501)35:1<231::aid-cncr2820350128>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–9. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Clement PB, Young RH. Sex Cord-Stromal and Steroid Cell Tumors. In: Clement PB, Young RH, editors. Atlas of Gynecologic Surgical Pathology. Second. Philadelphia: Saunders Elsevier; 2008. pp. 386–93. [Google Scholar]
- 6.Calaminus G, Wessalowski R, Harms D, Gobel U. Juvenile granulosa cell tumors of the ovary in children and adolescents: results from 33 patients registered in a prospective cooperative study. Gynecol Oncol. 1997;65:447–52. doi: 10.1006/gyno.1997.4695. [DOI] [PubMed] [Google Scholar]
- 7.Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984;8:575–96. doi: 10.1097/00000478-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Colombo N, Sessa C, Landoni F, Sartori E, Pecorelli S, Mangioni C. Cisplatin, vinblastine, and bleomycin combination chemotherapy in metastatic granulosa cell tumor of the ovary. Obstet Gynecol. 1986;67:265–8. doi: 10.1097/00006250-198602000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Gershenson DM, Morris M, Burke TW, Levenback C, Matthews CM, Wharton JT. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet Gynecol. 1996;87:527–31. doi: 10.1016/0029-7844(95)00491-2. [DOI] [PubMed] [Google Scholar]
- 10.Brown J, Shvartsman HS, Deavers MT, Burke TW, Munsell MF, Gershenson DM. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol. 2004;22:3517–23. doi: 10.1200/JCO.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 11.Brown J, Shvartsman HS, Deavers MT, Ramondetta LM, Burke TW, Munsell MF, Gershenson DM. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol Oncol. 2005;97:489–96. doi: 10.1016/j.ygyno.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Rustum NR, Restivo A, Ivy J, Soslow R, Sabbatini P, Sonoda Y, Barakat RR, Chi DS. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006;103:31–4. doi: 10.1016/j.ygyno.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 13.Brown J, Sood AK, Deavers MT, Milojevic L, Gershenson DM. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecologic Oncology. 2009 doi: 10.1016/j.ygyno.2008.12.007. in press. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Kammerer U, Segerer S, Cramer A, Kohrenhagen N, Dietl J, Voelker HU. Glucose metabolism and angiogenesis in granulosa cell tumors of the ovary: activation of Akt, expression of M2PK, TKTL1 and VEGF. Eur J Obstet Gynecol Reprod Biol. 2008;139:72–8. doi: 10.1016/j.ejogrb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Brown J, Deavers MT, Nick AM, Milojevic L, Gershenson DM, S AK. VEGF Overexpression and angiogenesis are prevalent and predict clinical outcome in sex cord-stromal ovarian tumors. Gynecologic Oncology. 2009 abstract, in press. [Google Scholar]
- 16.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 17.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 18.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 19.New Approval for Bevacizumab. 2004 http://www.fda.gov/cder/Offices/OODP/whatsnew/bevacizumab200802.htm.
- 20.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 21.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D, Scudder S, Morgan R, Chen H, Lenz HJ, Oza AM. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, Nugent E, Han LY, Landen CN, Jr, Spannuth WA, Lu C, Coleman RL, Gershenson DM, Sood AK. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 24.Monk BJ, Han E, Josephs-Cowan CA, Pugmire G, Burger RA. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol Oncol. 2006;102:140–4. doi: 10.1016/j.ygyno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DL, Backes FJ, Seamon LG, Zanagnolo V, O'Malley DM, Cohn DE, Fowler JM, Copeland LJ. Combination gemcitabine, platinum, and bevacizumab for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2008;111:461–6. doi: 10.1016/j.ygyno.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Kesterson JP, Mhawech-Fauceglia P, Lele S. The use of bevacizumab in refractory ovarian granulosa-cell carcinoma with symptomatic relief of ascites: A case report. Gynecol Oncol. 2008 doi: 10.1016/j.ygyno.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–34. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]


