Abstract
Seventeen days after double lung transplantation, a 56-year-old patient with idiopathic pulmonary fibrosis developed respiratory distress. Imaging revealed bilateral pulmonary infiltrates with pleural effusions and physical examination demonstrated sternal instability. Broad-spectrum antibacterial and antifungal therapy was initiated and bilateral thoracotomy tubes were placed. Both right and left pleural cultures grew a mold subsequently identified as Scopulariopsis brumptii. The patient underwent pleural irrigation and sternal debridement three times but pleural and wound cultures continued to grow S. brumptii. Despite treatment with five antifungal agents, the patient succumbed to his illness sixty-seven days after transplantation. Autopsy confirmed the presence of markedly invasive fungal disease and pleural rind formation. The patient’s organ donor had received bilateral thoracostomy tubes during resuscitation in a wilderness location. There were no visible pleural abnormalities at the time of transplantation. However, the patient’s clinical course and the location of the infection, in addition to the lack of similar infection in other organ recipients, strongly suggest that Scopulariopsis was introduced into the pleural space during pre-hospital placement of thoracostomy tubes. This case of lethal infection transmitted through transplantation highlights the unique risk of using organs from donors who are resuscitated in an outdoor location.
Keywords: lung transplantation, donor-derived infection, procurement, Scopulariopsis
Introduction
Donor-derived infections are a problem unique to transplant recipients. Some of the more common infections, such as hospital-acquired bacterial infections and cytomegalovirus, can be anticipated and prevented through screening and prophylaxis. However, unforeseen transmission of a number of unusual or rare pathogens at the time of transplantation has resulted in lethal infections in organ recipients. Donor-derived infections with pathogens such as Mycobacterium tuberculosis, Rabies virus, West Nile virus, Strongyloides, Treponema pallidum, and Trypanosoma cruzi have been reported and may be difficult to detect prospectively in the donor (1–6). Even less common are donor-derived infections acquired at the time of death.
Scopulariopsis is a common environmental mold. While rarely causing significant disease in immunocompetent hosts, Scopulariopsis species occasionally cause invasive and often fatal illness in immunosuppressed patients (7). The diagnosis and management of infection caused by Scopulariopsis is challenging due to delays in microbiologic diagnosis and the organism’s inherent resistance to many antifungal therapies including amphotericin (8–10). The majority of the reported cases of Scopulariopsis infections have occurred in immunocompromised patients, especially those with hematologic malignancies or who have undergone hematopoietic stem cell transplantation.
Here, we describe a case of Scopulariopsis infection in a lung transplant recipient. Severe fungal infection was discovered seventeen days after transplantation and the patient succumbed to his illness despite aggressive surgical and antifungal management. Prior to transplantation, the organ donor had received bilateral thoracostomy tubes while lying on soil during resuscitation in the field. We hypothesize that Scopulariopsis was introduced onto the pleural surface during thoracostomy tube placement and was unknowingly transferred into the organ recipient at the time of lung transplantation. To our knowledge, this is the first reported case of infection likely acquired during the process of organ procurement and raises important questions about donor exposures and management prior to hospitalization.
Case Report
A 56-year-old man with idiopathic pulmonary fibrosis on 10mg prednisone and diabetes underwent double lung transplantation in 2011. The organ donor had bilateral thoracostomy tubes placed during resuscitation while lying on the ground in a wilderness location. Careful inspection of the donor lungs at the time of procurement revealed no surface abnormalities. Post-transplantation, the patient received induction immunosuppression with basiliximab and maintenance immunosuppression with tacrolimus, mycophenylate mofetil, and methylprednisolone. He required repeat surgical intervention for bleeding, received sixteen units of packed red blood cell transfusion, and had delayed chest closure on post-operative day three. He received routine prophylaxis with cefepime, vancomycin, itraconazole and ganciclovir. He developed sternal instability with nonunion and underwent surgical repair on post-operative day ten. The team was informed of a possibility of soil contamination of the donor lungs, but this did not change management as the patient remained clinically stable without evidence of unusual infection. The patient was discharged to inpatient rehabilitation twelve days after transplantation on prophylactic trimethoprim-sulfamethoxazole, itraconazole, and ganciclovir. Pre-transplant donor bronchoalveolar lavage (BAL) cultures had light growth of Staphyloccus aureus. Intra-operative donor BAL fungal cultures were negative.
The patient was readmitted to the hospital five days later with respiratory distress. Physical examination revealed sternal instability and decreased breath sounds at the lung bases bilaterally. Computed tomography of the chest demonstrated new bilateral pulmonary infiltrates and pleural effusions. Antimicrobial therapy with vancomycin and piperacillin-tazobactam was initiated and itraconazole was continued. Tacrolimus levels ranged from 9.0 to 16.8 ng/mL. Both pleural spaces were drained with thoracostomy tubes and pleural fluid sent for culture. The patient improved and chest tubes were removed after forty-eight hours. Two days later, he developed rapidly reaccumulating pleural effusions and anemia due to acute blood loss requiring additional transfusion. Thoracostomy tubes were replaced and revealed bilateral hemothoraces. A bronchoscopic biopsy showed A1 acute cellular rejection and organizing pneumonia. The patient’s corticosteroid dose was increased to treat rejection. Pleural cultures collected at the time of initial chest tube placement were growing mold, later identified as Scopulariopsis brumptii (Figure 1A and 1B). Antifungal therapy was switched to micafungin and voriconazole. Fungal blood cultures were negative. The patient was taken to the operating room for sternal debridement and repair. Tissue appeared grossly necrotic and operative cultures grew S. brumptii from the sternal tissues and pleural space. Amphotericin lipid complex was added to the treatment regimen. Despite change in antifungal therapy, the patient continued to decline, and he underwent three additional operative debridements with cultures from each containing S. brumptii. During the final operation, black nodules were apparent in subcutaneous tissues. Antifungal therapy was escalated with the addition of terbinafine. In addition, local intrapleural therapy with amphotericin and Dakin’s solution was given, both of which were poorly tolerated because of pain and hypotension. S. brumptii susceptibilities demonstrated resistance to voriconazole (minimum inhibitory concentration, MIC, > 2 µg/mL), posaconazole (MIC > 16 µg/mL), terbinafine (MIC > 2 µg/mL), and amphotericin (MIC > 8 µg/mL), but susceptibility to echinocandins (minimum bactericidal concentration = 2). The patient’s therapy was changed to micafungin and amphotericin was discontinued. He developed worsening respiratory distress requiring re-intubation. A repeat BAL sample grew branching mold consistent with ongoing Scopulariopsis infection and fungal blood cultures remained negative. He clinically improved, and moved to the medical ward. Testing of fungal samples for sensitivity to the alkylphosphocholine miltefosine was requested. Unfortunately, the patient developed fever and recurrent sepsis with new pulmonary infiltrates and respiratory failure. Despite treatment with broad-spectrum antibiotics and antifungal medications, the patient remained febrile, developed pancytopenia, and died from sepsis sixty-seven days after transplantation.
Figure 1. Scopulariopsis brumptii isolated from the pleural space and sternum in a lung transplant recipient.
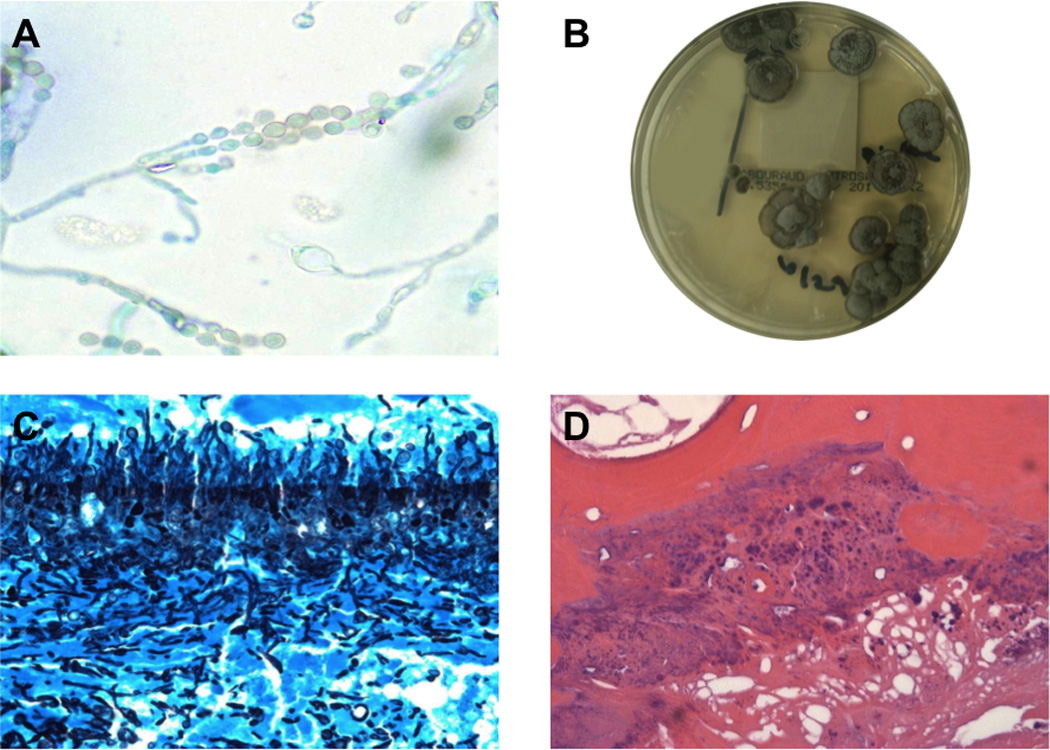
(A) S. brumptii identified in pleural fluid cultures (lactophenol blue, 100× magnification) (B) Colonies of S. brumptii isolated from pleural fluid cultures, grown on Sabouraud agar (C) Pleural rind with abundant fungal forms consistent with S. brumptii (Gomori methamine silver stain, 40× magnification) (D) Osteomyelitis due to S. brumptii (hematoxylin and eosin, 20× magnification).
On the post-mortem examination, there was a 14cm dehiscence of the sternal wound with a greenish-hue at the leading edges. Gross examination of the pleural space revealed extensive fibrosis of the visceral pleura, diaphragm, and pericardium with fibrous adhesions bilaterally. The lungs were covered with a thick rind. Microscopic examination showed widespread granulomatous response throughout the pleural space and diaphragm with Gomori methenamine silver (GMS) stains positive for abundant fungal forms (Figure 1C). There was no evidence of acute rejection in the lung parenchyma. S. brumptii was isolated from pleural fluid samples and the sternal wound. There was also evidence for S. brumptii osteomyelitis of the ribs and sternum (Figure 1D). Recipients of other donor organs, including the heart, had no clinical or microbiological evidence of S. brumptii infection. Whether the donor had any prior evidence of fungal disease is unknown. There was no evidence of fungal infection on culture or pathology of the recipient’s explanted lungs.
Discussion
This is the first reported case of fatal infection derived from organ environmental exposure at the time of donor death and is the fifth reported case of invasive Scopulariopsis in a solid organ transplant recipient. Lessons from this case include heightened awareness and caution regarding environmental exposures of the donor at the time of death.
Severe donor-derived infections following solid organ transplant often occur due to unrecognized infection of the host prior to death. In our patient, the pleural surface of the donor lungs was presumably inoculated during the placement of thoracostomy tubes during resuscitation on soil in a wooded area. Transmission of fungal disease with solid organ transplantation is rare (11, 12). Donor drowning events have been associated with increased risk of fungal infection in organ recipients (12). As in our patient, the majority of donor-derived fungal infections are locally injurious, and lead to graft failure in 83% of patients and death in 17% (12). The severe consequences of donor-derived fungal infections emphasize the need to consider the risk of donor-derived infection from any exposures during the short interval from the event causing brain death to organ procurement. This is especially true when exposures occur in uncontrolled environments and potentially contaminate the organs, as in the present case.
This case adds to the growing literature of Scopulariopsis as an important opportunistic pathogen in transplantation patients (7). There have been four previous descriptions of Scopulariopsis infections in solid organ transplant recipients, three of which were invasive and ultimately fatal (Table 1) (9, 13–15). The first report was in a liver transplant recipient who worked as a hog farmer prior to development of skin lesions and brain abscesses within several weeks of transplantation (13). It was presumed that the organ recipient had been colonized with Scopulariopsis during his occupational activity. Another liver transplant patient developed recurrent skin abscesses that grew S. brevicaulis seven years after organ transplantation (14). His fungal illness was thought to originate through skin inoculation and ultimately responded to surgical removal and chronic terbinafine therapy. Wuyts et al. describe a patient seven months after lung transplantation who developed severe fungal pericarditis due to S. acremonium resulting in tamponade, shock, and death (15). Finally, Miossec et al. describe a heart-lung transplant recipient who developed pleural effusions and fungemia due to the Scopulariopsis species, Microascus cirrosus, approximately three weeks after transplantation (9). The sources of the latter two infections were unknown. As our case illustrates, invasive Scopulariopsis infections are challenging to treat and are often fatal despite aggressive medical and surgical management. While our patient may have had increased susceptibility to infection because of his complicated series of operations, multiple transfusions, and underlying diabetes, his major infectious complications were fungal rather than bacterial in origin. In addition, his fungal illness was localized to the pleural space and contiguous tissues with no evidence of distant systemic spread.
Table 1.
Scopulariopsis infections in solid organ transplant recipients, including the current case.
| Organ Transplanted |
Interval from transplant to infection (days) |
Localized | Disseminated | Outcome | Interval from presentation to death (days) |
|---|---|---|---|---|---|
| Lung | 17 | Yes | No | Death | 50 |
| Lung | 240 | No | Yes | Death | 1 |
| Heart/Lung | 19 | No | Yes | Death | 9 |
| Liver | 2190 | Yes | No | Survived | |
| Liver | 120 | No | Yes | Death | 48 |
| TOTAL (n, %) | 2 (40%) | 3 (60%) | 4 (80%) |
Scopulariopsis species are inherently resistant to many antifungal medications (8–10). Our patient received five different antifungal agents, given either systemically or directly into the pleural space, and underwent multiple surgical debridements. Ultimately, laboratory testing showed the organism to be susceptible to micafungin with resistance to amphotericin, voriconazole, posaconazole, and terbinafine. The isolate was also sensitive in vitro to the investigational antifungal agent miltefosine, but the patient succumbed to his illness before this could be administered. Miltefosine is an alkylphosphocholine medication which is FDA-approved for treatment of Leishmania and has been shown to have antifungal properties (16). Its mechanism of action is primarily through apoptosis and disruption of lipid metabolism. Because of the high frequency of resistance to conventional antifungal therapies and high mortality when Scopulariopsis infection is present, investigational agents such as miltefosine should be considered early in the course of disease to try to improve patient outcomes.
The unfortunate clinical scenario described here represents an opportunity to reassess organ assessment and procurement procedures. When a donor requires out-of-hospital resuscitation, careful exploration of the circumstances of death and clinical management prior to organ harvest are needed. Improved communication between the donor and recipient clinicians and organ procurement organizations may allow for earlier recognition of uncommon exposures. Given the shortage of available organs and the rarity of untreatable infections, it seems unwise to categorically deny any organ from an outdoor resuscitation. However, when foreign objects such as thoracostomy tubes are placed in non-sterile conditions, serious consideration must be given to whether to transplant potentially contaminated organs. It is not clear whether sending additional samples from the lung or pleurae for culture would change patient outcomes from these rare but catastrophic infections. At a minimum, lung transplant physicians, as well as transplant infectious disease specialists, must be aware to consider unusual infections that may have been acquired during organ procurement so that additional treatment can be rapidly escalated for worsening disease.
Abbreviations
- MIC
minimum inhibitory concentration
- GMS
Gomori methenamine silver
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- West Nile virus infections in organ transplant recipients--New York and Pennsylvania, August–September, 2005. MMWR Morb Mortal Wkly Rep. 2005 Oct 14;54(40):1021–1023. [PubMed] [Google Scholar]
- 2.Transmission of Strongyloides stercoralis through transplantation of solid organs--Pennsylvania, 2012. MMWR Morb Mortal Wkly Rep. 2013 Apr 12;62(14):264–266. [PMC free article] [PubMed] [Google Scholar]
- 3.Morris MI, Daly JS, Blumberg E, Kumar D, Sester M, Schluger N, et al. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am J Transplant. 2012 Sep 12;(9):2288–2300. doi: 10.1111/j.1600-6143.2012.04205.x. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, et al. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005 Mar 17;352(11):1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 5.Tariciotti L, Das I, Dori L, Perera MT, Bramhall SR. Asymptomatic transmission of Treponema pallidum (syphilis) through deceased donor liver transplantation. Transpl Infect Dis. 2012 Jun;14(3):321–325. doi: 10.1111/j.1399-3062.2012.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Huprikar S, Bosserman E, Patel G, Moore A, Pinney S, Anyanwu A, et al. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001–2011. Am J Transplant. 2013 Sep;13(9):2418–2425. doi: 10.1111/ajt.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwen PC, Schutte SD, Florescu DF, Noel-Hurst RK, Sigler L. Invasive Scopulariopsis brevicaulis infection in an immunocompromised patient and review of prior cases caused by Scopulariopsis and Microascus species. Med Mycol. 2012 Aug;50(6):561–569. doi: 10.3109/13693786.2012.675629. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar C, Pujol I, Guarro J. In vitro antifungal susceptibilities of Scopulariopsis isolates. Antimicrob Agents Chemother. 1999 Jun;43(6):1520–1522. doi: 10.1128/aac.43.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miossec C, Morio F, Lepoivre T, Le Pape P, Garcia-Hermoso D, Gay-Andrieu F, et al. Fatal invasive infection with fungemia due to Microascus cirrosus after heart and lung transplantation in a patient with cystic fibrosis. J Clin Microbiol. 2011 Jul;49(7):2743–2747. doi: 10.1128/JCM.00127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoval-Denis M, Sutton DA, Fothergill AW, Cano-Lira J, Gene J, Decock CA, et al. Scopulariopsis, a poorly known opportunistic fungus: spectrum of species in clinical samples and in vitro responses to antifungal drugs. J Clin Microbiol. 2013 Dec;51(12):3937–3943. doi: 10.1128/JCM.01927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz I, Gavalda J, Monforte V, Len O, Roman A, Bravo C, et al. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant. 2006 Jan;6(1):178–182. doi: 10.1111/j.1600-6143.2005.01145.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomez CA, Singh N. Donor-derived filamentous fungal infections in solid organ transplant recipients. Curr Opin Infect Dis. 2013 Aug;26(4):309–316. doi: 10.1097/QCO.0b013e3283630e4d. [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Gustaferro CA, Krom RA, Wiesner RH, Roberts GD, Paya CV. Phaeohyphomycosis due to Scopulariopsis brumptii in a liver transplant recipient. Clin Infect Dis. 1994 Jul;19(1):198–200. doi: 10.1093/clinids/19.1.198. [DOI] [PubMed] [Google Scholar]
- 14.Sellier P, Monsuez JJ, Lacroix C, Feray C, Evans J, Minozzi C, et al. Recurrent subcutaneous infection due to Scopulariopsis brevicaulis in a liver transplant recipient. Clin Infect Dis. 2000 May;30(5):820–823. doi: 10.1086/313764. [DOI] [PubMed] [Google Scholar]
- 15.Wuyts WA, Molzahn H, Maertens J, Verbeken EK, Lagrou K, Dupont LJ, et al. Fatal Scopulariopsis infection in a lung transplant recipient: a case report. J Heart Lung Transplant. 2005 Dec;24(12):2301–2304. doi: 10.1016/j.healun.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012 Nov;67(11):2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]


