Abstract
Combined pulmonary fibrosis and emphysema is a condition occurring mainly in male smokers, presenting different lung mechanics and gas exchange abnormalities than emphysema or pulmonary fibrosis alone. We report the case of an elderly man, former heavy smoker, who presented with progressive exertional dyspnea for 1 year. Lung function tests showed near normal spirometry and lung volumes but marked reduction of diffusing capacity for carbon monoxide and even more nitric oxide. The arterial partial pressure of oxygen was reduced with a markedly increased alveolar-to-arterial difference. High-resolution computed tomography of the chest showed a pattern consistent with upper lobe emphysema and lower lobe pulmonary fibrosis. In conclusion, this case report confirms the limitations of a simplistic approach to lung function in the diagnosis of symptomatic smokers.
Keywords: Emphysema, lung volumes, pulmonary diffusion, pulmonary fibrosis, spirometry
Introduction
Combined pulmonary fibrosis and emphysema (CPFE) is a rare condition occurring mainly in male smokers [1]. The diagnosis is difficult because either disorder may predominate with widely different effects on lung function [2]. Early diagnosis of this condition is important because these patients may have different natural history, complications, and mortality than those with pulmonary fibrosis or emphysema alone [2]. We hereby report the case of an elderly man, with a history of heavy smoking, who presented with progressive exertional dyspnea but normal spirometry and lung volumes. An abnormal lung function was revealed by reduced pulmonary diffusion and high-resolution computed tomography (HRCT) of the chest showed a pattern consistent with a diagnosis of CPFE syndrome.
Case Report
In 2014, a 70-year-old Caucasian man, markedly overweight (body mass index: 29.3 kg·m−2) and with concomitant arterial hypertension and coronary artery disease, presented with dyspnea on exertion progressing over the last year. He had a 60-pack-year history of cigarette smoking, which he had quit in 2009. He was a shipping agent at a harbor with indirect exposure to asbestos for his whole occupational life. On auscultation, fine crackles were heard on mid-to-late inspiration over the lower lung zones bilaterally. Pulmonary function tests showed post-bronchodilator (salbutamol 400 μg) forced expiratory volume in one second (FEV1), its ratio to forced vital capacity (FEV1/FVC) and total lung capacity (TLC) slightly above the respective lower limits of normality (Table 1). Mean inspiratory resistance and reactance, measured by forced oscillation technique, were within the normal range at all forcing frequencies, without frequency dependence of resistance. However, using the single-breath nitric oxide (NO)-carbon monoxide (CO) method, the lung diffusing capacity for NO (DLNO) and CO (DLCO) were markedly reduced (32% and 57% of predicted values, respectively), and so were the derived values alveolar-capillary membrane conductance (DM,CO: 28.3 mL⋅min−1⋅mmHg−1) and pulmonary capillary volume (VC: 29.8 mL). Resting arterial blood gas analysis showed a mild hypoxemia (Pa,O2: 60 mmHg) associated with a widening of alveolar-to-arterial O2 tension difference (PA-a,O2: 44.5 mmHg). HRCT showed the presence of emphysema in both upper lobes and a sub-pleural reticular pattern in both lower lobes (Fig. 1), with quantitative analysis demonstrating a marked difference in lung density between lower (0.24 g⋅mL−1) and upper (0.18 g⋅mL−1) lung regions. Serum levels of α1-antitrypsin, angiotensin-1-converting enzyme, and C-reactive protein were within their normal ranges, and search for antinuclear antibodies was negative. Bronchoalveolar lavage fluid showed a reduced total cell count (6.0 × 104·mL−1), with relative T-lymphocytosis (70%) and an increased CD4/CD8 ratio of 5. The patient refused open lung biopsy and was treated with inhaled tiotropium bromide and long-term O2 supplementation (2 L·min−1, FI,O2: 0.24). After one month of treatment, his condition improved and PaO2 at rest increased to 66 mmHg on room air.
Table 1.
Post-bronchodilator lung function data
| Measured | z-score | |
|---|---|---|
| FVC (L) | 3.14 | −0.19 |
| FEV1 (L) | 2.06 | −1.06 |
| FEV1/FVC | 0.66 | −1.42 |
| TLC (L) | 4.48 | −1.61 |
 (cmH2O·s·L−1) (cmH2O·s·L−1) |
2.31 | 0.02 |
| DLNO (mL·min−1·mmHg−1) | 40.7 | −7.11 |
| DLCO (mL·min−1·mmHg−1) | 11.5 | −3.58 |
 , mean inspiratory resistance at 5 Hz. See text for other abbreviations.
, mean inspiratory resistance at 5 Hz. See text for other abbreviations.
Figure 1.
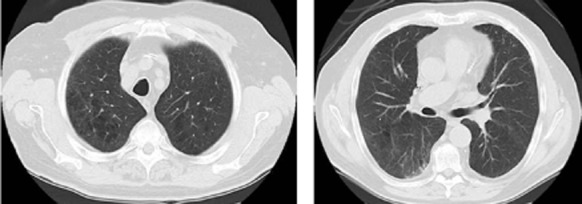
High-resolution computed tomography showing emphysematous areas in both upper lobes (left) and sub-pleural reticular pattern opacities with ground-glass appearance in the right lower lobe.
Discussion
The main features of this case report are (1) the onset of exertional dyspnea in (2) a male smoker, overweight, and occupationally exposed to asbestos fibers, with (3) relatively normal spirometry and lung volumes but (4) severe abnormalities of pulmonary diffusion, and (5) HRCT evidence of bilateral lower lobe reticular pattern with upper lobe emphysema. In the present case, the symptom leading the patient to consult a physician was breathlessness on exercise, which is considered the first sign of chronic obstructive pulmonary disease (COPD) in smokers above 40 years of age. Because dyspnea is a nonspecific symptom of various diseases, it is generally recommended that a diagnosis of COPD should be supported by spirometry. In case of negative spirometry results in a symptomatic subject, measurements of TLC and DLCO are also recommended [3]. In our case, neither spirometry nor lung volumes were conclusive, whereas DLNO and DLCO values were severely and moderately reduced, respectively. Although this pattern may be suggestive of a primary pulmonary vascular disorder, HRCT showed the coexistence of lower lobe fibrosis and upper lobe emphysema. There are physiological reasons why spirometry and lung volumes remained within the ranges of normality while gas exchange was severely impaired in this patient. Although he was near obese, a condition that may lead to under-diagnosis of COPD [4], the most likely reason was that the effects of reduced lung elastic recoil caused by emphysema were counterbalanced by the increased elastic recoil caused by fibrosis, thus preserving forced expiratory flow and lung volumes [2]. Inspiratory resistance was normal likely because the increased tethering of stiffer lung parenchyma on airways of fibrotic regions compensated for the decreased tethering of emphysematous regions. Likewise, reactance was also normal because the reduced lung volume and the increased elastance of fibrotic regions was compensated by the increased volume and reduced elastance of emphysematous regions. Were lung function tests limited to simple spirometry and lung volumes, this patient would have been classified as at risk of COPD, thus neglecting the severity of disease as shown by abnormalities of blood gases similar to those reported by Cottin et al. [5] in their series. The presence of mild arterial hypoxemia at rest and the marked increase in PA-a,O2 were likely caused by a severe DM,CO damage possibly associated with decreased VC, which may be present in either emphysema or pulmonary fibrosis even though by different mechanisms. Recognition of CPFE syndrome is of clinical and prognostic relevance, particularly because of its association with pulmonary hypertension, which may substantially decrease the 1-year survival rate and increase the risk of acute lung injury after lung surgery [2]. Overall, the mortality of this condition is significantly higher than in idiopathic pulmonary fibrosis alone with a median survival ranging from 2.1 to 8.5 years after the diagnosis [2]. In conclusion, this case report confirms the limitations of the use of spirometry as the only functional evaluation in smokers with suspected COPD because of progressive exertional dyspnea. Cigarette smoking and, to a lesser extent, the exposure to fibrogenic dust may cause a wide spectrum of pulmonary injuries with differing effects on lung function that can be revealed by comprehensive physiological studies.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Wiggins J, Strickland B. Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir. Med. 1990;84:365–369. doi: 10.1016/s0954-6111(08)80070-4. [DOI] [PubMed] [Google Scholar]
- Jankowich MD. Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome. Chest. 2012;141:222–231. doi: 10.1378/chest.11-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140:461–468. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur. Respir. J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]


