Abstract
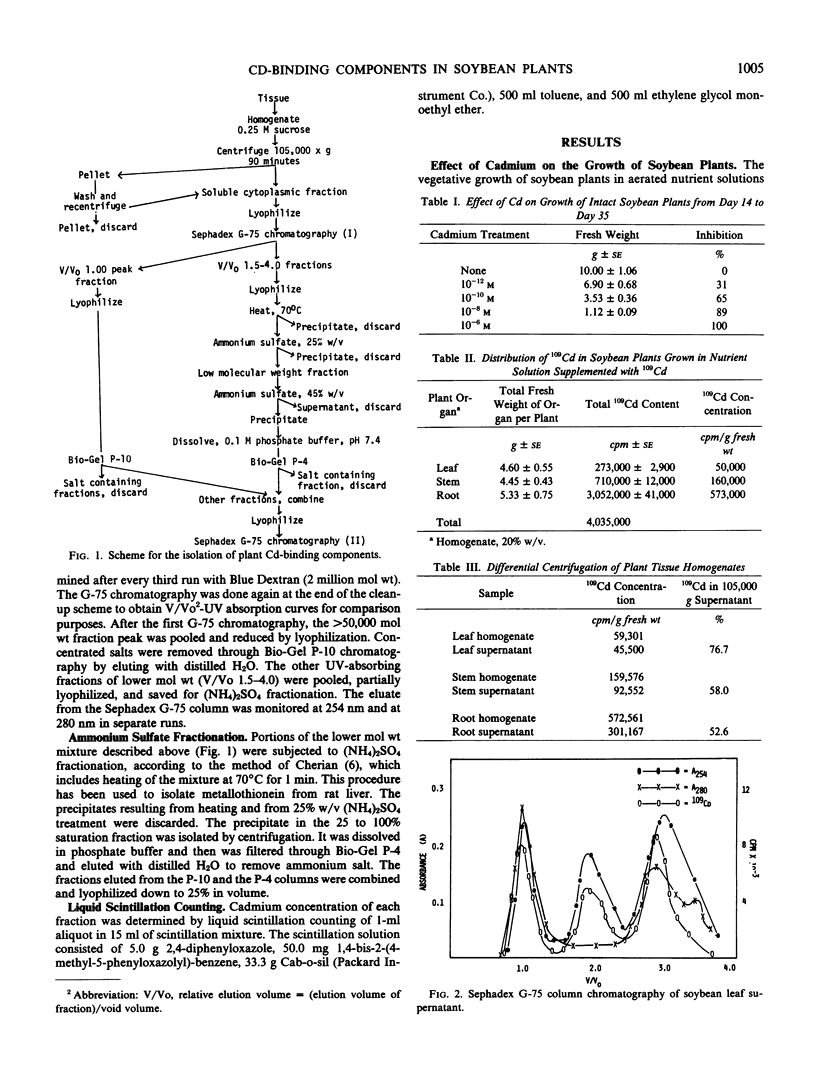
Soybean (Glycine max L.) plants exposed to 109Cd readily absorb the element. Differential centrifugation of leaf, stem, and root homogenates followed by radioassay showed that Cd was associated primarily with the 105,000g supernatant. Separation of this fraction by gel chromatography and subsequent analysis by radioassays revealed that 109Cd was bound to macromolecules of >50,000, 13,800, and 2,280 molecular weights. The >50,000 and 2,280 molecular weight fractions probably are nonspecific binding of Cd to normal cell components. The 13,800 molecular weight 109Cd-bound component was found to be inducible by cadmium. It had a high ultraviolet absorbance at 254 nm and a low absorbance at 280 nm at pH 8.6.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolf M., Brennan E., Price C. A. Partial Characterization of a Cadmium-binding Protein from the Roots of Cadmium-treated Tomato. Plant Physiol. 1980 Sep;66(3):438–441. doi: 10.1104/pp.66.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Chen R. W., Whanger P. D., Weswig P. H. Biological function of metallothionein. I. Synthesis and degradation of rat liver metallothionein. Biochem Med. 1975 Feb;12(2):95–105. doi: 10.1016/0006-2944(75)90100-3. [DOI] [PubMed] [Google Scholar]
- Cherian M. G. Isolation and purification of cadmium binding proteins from rat liver. Biochem Biophys Res Commun. 1974 Dec 11;61(3):920–926. doi: 10.1016/0006-291x(74)90243-5. [DOI] [PubMed] [Google Scholar]
- Cutler J. M., Rains D. W. Characterization of cadmium uptake by plant tissue. Plant Physiol. 1974 Jul;54(1):67–71. doi: 10.1104/pp.54.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Y., Bazzaz F. A., Vanderhoef L. N. The inhibition of soybean metabolism by cadmium and lead. Plant Physiol. 1974 Jul;54(1):122–124. doi: 10.1104/pp.54.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Pulido P., Kägi J. H., Vallee B. L. Isolation and some properties of human metallothionein. Biochemistry. 1966 May;5(5):1768–1777. doi: 10.1021/bi00869a046. [DOI] [PubMed] [Google Scholar]
- Ridlington J. W., Fowler B. A. Isolation and partial characterization of a cadmium-binding protein from the American oyster (Crassostrea virginica). Chem Biol Interact. 1979 May;25(2-3):127–138. doi: 10.1016/0009-2797(79)90041-3. [DOI] [PubMed] [Google Scholar]
- Shaikh Z. A., Lucis O. J. Isolation of cadmium-binding proteins. Experientia. 1971 Sep 15;27(9):1024–1025. doi: 10.1007/BF02138857. [DOI] [PubMed] [Google Scholar]
- Squibb K. S., Cousins R. J. Control of cadmium binding protein synthesis in rat liver. Environ Physiol Biochem. 1974;4(1):24–30. [PubMed] [Google Scholar]



