Abstract
Deoxynivalenol (DON) has various toxicological effects in humans and pigs that result from the ingestion of contaminated cereal products. This study was conducted to investigate the protective effects of dietary supplementation with glutamic acid on piglets challenged with DON. A total of 20 piglets weaned at 28 d of age were randomly assigned to receive 1 of 4 treatments (5 piglets/treatment): 1) basal diet, negative control (NC); 2) basal diet +4 mg/kg DON (DON); 3) basal diet +2% (g/g) glutamic acid (GLU); 4) basal diet +4 mg/kg DON +2% glutamic acid (DG). A 7-d adaptation period was followed by 30 days of treatment. A metabolite analysis using nuclear magnetic resonance spectroscopy (1H-NMR)-based metabolomic technology and the determination of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities for plasma, as well as the activity of Caspase-3 and the proliferation of epithelial cells were conducted. The results showed that contents of low-density lipoprotein, alanine, arginine, acetate, glycoprotein, trimethylamine-N-oxide (TMAO), glycine, lactate, and urea, as well as the glutamate/creatinine ratio were higher but high-density lipoprotein, proline, citrate, choline, unsaturated lipids and fumarate were lower in piglets of DON treatment than that of NC treatment (P<0.05). Compared with DON treatment, dietary supplementation with glutamic acid increased the plasma concentrations of proline, citrate, creatinine, unsaturated lipids, and fumarate, and decreased the concentrations of alanine, glycoprotein, TMAO, glycine, and lactate, as well as the glutamate/creatinine ratio (P<0.05). Addition glutamic acid to DON treatment increased the plasma activities of SOD and GSH-Px and the proliferating cell nuclear antigen (PCNA) labeling indexes for the jejunum and ileum (P<0.05). These novel findings indicate that glutamic acid has the potential to repair the injuries associated with oxidative stress as well as the disturbances of energy and amino acid metabolism induced by DON.
Introduction
The trichothecene mycotoxin deoxynivalenol (DON) is often found as a contaminant in agricultural staples, and the toxic effects of DON have been well-characterized in humans as well as pigs which is the most susceptible animal [1]. Due to the high percentage of grain in the pig diet, pigs can easily be exposed to DON. If we consider the similarities between humans and pigs, the pig can be regarded as a good model for investigating the toxicity of DON in humans [2]. Although the pathogenesis caused by DON in vivo is being revealed, little is known about the metabolic mechanism of DON in piglets.
While many strategies have been developed to reduce the toxic effects of DON, including physical adsorption, chemical decomposition and microbial detoxification [3]–[5], dietary strategies are the most promising approach to the problem[6]. Glutamic acid, a functional amino acid, is one of the most abundant amino acids in intestinal tract protein [7]. This nutrient plays multiple roles in the intestine, including energy production [8], taste activation [9], metabolism[10], redox state and detoxification process [11]. Thus,glutamic acid may be useful for alleviating the injury to the intestine induced by DON. Indeed, we have found that supplementation with glutamic acid can alleviate the negative effects of DON in piglets. However, little is known about the mechanism by which glutamic acid exerts its beneficial effects in DON-challenged piglets.
Substantial effort is being directed toward the study of metabolomics, which provides a useful systematic approach to understanding the global metabolic responses of living systems to influence such as disease, nutrition and environment [12]–[14]. Thus, the plasma metabolome that is associated with treatment with glutamic acid and DON in piglets was determined by a nuclear magnetic resonance-based (NMR) metabolomic method. This study was conducted to analyze the toxic effects of DON-contaminated feed on pigs and to investigate the effects of supplemental glutamic acid on DON-induced toxic damage in piglets.
Materials and Methods
This study was conducted in accordance with the Chinese Guidelines for Animal Welfare and was approved by the Animal Care and Use Committee of the Chinese Academy of Sciences (Beijing, China) [15].
Preparation of DON-contaminated feed
The mold strain F.graminearum R6576, that is only able to produce DON, was provided by the College of Plant Science & Technology of Huazhong Agricultural University, China. DON-contaminated feed was prepared according to previous reports from our group [6]. The resulting feed was determined to contain 4mg/kg DON [16], and the dose was chosen according to the report by Prelusky et al. (1994). Processed diet was mixed with unprocessed diet at a ratio of 1∶1, and glutamic acid was added according to the experimental design.
Experimental design
A total of 20 piglets (Duroc × Landrace × Large Yorkshire) weaned at 28 d of age were randomly assigned to receive 1 of 4 treatments (5 piglets/treatment): 1) basal diet, negative control (NC); 2) basal diet +4 mg/kg DON (DON); 3) basal diet +2% (g/g) glutamic acid (GLU); 4) basal diet +4 mg/kg DON +2% (g/g) glutamic acid (DG). The basal diets were prepared from corn, soybean meal, wheat bran, limestone, CaHPO4, salt, and additive premix to meet or exceed the nutritional requirements for growing pigs as recommended by the NRC (1998) (Table 1). The amount of DON (mg/kg) in the NC, DON, GLU, and DG diets was determined to be 1.02±0.03, 4.01±0.06, 1.03±0.02, and 4.03±0.04, respectively.
Table 1. Composition and nutrient levels of basal diet (as-fed basis)1.
| Item | NC | DON | GLU | DG |
| Ingredients, % | ||||
| Corn | 61.25 | 61.25 | 60.03 | 60.03 |
| Soybean | 15.79 | 15.79 | 15.47 | 15.47 |
| Extruded-soybean | 10.00 | 10.00 | 9.80 | 9.80 |
| Fish meal | 5.00 | 5.00 | 4.90 | 4.90 |
| Wheat bran | 3.00 | 3.00 | 2.94 | 2.94 |
| Soybean oil | 1.74 | 1.74 | 1.71 | 1.71 |
| Premix2 | 1.00 | 1.00 | 0.98 | 0.98 |
| Limestone powder | 0.98 | 0.98 | 0.96 | 0.96 |
| Calcium hydrogen phosphate | 0.78 | 0.78 | 0.76 | 0.76 |
| Salt | 0.37 | 0.37 | 0.36 | 0.36 |
| Glutamic acid | 0.00 | 0.00 | 2.00 | 2.00 |
| Lys·HCl (98%) | 0.09 | 0.09 | 0.09 | 0.09 |
| Analyzed chemical composition | ||||
| DM, % | 89.85 | 89.84 | 89.83 | 89.82 |
| CP, % | 18.90 | 18.91 | 18.96 | 18.97 |
| Crude ash, % | 6.79 | 6.78 | 6.77 | 6.75 |
| Calculated DE, kcal/kg | 3400 | 3400 | 3400 | 3400 |
NC = uncontaminated basal diet, DON = basal diet contaminated with deoxynivalenol (4 mg/kg), GLU = uncontaminated basal diet supplemented with 2% glutamic acid; DG = DON diet supplemented with 2% glutamic acid.
Providing the following amount of vitamins and minerals per kilogram on an as-fed basis: Zn (ZnO), 50 mg; Cu (CuSO4), 20 mg; Mn (MnO), 55 mg; Fe (FeSO4), 100 mg; I (KI), 1 mg; Co (CoSO4), 2 mg; Se (Na2SeO3), 0.3 mg; vitamin A, 8,255 IU; vitamin D3, 2,000 IU; vitamin E, 40 IU; vitamin B1, 2 mg; vitamin B2, 4 mg; pantothenic acid, 15 mg; vitamin B6, 10 mg; vitamin B12, 0.05 mg; vitamin PP, 30 mg; folic acid, 2 mg; vitamin K3, 1.5 mg; biotin, 0.2 mg; choline chloride, 800 mg; and vitamin C, 100 mg. The premix did not contain additional copper, zinc, antibiotics, or probiotics.
The experiment was arranged as a randomized design, and pigs were allowed free access to water throughout the experimental period. After an adaptation period of 7 days, piglets were fed their respective diets 3 times per day (at 8:00, 13:00 and 18:00) for a 30-d period. Fifteen and 30 d after the initiation of treatment, 10 mL of blood was collected from a jugular vein into a collection tube with heparin sodium 2 h after feeding, and centrifuged at 1000×g for 10 min at 4°C to obtain plasma samples, which were stored at −80°C for further analysis. On d 30, piglets were anesthetized with sodium pentobarbital and exsanguinated. The small intestine was excised, and rinsed thoroughly with ice-cold physiological saline solution, and the jejunum and ileum were dissected out. Two-centimeter segments of the mid-jejunum and mid-ileum were cut and fixed in 4% formaldehyde for measurements of crypt cell proliferation. In addition, samples of the jejunal and ileal mucosa were immediately snap-frozen in liquid N and stored at −80°C for the determination of Caspase-3.
Plasma GSH-Px and SOD activities
Glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities were measured using spectrophotometric kits in accordance with the manufacturer's instructions (Nanjing Jiancheng Biotechnology Institute, Nanjing, China).
Intestinal Crypt Cell Proliferation and caspase-3
After tisssue samples were subjected to dehydration, embedding, and sectioning, crypt cell proliferation was determined using proliferating cell nuclear antigen (PCNA) as described by Xu et al. (2003). [17] The primary monoclonal antibody against PCNA (Calbiochem, Cambridge, UK) was obtained commercially (Wuhan Boster Biological Technology Co., Ltd., Wuhan, China) together with a streptavidin-biotin complex detection kit. Prior to staining, the sections were heated by microwave in 0.01 M citric acid solution for antigen retrieval. As a negative control, primary antibodies were replaced with phosphate buffer solution. The stained sections were reviewed and scored independently by 2 investigators using a microscope (Olympus, Tokyo, Japan). The PCNA labeling index was expressed as the ratio of cells that were positively stained for PCNA to all epithelial cells in at least 5 areas that were randomly selected for counting at less than 200-fold magnification. Caspase-3 activity was measured using a colorimetric assay kit in accordance with the manufacturer's instructions (Keygentec, Nanjing, China).
1H NMR Spectroscopic measurement of plasma samples
1H NMR spectroscopic measurement of plasma samples was conducted as described previously [18]. Briefly, plasma samples (500 µL) were placed in 5-mm NMR tubes with 50 µL D2O (as a lock signal) and 50 µL 0.9% saline. All NMR spectra were measured at a 1H frequency of 600.11 MHz using a Bruker Avance AVIII 600 spectrometer at 298 K (Bruker Biospin, Rheinstetten, Germany). Water pre-saturation was achieved by using a standard one-dimensional (1D) NMR spectrum, which is a general representation of the total metabolite composition. An inter-pulse delay of 3 µs, a mixing time of 100ms and irradiation of the water resonance were used to attenuate signals from macromolecules by the CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence. Large macromolecule signals were detected by using a BPP-LED (bipolar-pair longitudinal eddy) current pulse sequence. For resonance assignment purposes, two-dimensional 1H-1H COSY (correlation spectroscopy) and TOCSY (total correlation spectroscopy) were also performed for selected plasma samples.
Free induction decays (FID) were multiplied by an exponential window function of 1.0 Hz prior to Fourier transformation and corrected for phase and baseline distortions using TopSpin 2.0 (Bruker). Chemical shifts were referenced to the peak of the anomeric proton of α-glucose at δ 5.23. NMR spectra (δ 0.5–8.5) were binned with regions 0.002 ppm wide and automatically integrated with the AMIX package (v.3.8.3, Bruker Biospin, Germany). The region at δ 4.55–5.13 was removed to avoid the effects of imperfect water suppression. Consequently, the spectra over the ranges δ 0.5–4.55, and δ 5.13–8.5 were selected and reduced to 3663 regions, each of which was 0.002ppm wide. Each integral region was normalized to the sum of all integral regions for each spectrum prior to pattern recognition analysis.
An overview of the data distribution and intersample similarities (e.g., clusterings and outliers) for each serum sample was first investigated by PCA (principal component analysis) using Simca-P 11.0 software (Umetrics, Sweden). NMR spectral data were further analyzed using the OPLS-DA (orthogonal projection to latent structure with discriminant analysis) method with unit variance scaling. Since the OPLS-DA results for the BPP-LED spectra of serum are similar to those for standard 1D spectra, the analysis of BPP-LED spectra will not be discussed in the Results section.
Statistical Analysis
All statistical analyses were performed using the SAS software package (Version 9.2; SAS Institute, Cary, NC, USA). Data were subjected to a Proc Mixed analysis of variance-covariance followed by Tukey's multiple comparisons test. Data are expressed as the mean ± standard error of the mean.
Results
Plasma SOD and GSH-Px activities
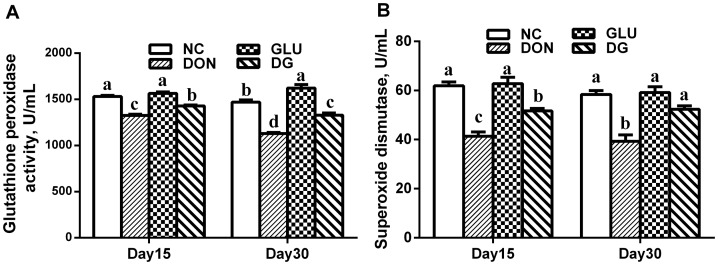
Oxidative stress has been shown to be involved in the progression of DON-induced injuries, and investigations have found that dietary supplementation with DON increases the production of reactive oxygen metabolities, such as hydroxyl radical, hydrogen peroxide, and superoxide [19]. Furthermore, glutamic acid plays a crucial role in the intestinal tract as a regulator of oxidative reactions [11]. Thus, we determined the activities of two major factors in the anti-oxidative system: SOD and GSH-Px. As shown in Fig. 1, there was no significant difference in SOD between the NC and GLU groups on days 15 and 30 (Fig. 1 A), or in GSH-Px on day 15 (Fig. 1 B). However, treatment with GLU increased (P<0.05) GSH-Px activity on day 30, compared to that in the NC group (Fig. 1 B). DON decreased (P<0.05) SOD and GSH-Px activities compared with those in the control on days 15 and 30. This decrease was reversed (P<0.05) by supplementation with glutamic acid on days 15 and 30 (Figs. 1 A and B).
Figure 1. Antioxidant enzymes activities in each group.
A: SOD activity in each group at day 15 and 30. B: GSH-Px activity in each group at day 15 and 30. Dietary treatments were NC, an uncontaminated basal diet, DON, the basal contaminated with 4mg/kg deoxynivalenol, GLU, uncontaminated basal diet with 2% glutamic acid supplementation, and DG, deoxynivalenol-contaminated (4 mg/kg) basal diet with 2% glutamic acid supplementation. Data are presented as means ± SEM, n = 5 for treatments, with a-d used to indicate statistically significant difference (P<0.05, one way ANOVA method). SOD: superoxide dismutase (U/ml); GSH-PX: glutathione peroxidase (U/ml).
Intestinal crypt cell proliferation and caspase-3
A decrease in the production of PCNA labeling index has been reported in piglets treated with DON [20]. In this study, we found that the DON significantly reduced (P<0.05) the PCNA labeling index in the jejunum and ileum, compared to that in the NC group (Table 2). The PCNA labeling index in both the jejunum and ileum in the DG group was greater than that with DON (P<0.05). Caspases orchestrate the dismantling and clearance of apoptotic cells, and among these caspase-3 appears to be responsible for the apoptotic hallmarks [21]. A significant increase in caspase-3 activity was observed in the jejunum and ileum of piglets in the DON group (P<0.05; Table 3). The caspase-3 activity in the jejunum and ileum in the GLU and DG groups did not differ from that in the NC group.
Table 2. Effects of glutamic acid on the proliferating cell nuclear antigen labeling index in the jejunum and ileum in piglets challenged with deoxynivalenol (%)1 , 2.
| Item | Diet | SEM | P-value | |||
| NC | DON | GLU | DG | |||
| Jejunum | 39.83a | 6.15c | 37.83a | 29.48b | 3.48 | <0.01 |
| Ileum | 61.88a | 22.63c | 57.58a | 50.13b | 4.01 | <0.01 |
Values with different letters within the same row are different (P<0.05, one way ANOVA method).
Dietary treatments were NC, an uncontaminated basal diet, DON, the basal contaminated with 4mg/kg deoxynivalenol, GLU, uncontaminated basal diet with 2% glutamic acid supplementation, and DG, deoxynivalenol-contaminated (4 mg/kg) basal diet with 2% glutamic acid supplementation (n = 5).
Proliferating cell nuclear antigen labeling was defined as the ratio of positive cells to total cells in each section.
Table 3. Effects of glutamic acid on the Caspase-3 activity in the jejunum and ileum in piglets challenged with deoxynivalenol (%)1.
| Item | Diet | SEM | P-value | |||
| NC | DON | GLU | DG | |||
| Jejunum | 5.74b | 7.60a | 5.53b | 5.50b | 0.24 | <0.01 |
| Ileum | 5.42b | 7.33a | 5.63b | 5.75b | 0.22 | <0.01 |
Values with different letters within the same row are different (P<0.05, one way ANOVA method).
Dietary treatments were NC, an uncontaminated basal diet, DON, the basal contaminated with 4mg/kg deoxynivalenol, GLU, uncontaminated basal diet with 2% glutamic acid supplementation, and DG, deoxynivalenol-contaminated (4 mg/kg) basal diet with 2% glutamic acid supplementation (n = 5).
1H NMR Spectroscopic measurement of plasma samples
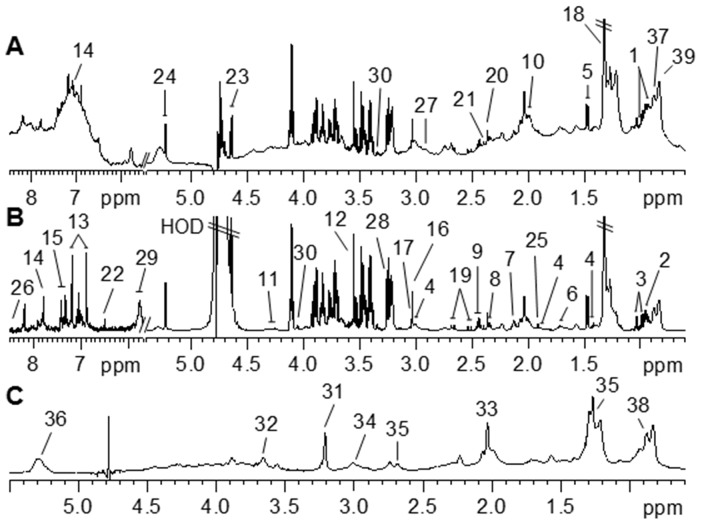
1H NMR spectra of biological fluids and tissues provide a unique fingerprint of the metabolic state of an organism along with considerable information on the nature of the drug or toxin to which an animal has been exposed [22], [23]. Examples of a 1H NMR CPMG (Fig. 2 A), standard 1D (Fig. 2 B), and BPP-LED (Fig. 2 C) spectra from a representative control pig fed the uncontaminated basal diet are shown in Fig. 2. In these spectra, 39 metabolites were unambiguously assigned by comparison to the published literature[12], [18], [24]–[26]. These assignments were confirmed by two-dimensional 1H-1H COSY and TOCSY methods (data not shown).
Figure 2. Typical 600 MHz 1H NMR spectra of plasma taken from piglets from standard 1D (A), CMPG (B) and BPP-LED (C) experiments.
The spectra in the aromatic region were magnified four times (A) (δ 5.7–8.5) or eight times (B) (δ 5.7–8.5) compared to the aliphatic region (δ 0.6–5.4). Keys for metabolites are given in Table 4 .
Visual inspection of the 1H NMR spectra revealed visible differences in plasma metabolites among piglets in the NC, DON, GLU and DG groups. For example, the concentrations of LDL, alanine, arginine, glycoprotein, TMAO, lactate and urea were higher, while those of HDL, proline, citrate, unsaturated lipids and fumarate were lower in the plasma from the DON group compared with that from the NC group. To perform a more detailed analysis of metabolic differences among the piglets in the four groups, multivariate data analyses were performed using PCA and OPLS-DA.
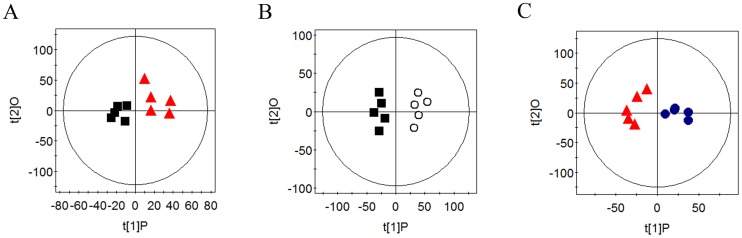
PCA of plasma CMPG and standard 1D spectral data of piglets in the four groups showed clear clustering (data not shown). Further analysis using OPLS-DA indicated that the concentrations of plasma HDL, proline, acetate, citrate, unsaturated lipids, and fumarate were decreased (P<0.05) in the DON group, compared with those in the NC and GLU groups, while the concentrations of LDL, alanine, arginine, glycoprotein, TMAO, glycine, lactate, and urea, as well as the glutamate/creatinine ratio were increased (P<0.05) in the DON group, compared with those in the NC and GLU groups. Concentrations of plasma proline, citrate, unsaturated lipids, and fumarate were higher (P<0.05) in the DG group than in the DON group, and concentrations of plasma alanine, arginine, TMAO, glycine, and lactate, as well as the glutamate/creatinine ratio were lower (P<0.05) in the DG group (Fig. 3 and Table 4).
Figure 3. OPLA-DA scores for CPMG spectra of NC (▪), DON (▴), DG (•) and GLU (○) groups.
Dietary treatments were NC, an uncontaminated basal diet, DON, the basal contaminated with 4 mg/kg deoxynivalenol, GLU, uncontaminated basal diet with 2% glutamic acid supplementation, and DG, deoxynivalenol-contaminated (4 mg/kg) basal diet with 2% glutamic acid supplementation.
Table 4. Relative integrals (%)1 from selected plasma metabolites in piglets of all groups.
| Metabolites | Relative integrals (%)1 | SEM | P-value | ||||
| NC | DON | GLU | DG | ||||
| HDL(δ0.83) | CPMG | 1.971b | 1.751c | 2.270a | 2.007ab | 0.091 | <0.01 |
| Standard 1D | 1.926a | 1.814b | 1.948a | 1.848b | 0.014 | <0.01 | |
| LDL (δ0.84) | CPMG | 1.010b | 1.266a | 0.964b | 1.261a | 0.036 | <0.01 |
| Standard 1D | 1.001b | 1.062a | 0.992b | 1.051a | 0.008 | <0.01 | |
| Alanine (δ1.48) | CPMG | 1.033b | 1.237a | 0.980b | 1.016b | 0.026 | <0.01 |
| Standard 1D | 1.013b | 1.040a | 1.003bc | 0.993c | 0.005 | <0.01 | |
| Arginine (δ1.63) | CPMG | 0.502b | 0.658a | 0.499b | 0.610ab | 0.022 | <0.01 |
| Standard 1D | 1.249b | 1.314a | 1.248b | 1.304a | 0.009 | <0.01 | |
| Acetate (δ1.92) | CPMG | 0.172b | 0.218a | 0.194b | 0.173b | 0.005 | <0.01 |
| Standard 1D | 0.310b | 0.318a | 0.320ab | 0.318ab | 0.005 | 0.168 | |
| Proline(δ2.00) | CPMG | 1.204a | 0.987b | 1.185a | 1.083ab | 0.024 | <0.01 |
| Standard 1D | 1.478a | 1.408c | 1.486a | 1.450b | 0.008 | <0.01 | |
| Glycoprotein (δ2.05) | CPMG | 2.190b | 2.766a | 2.254b | 2.630a | 0.063 | <0.01 |
| Standard 1D | 1.461c | 1.593a | 1.460c | 1.513b | 0.014 | <0.01 | |
| Citrate (δ2.52) | CPMG | 0.196a | 0.166b | 0.187a | 0.197a | 0.004 | <0.01 |
| Standard 1D | 0.463a | 0.451b | 0.464a | 4.681a | 0.002 | <0.01 | |
| TMAO (δ3.26) | CPMG | 0.575b | 0.811a | 0.549b | 0.613b | 0.029 | <0.01 |
| Standard 1D | 0.169b | 0.227a | 0.184b | 0.183b | 0.006 | <0.01 | |
| Glycine (δ3.56) | CPMG | 1.179b | 1.439a | 1.237b | 1.242b | 0.028 | <0.01 |
| Standard 1D | 0.343b | 0.380a | 0.347b | 0.337b | 0.005 | <0.01 | |
| Lactate (δ4.11) | CPMG | 2.462bc | 3.817a | 2.316c | 3.154ab | 0.158 | <0.01 |
| Standard 1D | 1.289b | 1.549a | 1.234b | 1.260b | 0.034 | <0.01 | |
| Unsaturated lipids (δ5.31) | CPMG | 0.706a | 0.576b | 0.712a | 0.678ab | 0.018 | 0.014 |
| Standard 1D | 0.757a | 0.640c | 0.781a | 0.679b | 0.014 | <0.01 | |
| Urea (δ5.78) | CPMG | 0.178b | 0.271a | 0.178b | 0.244a | 0.010 | <0.01 |
| Standard 1D | 0.165c | 0.212a | 0.179b | 0.204a | 0.005 | <0.01 | |
| Fumarate (δ6.52) | CPMG | 0.0061a | 0.0033b | 0.0061a | 0.0057a | 0.00003 | <0.01 |
| Standard 1D | 0.028a | 0.026b | 0.028a | 0.028a | 0.000 | 0.016 | |
| Glutamate/ Creatinine | CPMG | 3.300b | 4.152a | 3.352b | 3.435b | 0.086 | <0.01 |
| Standard 1D | 3.265 | 3.253 | 3.252 | 3.248 | 0.023 | 0.995 | |
Data are presented as means ± SEM, n = 5 for treatments, with a-c used to indicate statistically significant difference (P<0.05, one way ANOVA method). Normalized integral of metabolites in spectrum (normalized to 100, chemical shift region over the ranges of δ 0.5–4.55 and δ 5.13–8.5.
Dietary treatments were NC, an uncontaminated basal diet, DON, the basal contaminated with 4 mg/kg deoxynivalenol, GLU, uncontaminated basal diet with 2% glutamic acid supplementation, and DG, deoxynivalenol-contaminated (4 mg/kg) basal diet with 2% glutamic acid supplementation (n = 5).
Discussion
Among various mycotoxins, including DON, aflatoxin B1, zearalenone, fumonisin, fusariotoxin T2, and ochratoxin A, DON is encountered at the highest concentrations in the cereal foods worldwide [27], [28]. The toxic effects of DON in piglets have been studied in animal and cell culture experiments [20], [29], [30]. Previous studies have indicated that both short-term and subchronic exposure to DON disrupts immune function, antioxidative capacity, macromolecular synthesis, cell signaling, proliferation, gene up-regulation, and programmed cell death [19], [20], [30], [31]. Oxidative stress and reactive oxygen species may contribute to DON-induced toxicity in cells [32]–[34]. The present results regarding the plasma activities of anti-oxidative enzymes indicate that supplementing of DON-contaminated piglet diet with glutamic acid could significantly decrease the accumulation of reactive oxygen species (ROS) in the small intestine caused by DON. A possible explanation for this result is that glutamic acid is a precursor for glutathione, which is involved in the enterocyte redox state and in detoxification in enterocytes [35]. Thus, dietary supplementation with glutamic acid helps to scavenge the excess ROS induced by DON, thereby improving the balance between the production of ROS and the biological defense against the toxicity of these oxidants.
Due to its emetic effects, DON has been associated with human gastroenteritis [1]. It has been demonstrated that both chronic ingestion and dietary supplementation with DON induce morphological changes in the intestine of piglets, especially in the ileum and jejunum, as evidenced by shorter villi and a reduced number of goblet cells [20], [36]. The present changes in the intestinal morphology with DON and DG were supported by the PCNA labeling index, which was reduced in the DON group but increased in the DG group. PCNA is a suitable marker of proliferation potential, and is essential for DNA replication and repair [37], [38]. Mitochondria are very sensitive to oxidative stress damage, and it has been demonstrated that an excess of ROS can induce mitochondrial dysfunction [39]. It has been demonstrated that DON can induce caspase-3 activation and apoptosis in many cells [40], [41]. Similar to previous reports, the results of the current study indicate that glutamic acid can alleviate apoptosis in the small intestine induced by DON. A possible explanation is that glutamate is not only a precursor for enterocyte citrulline synthesis, but is also an ATP-producing substrate for enterocytes [35], [42]. In addition, glutamic acid is a precursor of N-acetylglutamate in enterocyte mitochondria, which is known to be an activator of the first step in citrulline biosynthesis. The plasma citrulline concentration has been proposed to be a reliable parameter for estimating the functional capacity of the intestine [43]–[45], and has been shown to correlate with enteral tolerance and bowel length in infants with short-bowel syndrome [46]. As a result, glutamic acid can be used within the intestinal mucosa to protect the intestinal epithelial barrier.
To further our understanding of the biological phenomena observed in the four treatment groups, we decided to perform targeted metabolomic analyses on a series of metabolites from the main metabolic pathways. These metabolites are the end-products or intermediates of cellular processes and therefore reflect the global integrated response of an organ or entire biological system to pathophysiologic stimuli [22]. Plasma metabolomic analysis revealed that many metabolites changed after exposure to DON. For example, treatment with DON resulted in a significant (P<0.05) increase in LDL and glycoprotein, and a marked decrease in HDL and unsaturated lipids, which suggested the presence of the lipid metabolism disorders. The results of the present study demonstrate for the first time that dietary supplementation with glutamic acid relieves the changes in the concentrations of lipids in the plasma of piglets, which is associated with the treatment of cardiovascular disorders. Triglycerides and cholesterol are transported by VLDL from the liver to various tissues. The triglycerides in VLDLs are hydrolyzed by lipase generating LDL. HDLs are involved in reverse cholesterol transfer from the tissues back to the liver. Total LDL and HDL particle concentrations have been used to assess the risk of cardiovascular disease [47], [48] and type 2 diabetes [49]. Our results indicate that LDL and HDL concentrations in the DON group were strongly and positively associated with atherogenic factors, while those with glutamic acid were inversely associated with such factors.
Another intriguing observation from the current study is that the DON group had an elevated concentration of TMAO and a greater glutamate/creatinine ratio, which suggested renal medullary injury and hepatic failure [50]. Several studies have demonstrated that TMAO is a marker of oxidative stress [51], is correlated with the degree of renal injury inflicted by different mechanisms [52], and is related to functions of the gut microbiota [53]. For example, TMAO in urine was associated with more intense medullary damage, and was also detected in clinical situations as acute toxic exposure due to xenobiotics, or under experimental exposure to nephrotoxins, where the increased excretion of TMAO is associated with medullary damage [54]–[56]. While dietary glutamic acid reduces the plasma TMAO concentration, a possible explanation is that glutamic acid has a significant beneficial effect on intestinal barrier function, and 95% of the dietary glutamic acid that is metabolized in the first pass contributes greatly to intestinal energy generation. Notably, TMAO is a microbial metabolite of carbohydrates and amino acids, which are likely produced in the lumen of the intestine [57]. Changes in this metabolite may result from an altered activity and/or reduced number of intestinal microorganisms [58]. This finding raises a crucial question regarding the role of glutamic acid in regulating nutrient metabolism and the ecology of the gut microbiota. In addition, plasma metabolomic analysis revealed that many other metabolites changed after exposure to DON, and dietary glutamic acid counteracts these changes. For example, treatment with DON resulted in an increase in lactate alanine. Lactate is an intermediate product of the citric acid cycle, and the abnormal metabolism of lactate is a hallmark of energy metabolic disorders. Metabolic disturbance of alanine is related to the dysfunction of glomerular filtration and recycling. An elevated concentration of acetate in the plasma suggests the presence of energy metabolism disorders related to an increase in ketone bodies. Dietary glutamic acid in enterocytes is a precursor for several other amino acids, including alanine, proline, and aspartate. Dietary glutamate is an important nutritional source of C and N, which enter the citric acid cycle and are metabolized mainly into CO2, lactate and amino acids [35].
Taken together, our findings demonstrated that DON induces oxidative stress, causes epithelial cell apoptosis, and induces energy, lipid and amino acid metabolism disorders. Furthermore, dietary supplementation with glutamic acid decreases oxidative stress, promotes intestinal epithelial cell proliferation, and regulates the metabolism disorders induced by DON, indicating that glutamic acid may be a useful nutritional supplement for regulating DON-induced injury.
Acknowledgments
We offer our profound admiration and respect to the many dedicated and hard-working researchers in this field and in our lab.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This research was jointly supported by grants from the Research Subject of State Science and Technology Support Program (No. 2013BAD21B04), the National Natural Science Foundation of China (No. 31272463 and 31072042), Hunan Provincial Natural Science Foundation of China (No. 12JJ2020 and 13JJ2034) and Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (Grant No. 2011T2S15). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Archives of Toxicology 84:663–679. [DOI] [PubMed] [Google Scholar]
- 2. Schollenberger M, Jara HT, Suchy S, Drochner W, Muller HM (2002) Fusarium toxins in wheat flour collected in an area in southwest Germany. International Journal of Food Microbiology 72:85–89. [DOI] [PubMed] [Google Scholar]
- 3. He P, Young LG, Forsberg C (1993) Microbially Detoxified Vomitoxin-Contaminated Corn for Young-Pigs. Journal of Animal Science 71:963–967. [DOI] [PubMed] [Google Scholar]
- 4. Awad WA, Ghareeb K, Bohm J, Zentek J (2010) Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 27:510–520. [DOI] [PubMed] [Google Scholar]
- 5. Volkl A, Vogler B, Schollenberger M, Karlovsky P (2004) Microbial detoxification of mycotoxin deoxynivalenol. Journal of Basic Microbiology 44:147–156. [DOI] [PubMed] [Google Scholar]
- 6. Wu M, Xiao H, Ren W, Yin J, Tan B, et al. (2014) Therapeutic effects of glutamic Acid in piglets challenged with deoxynivalenol. PLoS One 9:e100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young VR, Ajami AM (2000) Glutamate: an amino acid of particular distinction. J Nutr 130:892S–900S. [DOI] [PubMed] [Google Scholar]
- 8. Reeds PJ, Burrin DG, Stoll B, Jahoor F (2000) Intestinal glutamate metabolism. Journal of Nutrition 130:978s–982s. [DOI] [PubMed] [Google Scholar]
- 9. Gabriel AS, Uneyama H (2013) Amino acid sensing in the gastrointestinal tract. Amino Acids 45:451–461. [DOI] [PubMed] [Google Scholar]
- 10. Geng M, Li T, Kong X, Song X, Chu W, et al. (2011) Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids 40:1513–1522. [DOI] [PubMed] [Google Scholar]
- 11. Wu GY, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. Journal of Nutrition 134:489–492. [DOI] [PubMed] [Google Scholar]
- 12. He Q, Kong X, Wu G, Ren P, Tang H, et al. (2009) Metabolomic analysis of the response of growing pigs to dietary L-arginine supplementation. Amino Acids 37:199–208. [DOI] [PubMed] [Google Scholar]
- 13. Nicholson JK, Holmes E, Lindon JC, Wilson ID (2004) The challenges of modeling mammalian biocomplexity. Nat Biotechnol 22:1268–1274. [DOI] [PubMed] [Google Scholar]
- 14. Nicholson JK, Wilson ID (2003) Opinion: understanding 'global' systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2:668–676. [DOI] [PubMed] [Google Scholar]
- 15. Yin Y, Yao K, Liu Z, Gong M, Ruan Z, et al. (2010) Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486. [DOI] [PubMed] [Google Scholar]
- 16. Prelusky DB, Gerdes RG, Underhill KL, Rotter BA, Jui PY, et al. (1994) Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat Toxins 2:97–104. [DOI] [PubMed] [Google Scholar]
- 17. Xu MH, Deng CS, Zhu YQ, Lin J (2003) Role of inducible nitric oxide synthase expression in aberrant crypt foci-adenoma-carcinoma sequence. World Journal of Gastroenterology 9:1246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He QH, Ren PP, Kong XF, Wu YN, Wu GY, et al. (2012) Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. Journal of Nutritional Biochemistry 23:133–139. [DOI] [PubMed] [Google Scholar]
- 19. Xiao H, Wu MM, Tan BE, Yin YL, Li TJ, et al. (2013) Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. Journal of Animal Science 91:4772–4780. [DOI] [PubMed] [Google Scholar]
- 20. Xiao H, Tan BE, Wu MM, Yin YL, Li TJ, et al. (2013) Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. Journal of Animal Science 91:4750–4756. [DOI] [PubMed] [Google Scholar]
- 21. Chipuk JE, Green DR (2006) Dissecting p53-dependent apoptosis. Cell Death and Differentiation 13:994–1002. [DOI] [PubMed] [Google Scholar]
- 22. Nicholson JK, Lindon JC, Holmes E (1999) 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189. [DOI] [PubMed] [Google Scholar]
- 23. Nicholson JK, Lindon JC (2008) Systems biology - Metabonomics. Nature 455:1054–1056. [DOI] [PubMed] [Google Scholar]
- 24. Constantinou MA, Papakonstantinou E, Benaki D, Spraul M, Shulpis K, et al. (2004) Application of nuclear magnetic resonance spectroscopy combined with principal component analysis in detecting inborn errors of metabolism using blood spots: a metabonomic approach. Analytica Chimica Acta 511:303–312. [Google Scholar]
- 25. Williams R, Lenz EM, Wilson AJ, Granger J, Wilson ID, et al. (2006) A multi-analytical platform approach to the metabonomic analysis of plasma from normal and Zucker (fa/fa) obese rats. Mol Biosyst 2:174–183. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XY, Wang YL, Hao FH, Zhou XH, Han XY, et al. (2009) Human Serum Metabonomic Analysis Reveals Progression Axes for Glucose Intolerance and Insulin Resistance Statuses. Journal of Proteome Research 8:5188–5195. [DOI] [PubMed] [Google Scholar]
- 27. Guan S, Gong M, Yin YL, Huang RL, Ruan Z, et al. (2011) Occurrence of mycotoxins in feeds and feed ingredients in China. Journal of Food Agriculture & Environment 9:163–167. [Google Scholar]
- 28. Binder EM, Tan LM, Chin LJ, Handl J, Richard J (2007) Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal Feed Science and Technology 137:265–282. [Google Scholar]
- 29. Diesing AK, Nossol C, Panther P, Walk N, Post A, et al. (2011) Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicology Letters 200:8–18. [DOI] [PubMed] [Google Scholar]
- 30. Yin J, Ren W, Duan J, Wu L, Chen S, et al. (2014) Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 46:883–892. [DOI] [PubMed] [Google Scholar]
- 31. Halawa A, Danicke S, Kersten S, Breves G (2013) Intestinal transport of deoxynivalenol across porcine small intestines. Archives of Animal Nutrition 67:134–146. [DOI] [PubMed] [Google Scholar]
- 32. Dinu D, Bodea GO, Ceapa CD, Munteanu MC, Roming FI, et al. (2011) Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon 57:1023–1032. [DOI] [PubMed] [Google Scholar]
- 33. Kouadio JH, Mobio TA, Baudrimont I, Moukha S, Dano SD, et al. (2005) Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 213:56–65. [DOI] [PubMed] [Google Scholar]
- 34. Sahu SC, O'Donnell MW, Wiesenfeld PL (2010) Comparative hepatotoxicity of deoxynivalenol in rat, mouse and human liver cells in culture. Journal of Applied Toxicology 30:566–573. [DOI] [PubMed] [Google Scholar]
- 35. Blachier F, Boutry C, Bos C, Tome D (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. American Journal of Clinical Nutrition 90:814s–821s. [DOI] [PubMed] [Google Scholar]
- 36. Bracarense APFL, Lucioli J, Grenier B, Pacheco GD, Moll WD, et al. (2012) Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. British Journal of Nutrition 107:1776–1786. [DOI] [PubMed] [Google Scholar]
- 37. Kelman Z (1997) PCNA: structure, functions and interactions. Oncogene 14:629–640. [DOI] [PubMed] [Google Scholar]
- 38. Strzalka W, Ziemienowicz A (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot 107:1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo D, Bi H, Liu B, Wu Q, Wang D, et al. (2013) Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro 27:731–738. [DOI] [PubMed] [Google Scholar]
- 40. Pestka JJ, Smolinski AT (2005) Deoxynivalenol: Toxicology and potential effects on humans. Journal of Toxicology and Environmental Health-Part B-Critical Reviews 8:39–69. [DOI] [PubMed] [Google Scholar]
- 41. Bensassi F, El Golli-Bennour E, Abid-Essefi S, Bouaziz C, Hajlaoui MR, et al. (2009) Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 264:104–109. [DOI] [PubMed] [Google Scholar]
- 42. Boutry C, Matsumoto H, Bos C, Moinard C, Cynober L, et al. (2012) Decreased glutamate, glutamine and citrulline concentrations in plasma and muscle in endotoxemia cannot be reversed by glutamate or glutamine supplementation: a primary intestinal defect? Amino Acids 43:1485–1498. [DOI] [PubMed] [Google Scholar]
- 43. Crenn P, Messing B, Cynober L (2008) Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clinical Nutrition 27:328–339. [DOI] [PubMed] [Google Scholar]
- 44. Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, et al. (2003) Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124:1210–1219. [DOI] [PubMed] [Google Scholar]
- 45. Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B (2000) Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119:1496–1505. [DOI] [PubMed] [Google Scholar]
- 46. Rhoads JM, Plunkett E, Galanko J, Lichtman S, Taylor L, et al. (2005) Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. J Pediatr 146:542–547. [DOI] [PubMed] [Google Scholar]
- 47. Mihaleva VV, van Schalkwijk DB, de Graaf AA, van Duynhoven J, van Dorsten FA, et al. (2014) A systematic approach to obtain validated partial least square models for predicting lipoprotein subclasses from serum NMR spectra. Anal Chem 86:543–550. [DOI] [PubMed] [Google Scholar]
- 48. El Harchaoui K, van der Steeg WA, Stroes ESG, Kuivenhoven JA, Otvos JD, et al. (2007) Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women - The EPIC-Norfolk Prospective Population study. Journal of the American College of Cardiology 49:547–553. [DOI] [PubMed] [Google Scholar]
- 49. Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, et al. (2003) Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 52:453–462. [DOI] [PubMed] [Google Scholar]
- 50. Saxena V, Gupta A, Gowda GAN, Saxena R, Yachha SK, et al. (2006) H-1 NMR spectroscopy for the prediction of therapeutic outcome in patients with fulminant hepatic failure. Nmr in Biomedicine 19:521–526. [DOI] [PubMed] [Google Scholar]
- 51. Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU (2005) H-1-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney International 67:1142–1151. [DOI] [PubMed] [Google Scholar]
- 52. Hauet T, Gibelin H, Richer JP, Godart C, Eugene M, et al. (2000) Influence of retrieval conditions on renal medulla injury: Evaluation by proton NMR spectroscopy in an isolated perfused pig kidney model. Journal of Surgical Research 93:1–8. [DOI] [PubMed] [Google Scholar]
- 53. Rezzi S, Ramadan Z, Fay LB, Kochhar S (2007) Nutritional metabonomics: Applications and perspectives. Journal of Proteome Research 6:513–525. [DOI] [PubMed] [Google Scholar]
- 54. Foxall PJD, Lenz EM, Lindon JC, Neild GH, Wilson ID, et al. (1996) Nuclear magnetic resonance and high-performance liquid chromatography nuclear magnetic resonance studies on the toxicity and metabolism of ifosfamide. Therapeutic Drug Monitoring 18:498–505. [DOI] [PubMed] [Google Scholar]
- 55. Griffin JL (2003) Metabonomics: NMR spectroscopy and pattern recognition analysis of body fluids and tissues for characterisation of xenobiotic toxicity and disease diagnosis. Current Opinion in Chemical Biology 7:648–654. [DOI] [PubMed] [Google Scholar]
- 56. Cole E, Naimark D, Aprile M, Wade J, Cattran D, et al. (1995) An Analysis of Predictors of Long-Term Cadaveric Renal-Allograft Survival. Clinical Transplantation 9:282–288. [PubMed] [Google Scholar]
- 57. Sugita Y, Takao K, Toyama Y, Shirahata A (2007) Enhancement of intestinal absorption of macromolecules by spermine in rats. Amino Acids 33:253–260. [DOI] [PubMed] [Google Scholar]
- 58. Cornell HJ, Stelmasiak T (2007) A unified hypothesis of coeliac disease with implications for management of patients. Amino Acids 33:43–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.