Abstract
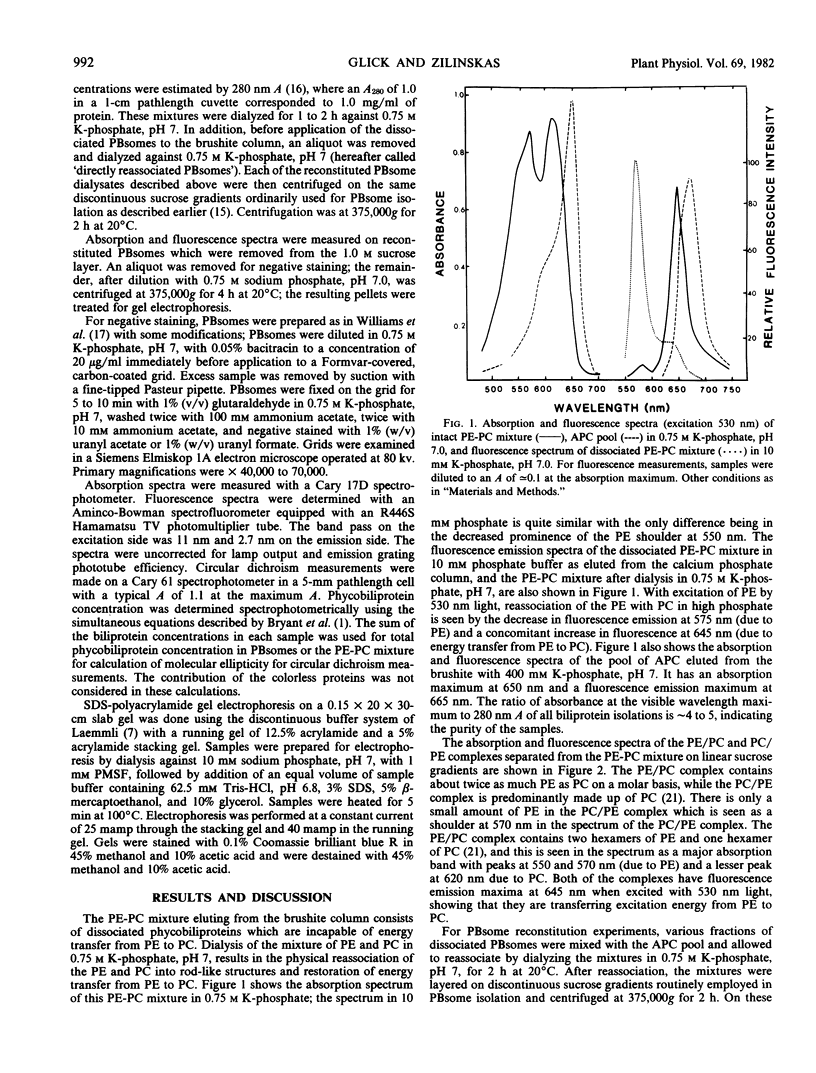
A phycoerythrin (PE) and phycocyanin (PC) mixture was separated from allophycocyanin on calcium phosphate chromatography from completely dissociated phycobilisomes of the blue-green alga, Nostoc sp. After dialysis of the PE-PC mixture in 0.75 m potassium phosphate, pH 7, which allows reassociation of the dissociated pigment-proteins, complexes of PE and PC in a 2:1 m ratio (PE/PC complex) as well as complexes predominantly of PC (PC/PE complex) were then separated by sedimentation on linear sucrose gradients. These complexes resemble the rods of intact phycobilisomes and transfer energy efficiently from PE to PC. They contain the Group II colorless polypeptides described by Tandeau de Marsac and Cohen-Bazire (1977 Proc Natl Acad Sci USA 74: 1635 61639). Phycobilisomes can be reconstituted by combining the allophycocyanin pool with (a) the PE-PC mixture, (b) the PE/PC complex, or (c) the PC/PE complex. Successful reconstitution is measured by absorption, fluorescence, circular dichroism, and electron microscopy. The major requirement for reconstitution is the 29-kilodalton colorless polypeptide. In its absence, no phycobilisomes are formed. It is the only colorless polypeptide common to both the PE/PC complex and the PC/PE complex, and appears to be the polypeptide responsible for rod attachment to the allophycocyanin. In addition, high phosphate concentrations and 20°C temperatures are needed for reconstitution.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Zilinskas B. Further evidence for a phycobilisome model from selective dissociation, fluorescence emission, immunoprecipitation, and electron microscopy. Biochim Biophys Acta. 1976 May 14;430(2):375–388. doi: 10.1016/0005-2728(76)90093-1. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Williams R. C., Yamanaka G., Schachman H. K. Characterization of cyanobacterial phycobilisomes in zwitterionic detergents. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6162–6166. doi: 10.1073/pnas.76.12.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Mörschel E. Biliprotein assemble in the disc-shaped phycobilisomes of Rhodella violacea. On the molecular composition of energy-transfering complexes (tripartite units) forming the periphery of the phycobilisome. Eur J Biochem. 1978 Nov 2;91(1):57–63. doi: 10.1111/j.1432-1033.1978.tb20936.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Isolation and Function of Allophycocyanin B of Porphyridium cruentum. Plant Physiol. 1977 May;59(5):974–980. doi: 10.1104/pp.59.5.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- Rigbi M., Rosinski J., Siegelman H. W., Sutherland J. C. Cyanobacterial phycobilisomes: Selective dissociation monitored by fluorescence and circular dichroism. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1961–1965. doi: 10.1073/pnas.77.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusckowski M., Zilinskas B. A. Chlorophyll-Protein Complexes of the Cyanophyte, Nostoc sp. Plant Physiol. 1980 Feb;65(2):392–396. doi: 10.1104/pp.65.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Greenwald L. S., Zilinskas B. A. Allophycocyanin from Nostoc sp. phycobilisomes. Properties and amino acid sequence at the NH2 terminus of the alpha and beta subunits of allophycocyanins I, II, and III. J Biol Chem. 1980 Oct 10;255(19):9380–9387. [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- Zilinskas B. A., Glick R. E. Noncovalent Intermolecular Forces in Phycobilisomes of Porphyridium cruentum. Plant Physiol. 1981 Aug;68(2):447–452. doi: 10.1104/pp.68.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]