Case Presentation
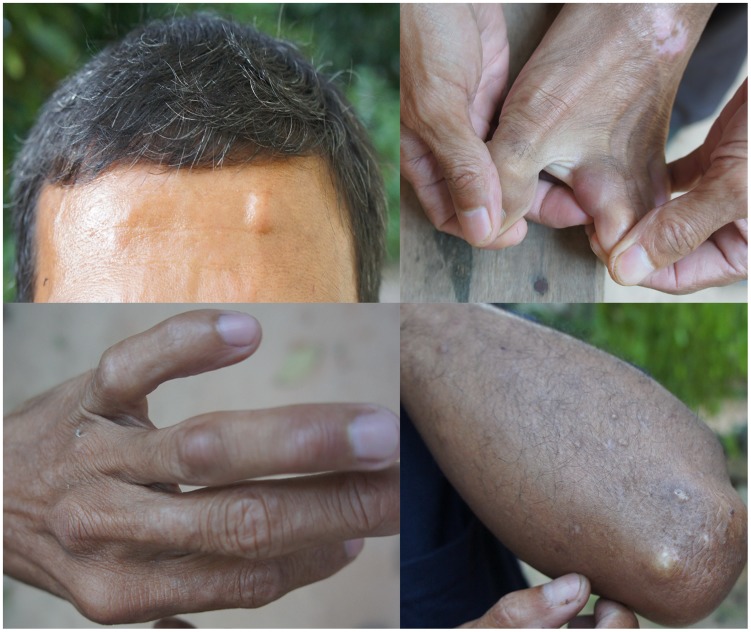
The patient was a 49-year-old male rubber planter living in southern Thailand who has had HIV infection for 10 years. He was diagnosed with disseminated cutaneous and visceral leishmaniasis 2 years previously and was treated with amphotericin B deoxycholate and itraconazole for leishmaniasis and with tenofovir, lamivudine, and nevirapine for HIV. Six months before this visit, he observed multiple nodules on his brow, left second toe, left ring finger, and left elbow (Fig. 1). His CD4+ Tcell count was 207 cells/mm3.
Figure 1. Nodules on brow, left second toe, left ring finger, and left elbow of nodular leishmaniasis case.
Diagnosis and Treatment
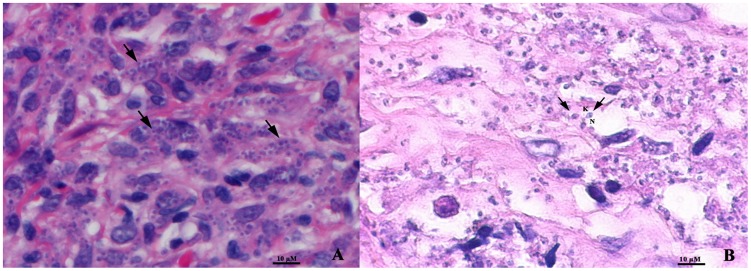
Diagnosis of relapsed Leishmania siamensis infection in this patient was performed by microscopic examination, culture, and polymerase chain reaction (PCR). Microscopic examination of tissue sections from his brow showed numerous intracellular organisms (Fig. 2A), and typical Leishmania amastigote parasites containing nucleus and kinetoplast were shown in a tissue biopsy that was submerged in Schneider's medium for two days and its sections then cut and stained with hematoxylin and eosin (H&E) (Fig. 2B). Numerous promastigotes were also observed in culture. PCR was performed using a primer set specific to the 18S rRNA gene of the internal transcribed spacer 1 (ITS1) of Leishmania spp. [1]. L. siamensis infection was identified by nucleotide sequencing and comparison with a sequence of L. siamensis reported previously (accession number JQ001751) and was shown to be 100% identical. After a final diagnosis of nodular leishmaniasis was established, the patient received 1 mg/kg/day of intravenous amphotericin B deoxylate for 28 days, followed by 300 mg of oral itraconazole twice a day for 5 months. The nodules regressed, and PCR detection of L. siamensis DNA in saliva and blood samples after the treatment was negative.
Figure 2. Numerous intracellular amastigotes (arrows) shown in a tissue biopsy of nodule from brow (A) and in a section of tissue submerged in Schneider's insect medium (B) with H&E staining. N, nucleus; K, kinetoplast (magnification x1000).
Discussion
Leishmaniasis is a parasitic disease spread by the bite of infected female sand flies. The life cycle of Leishmania is initiated by sand flies feeding on the blood of an infected vertebrate host. Amastigotes of Leishmania transform into promastigotes in the digestive tract of female sand flies and are transmitted to a new vertebrate host during the next blood feed [2]. Currently, infection with the disease has resulted in approximately 0.2 to 0.4 million cases of visceral leishmaniasis and 0.7 to 1.2 million cases of cutaneous leishmaniasis [3]. Globally, leishmaniasis is a significant cause of morbidity and mortality in several countries. The disease is often a coinfection among HIV patients, tourists, refugees, and military personnel as well as among residents of endemic areas. Atypical clinical presentation of leishmaniasis has been described in AIDS patients, such as in a case of dermonodular and visceral leishmaniasis caused by L. infantum in an AIDS patient [4]. Leishmaniasis is usually found in HIV patients who have a CD-4+T cell level less than 200 cells/mm3 [5]–[9].
Autochthonous leishmaniasis cases in Thailand have dramatically increased in recent years [6]–[11]. The disease was reported in both immunocompetent and immunocompromised hosts, especially AIDS patients. Approximately 20 cases of autochthonous leishmaniasis have been documented, and most have been reported from southern Thailand [6]–[11]. Sukmee et al. [10] reported that autochthonous leishmaniasis in Thailand was caused by a novel species that later became known as L. siamensis. Phylogenetic analysis of three protein-coding DNA sequences showed a monophyletic clade that included both the L. siamensis and L. enriettii complex [7]. Surveys conducted in areas where L. siamensis infection has been reported in Thailand showed that Sergentomyia (Neophlebotomus) gemmea and S. (Parrotomyia) barraudi sand flies and black rats (Rattus rattus) were suspected to be a potential vectors and animal reservoirs for L. siamensis, respectively [12]. More recently, indigenous L. siamensis infection has been described in Myanmar in a patient who was treated with corticosteroids and in an AIDS patient [8], [13]. L. siamensis was also detected as a causative agent of cutaneous leishmaniasis in cows and horses in Germany [14], Switzerland [15], and the United States [16]. Patients infected with L. siamensis may present with classical clinical presentations of leishmaniasis, including visceral [9], [10], [11], diffuse cutaneous [8], [13], and overlapping diffuse cutaneous and visceral forms [6], [7], [8].
Diagnosis of L. siamensis infection relies on microscopy, culture, and PCR. Microscopic examination and culture are time consuming and require expertise to be reliable, while other screening tests for leishmaniasis such as enzyme-linked immunosorbent assay (ELISA), direct agglutination test (DAT), and rK39 dipsticks are not widely available. Diagnosis of L. siamensis infection is based on PCR and sequencing. Samples used for PCR for L. siamensis include blood, bone marrow, tissue, urine, and saliva. Recently, Phumee and colleagues demonstrated that saliva is a good source for PCR detection of L. siamensis DNA [8]. They also demonstrated that L. siamensis DNA levels in saliva decreased after treatment [8]. Saliva could thus be used as a biomarker to detect L. siamensis infection and follow treatment.
Several drugs are used for treatment of leishmaniasis, such as pentavalent antimonials, amphotericin B deoxycholate, lipid formulations of amphotericin B, miltefosine, and paromomycin. However, amphotericin B is the only drug available for treatment of L. siamensis infection in Thailand.
Recurrence of L. siamensis infection after amphotericin B therapy occurred in some patients. As documented in [9], a seronegative girl was successfully treated for a recurrent L. siamensis infection after amphotericin B treatment by extending amphotericin B therapy from 3 weeks to 5 weeks and following it with 6 months of prophylaxis.
Boxes
Key Learning Points
Leishmaniasis caused by L. siamensis is an emerging disease in Thailand and Myanmar.
L. siamensis infections were described in immunocompromised hosts such as individuals on systemic steroids and AIDS patients.
Patients infected by L. siamensis can present with visceral, disseminated cutaneous, and overlapping of disseminated cutaneous and visceral forms.
Diagnosis of L. siamensis can be performed by PCR using primers specific to the 18S rRNA gene of the ITS1, and saliva has been shown to be a good source of DNA for PCR.
Amphotericin B is an effective therapy for L. siamensis infection. Although some patients have developed relapsed leishmaniasis after treatment, extended amphotericin B therapy followed by monthly prophylaxis for 6 months is effective.
Funding Statement
This study was supported by the tuition fee scholarship, Graduate School, Chulalongkorn University and the Thailand Research Fund (TRF Senior Research Scholar: RTA5480006); the Ratchadapisak Sompotch Fund, Faculty of Medicine, Chulalongkorn University (grant no. RA (MF) 02/56); and National Science and Technology Development Agency (Thailand), P-12-01458 and Research Chair Grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Spanakos G, Piperaki ET, Menounos PG, Tegos N, Flemetakis A, et al. (2007) Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg 102: 46–53. [DOI] [PubMed] [Google Scholar]
- 2. Kamhawi S (2006) Phlebotomine sand flies and Leishmania parasites: friends or foes?. Trends Parasitol 22: 439–445. [DOI] [PubMed] [Google Scholar]
- 3. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scaglia M, Malfitano A, Douville H, Sacchi P, Gatti S, et al. (1996) Dermonodular and visceral leishmaniasis due to Leishmania infantum with a new isoenzyme pattern: report of a case involving a patient with AIDS. Clin Infect Dis 22: 376–377. [DOI] [PubMed] [Google Scholar]
- 5. Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, et al. (2008) The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chusri S, Hortiwakul T, Silpapojakul K, Siriyasatien P (2012) Case Report: consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am J Trop Med Hyg 87: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, et al. (2012) Case Report: autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis . Am J Trop Med Hyg 86: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phumee A, Kraivichian K, Chusri S, Noppakun N, Vibhagool A, et al. (2013) Detection of Leishmania siamensis DNA in saliva by polymerase chain reaction. Am J Trop Med Hyg 89: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osatakul S, Mungthin M, Siripattanapipong S, Hitakarun A, et al. (2014) Recurrences of visceral leishmaniasis caused by Leishmania siamensis after treatment with amphotericin B in a seronegative child. Am J Trop Med Hyg 90: 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, et al. (2008) A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol 38: 617–622. [DOI] [PubMed] [Google Scholar]
- 11. Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P (2010) Case Report: autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg 82: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P (2014) Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health 45: 13–19. [PubMed] [Google Scholar]
- 13. Noppkun N, Kraivichian K, Siriyasatien P (2014) Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg 91: 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, et al. (2009) Occurrence of Leishmania spp. in cutaneous lesions of horses in central Europe. Vet Parasitol 166: 346–351. [DOI] [PubMed] [Google Scholar]
- 15. Lobsiger L, Muller N, Schweizer T, Frey CF, Wiederkehr D, et al. (2010) An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol 169: 408–414. [DOI] [PubMed] [Google Scholar]
- 16. Reuss SM, Dunbar MD, Calderwood Mays MB, Owen JL, Mallicote MF, et al. (2012) Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis 18: 1545–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]