Abstract
Quorum signaling (QS) describes how bacteria can use small signaling molecules (autoinducers) to coordinate group-level behaviors. In Vibrio fischeri, QS is achieved through a complex regulatory network that ultimately controls bioluminescence, motility, and host colonization. We conducted a genetic screen focused on qrr1, which encodes a small regulatory RNA that is necessary for the core quorum-signaling cascade to transduce autoinducer information into cellular responses. We isolated unique mutants with a transposon inserted into one of two genes within the syp locus, which is involved in biofilm formation. We found that overexpression of sypK, which encodes a putative oligosaccharide translocase, is sufficient to activate qrr1, and, in addition, this effect appears to depend on the kinase activity of the sensor LuxQ. Consistent with the established model for QS in V. fischeri, enhanced expression of qrr1 by the overexpression of sypK resulted in reduced bioluminescence and increased motility. Finally, we found that induction of the syp locus by overexpression of sypG was sufficient to activate qrr1 levels. Together, our results show how conditions that promote biofilm formation impact the quorum-signaling network in V. fischeri, and further highlight the integrated nature of the regulatory circuits involved in complex bacterial behaviors.
Keywords: Biofilm, gene regulation, molecular genetics, quorum sensing
Introduction
Quorum signaling (QS) describes the process that enables a bacterium to sense and respond to other bacteria (Fuqua et al. 2001; Ng and Bassler 2009). The cell-signaling systems associated with QS depend on the synthesis and detection of signaling molecules, called autoinducers. For many bacterial species, these QS systems enable the coordination of population-level responses through gene regulation. Because autoinducer concentrations are often proportional to cell density, the responses to QS are also traditionally characterized according to cell density. However, this correlation can be disrupted by additional signaling components that occur downstream of the autoinducer receptor(s) within the regulatory network. Therefore, studies aimed to identify such inputs are critical for understanding how QS systems function in nature.
Vibrio fischeri is a marine bacterium that uses QS to regulate a multitude of cellular processes, including bioluminescence, motility, and colonization of its natural host, the Hawaiian bobtail squid, Euprymna scolopes (Nyholm and McFall-Ngai 2004; Miyashiro and Ruby 2012; Stabb and Visick 2013; Verma and Miyashiro 2013). The LuxR-LuxI QS system directly regulates the lux genes, which encode the light-producing enzyme luciferase and several proteins involved in light production and other activities. LuxR is a transcription factor activated by the autoinducer N-3-oxohexanoyl-homoserine lactone (3-oxo-C6), which is produced by the synthase LuxI. V. fischeri possesses additional QS systems that converge on a signaling cascade that, unlike the LuxR-LuxI system, is conserved among all Vibrionaceae members (Milton 2006). At its core is a phosphorelay composed of the histidine phosphotransfer protein LuxU and the response regulator LuxO (Fig. 1). Based primarily on the studies of the analogous phosphorelay in Vibrio harveyi, LuxU is predicted to become phosphorylated on a conserved histidine residue by the kinases AinR and LuxQ under conditions of low autoinducer concentrations, for example, low cell density (Freeman and Bassler 1999a, 1999b; Ray and Visick 2012). Whereas AinR appears to serve as the receptor for the AinS-derived autoinducer N-octonoyl-homoserine lactone (C8) (Gilson et al. 1995; Kimbrough and Stabb 2013), the periplasmic protein LuxP is thought, based on work in V. harveyi, to bind to the furanosyl borate diester, autoinducer-2 (AI-2), which modulates the kinase activity of LuxQ toward LuxU (Neiditch et al. 2005, 2006). Upon phosphorylation, LuxU is predicted to donate the phosphoryl group to a conserved aspartic acid residue of LuxO, which can then activate transcription of qrr1 (Miyashiro et al. 2010). The RNA chaperone Hfq assists the small regulatory RNA (sRNA) Qrr1 in the posttranscriptional repression of LitR, a global transcription factor that regulates motility, host colonization factors, and bioluminescence (Fidopiastis et al. 2002; Miyashiro et al. 2010; Cao et al. 2012). The net effect of the integrated QS systems is that under high cell density (i.e., in the presence of autoinducers) LuxO becomes de-phosphorylated, which leads to low qrr1 expression and the ability of V. fischeri to fully activate the lux genes.
Figure 1.
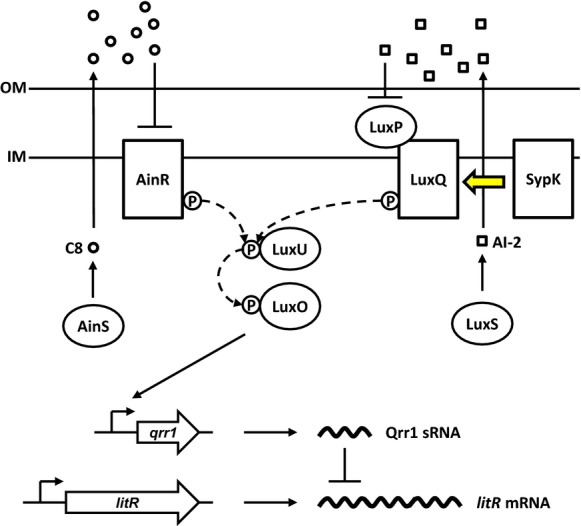
Model of the core quorum-signaling (QS) system in Vibrio fischeri. The outputs of the QS systems AinS/AinR and LuxS/LuxP/LuxQ converge on the LuxU/LuxO phosphorelay. Phosphorylated LuxO activates transcription of the small regulatory RNA Qrr1 that posttranscriptionally represses litR, which encodes the transcription factor LitR. In this study, we show that SypK modulates QS by affecting the kinase activity of LuxQ (indicated by the yellow arrow).
Within the past decade, V. fischeri has also become a useful model organism to explore the genetic determinants for developing biofilms, which are elaborate structures that bacterial populations or communities can produce to associate with surfaces and each other (Visick 2009; Yildiz and Visick 2009). By synthesizing and exporting various exopolysaccharides and other molecules (Flemming et al. 2007), bacteria can remain attached to a surface and sheltered from unpredictable and potentially stressful environments. Wild-type V. fischeri does not produce a substantial biofilm under standard laboratory conditions. However, activation of a cluster of 18 genes that comprise the syp locus (Yip et al. 2005) confers phenotypes associated with biofilms, such as the ability to form wrinkled colonies on solid agar surfaces (Yip et al. 2006; Hussa et al. 2008). QS was recently shown to impact the dynamics of syp-mediated biofilm development as mutants containing an insertion in luxQ were delayed in wrinkled colony formation (Ray and Visick 2012). Further investigation revealed that deletion of luxU but not luxO leads to a similar delay, highlighting a branch within the signaling network that impacts biofilm development but not bioluminescence. In this current study, we report our discovery of another connection between the QS system and the syp locus, which expands our knowledge of the regulatory networks that V. fischeri has evolved to interact with its environment.
Experimental Procedures
Growth and media
V. fischeri strains were grown aerobically at 28°C in Luria-Bertani-Salt (LBS) broth (Graf et al. 1994) without supplemented glycerol. When necessary, chloramphenicol, tetracycline, and erythromycin were used at 2.5, 5.0, and 5.0 μg mL−1, respectively. Escherichia coli strains were grown aerobically at 37°C in Luria-Bertani (LB) or brain–heart infusion (BHI) media. For fluorescence assay measurements, cells were resuspended in defined minimal medium (DMM): (50 mmol/L Tris-HCl [pH 7.5], 50 mmol/L MgSO4, 10 CaCl2, 300 mmol/L NaCl, 10 mmol/L KCl, 0.0058% K2HPO4, 10 μmol/L FeSO4). TBSW (DeLoney-Marino et al. 2003) was used for motility assays.
Strains and plasmids
V. fischeri strains and plasmids used in this study are listed in Table 1, and additional details of their construction are located in Supporting Information. All V. fischeri strains were derived from wild-type strain ES114 (Ruby et al. 2005; Mandel et al. 2008). Escherichia coli strains used in this work include EC100Dpir+ (Epicentre Biotechnologies, Madison, WI), TAM1 (Active Motif, Carlsbad, CA), β3914 (Le Roux et al. 2007), π3813 (Le Roux et al. 2007), CC118 λpir (Herrero et al. 1990), and GT115 (InvivoGen, San Diego, CA). Oligonucleotides used in this study are listed in Table S1 and were purchased from Integrated DNA Technologies, Inc. (Coralville, IA) (IDT).
Table 1.
Vibrio fischeri strains and plasmids used in this study
| Strain | Genotype | References |
|---|---|---|
| ES114 | Wild-type V. fischeri | Boettcher and Ruby (1990); Ruby et al. (2005); Mandel et al. (2008) |
| CL59 | luxO::luxOD47E | Lupp and Ruby (2005) |
| DRO1B3 | sypJ::Tn5 | This study |
| DRO216 | luxO::luxOD47E attTn7::Pqrr1-gfp erm | This study |
| DRO222 | sypI::Tn5 [NT] | This study |
| DRO5F11 | sypI::Tn5 | This study |
| EVS102 | ΔluxCDABEG | Bose et al. (2008) |
| KV4829 | ΔluxU | This study |
| KV5069 | ΔsypL | Shibata et al. (2012) |
| KV5972 | ΔluxQ | This study |
| KV6010 | ΔluxP | This study |
| KV6529 | ΔluxQ attTn7::Pqrr1-gfp erm | This study |
| KV6530 | ΔluxU attTn7::Pqrr1-gfp erm | This study |
| KV6549 | ΔluxP attTn7::Pqrr1-gfp erm | This study |
| KV6629 | ΔsypL attTn7::Pqrr1-gfp erm | This study |
| TIM303 | attTn7::Pqrr1-gfp erm | This study |
| TIM305 | Δqrr1 | Miyashiro et al. (2010) |
| TIM306 | ΔluxO | Miyashiro et al. (2010) |
| TIM311 | ΔluxO attTn7::Pqrr1-gfp erm | This study |
| TIM358 | ΔlitR | Miyashiro et al. (2010) |
| TIM374 | ΔluxPQ attTn7::Pqrr1-gfp erm | This study |
| TIM394 | ΔsypK | This study |
| TIM395 | ΔsypK attTn7::Pqrr1-gfp erm | This study |
| Plasmid | Description | References |
|---|---|---|
| pCLD56 | pKV282 sypG | Morris and Visick (2013) |
| pEVS107 | R6Kori oriT mini-Tn7 mob erm kan | McCann et al. (2003) |
| pEVS79 | pBC SK (+) oriT cat | Stabb and Ruby (2002) |
| pKV282 | Mobilizable vector; TetR | Morris et al. (2011) |
| pLosTfoX | pEVS79 tfoX | Pollack-Berti et al. (2010) |
| pTM146 | ColE1ori bla cat kan gfp PtetA-mCherry | Miyashiro et al. (2010) |
| pTM214 | lacIqPtrc-mCherry | Miyashiro et al. (2011) |
| pTM239 | pEVS107 Pqrr1-gfp erm | This study |
| pTM268 | pVSV105 Pqrr1-gfp PtetA-mCherry | Miyashiro et al. (2010) |
| pTM327 | pEVS79 ΔluxPQ | This study |
| pTM367 | pTM214 ΔmCherry::sypK | This study |
| pTM368 | pTM214 ΔmCherry::sypL | This study |
| pTM375 | pEVS79 ΔsypK | This study |
| pVAR18 | Mobilizable suicide vector; ΔluxU | Ray and Visick (2012) |
| pVAR29 | Mobilizable suicide vector; ΔluxQ | Ray and Visick (2012) |
| pVAR30 | Mobilizable suicide vector; ΔluxP | Ray and Visick (2012) |
| pVAR48 | pVSV105 luxQ-FLAG | This study |
| pVAR50 | pVSV105 luxQA216P-FLAG | This study |
| pVAR51 | pVSV105 luxQH378A-FLAG | This study |
| pVAR70 | pKV282 sypK-FLAG | This study |
| pVSV105 | R6Kori ori(pES213) RP4 oriT cat | Dunn et al. (2006) |
Transposon mutagenesis screen
The reporter plasmid pTM268 was introduced by conjugation into a Tn5 transposon-mutant library of ES114 that has been previously described (Miyashiro et al. 2011). Recipients of the reporter plasmid were selected by plating the mating mixture onto LBS with 2.5 μg mL−1 chloramphenicol. The resulting colonies were screened for elevated Green Fluorescent Protein (GFP) levels using a Leica MZFLIII fluorescence dissecting microscope (Leica Microsystems, Wetzlar, Germany), equipped with a GFP2 filter set.
To determine the transposon insertion site within each mutant, genomic DNA was extracted from 0.5 mL overnight LBS cultures using the MasterPure DNA Purification Kit (Epicentre Biotechnologies). Approximately 3-μg genomic DNA was digested by EcoRI-HF (New England Biolabs, Ipswich, MA) in a 30-μL reaction at 37°C. After 1 h at 37°C, EcoRI was heat inactivated at 65°C for 20 min. The enzyme was removed using the Wizard SV Gel and polymerase chain reaction (PCR) Clean-Up System (Promega, Madison, WI). The DNA was self-ligated using T4 DNA ligase (New England Biolabs), transformed by electroporation into EC100Dpir+ (Epicentre Biotechnologies), and selected on BHI containing 150 μg mL−1 erythromycin. Plasmid DNA was extracted using the QIAprep Spin Miniprep Kit (Qiagen, Venlo, Netherlands) and sequenced at the UWBC DNA Sequencing Facility (University of Wisconsin-Madison) with transposon-specific primers pMJM10-Ext2 (CTAAAGAGGTCCCTAGCGATAAGC) and 170Ext (GCACTGAGAAGCCCTTAGAGCC).
Fluorescence assay
Overnight LBS cultures containing 2.5 μg mL−1 chloramphenicol were diluted 1:100 into fresh media and grown aerobically at 28°C. At OD600 ˜0.6, cultures were quickly cooled on ice. One-milliliter samples were spun at 15,000g for 5 min, and the pellets were resuspended in 350 μL cold DMM. The OD600 and fluorescence of 100 μL of each sample were determined in triplicate using a Tecan M1000 Pro Quadruple Monochromator Microplate Reader (Tecan Group, Mannedorf, Switzerland). For excitation and emission of GFP measurements, the monochromators were set to 488 ± 5 nm and 509 ± 5 nm, respectively. For excitation and emission of mCherry measurements, the monochromators were set to 587 ± 5 nm and 610 ± 5 nm, respectively. DMM was used as a blank for OD600 measurements. The fluorescence/OD600 was calculated by subtracting the autofluorescence levels associated with a nonfluorescent sample.
Luminescence assay
Overnight LBS cultures were diluted 1:100 into fresh media and grown aerobically at 28°C. After 2 h, cultures were diluted 1:10 into media containing 3-oxo-C6 (Sigma, St. Louis, MO) at a final concentration of 120 nmol/L. At OD600 ˜0.6–0.8, a 100-μL sample was sampled for luminescence using a GloMax 20/20 (Promega). Luminescence levels were normalized by the corresponding OD600 levels.
Quantitative reverse transcription-PCR (qRT-PCR)
Overnight LBS cultures were diluted 1:100 into fresh media and grown aerobically at 28°C. At OD600 ˜0.5, cultures were quickly cooled on ice. To extract RNA, 1.5-mL samples were spun at 15,000g for 10 min, and the corresponding pellets were resuspended in 200 μL QuickExtract RNA solution (Epicentre Biotechnologies). Samples were heated at 65°C with occasional mixing by vortexer. After 15 min, samples were cooled on ice. After 5 min, each sample was supplemented with 24-μL DNase I buffer, 5 μL Riboguard, and 10 μL DNase I (Epicentre) and heated at 37°C. After 30 min, samples were cooled on ice. RNA was precipitated using 2× Tissue and Culture Solution and MPC Precipitation Reagent according to manufacturer's instructions. A second round of DNase I treatment was performed by resuspending RNA in 94 μL DNase I 1x buffer, 2 μL Riboguard, and 4 μL DNase I. After 1 h at 37°C, RNA was precipitated as described above. RNA was resuspended in 15 μL of nuclease-free water (IDT). The concentration of RNA was measured using a Nanodrop (Thermo Scientific, Waltham, MA).
RT reactions were performed starting from 4 μg of total RNA, using AMV Reverse Transcriptase (Promega, Madison, WI) and Random Primers (Promega), according to the manufacturer's instructions. Negative controls were performed in the same manner but without AMV Reverse Transcriptase. The resulting cDNA samples were diluted 1:80 in nuclease-free water. Each 25-μL reaction mixture for qRT-PCR consisted of 10-μL cDNA, iQ SYBR Green Supermix diluted to 1×, and 500 nmol/L of each primer. qRT-PCR was performed in an iCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA) with two technical replicates/biological replicate as follows: 3 min at 95°C, 40 times (15 sec at 95°C, 30 sec at 60°C, 30 sec at 72°C), 1 min at 60°C. A melting curve was recorded at the end of the PCR amplification (from 60°C to 100°C) to confirm that a unique transcript product had been amplified. To calculate PCR efficiencies, standard curves were plotted using five 10-fold dilutions of a mixture containing 2.5 μL of each cDNA reaction diluted 1:2 with nuclease-free water. Primer sets exhibited amplification efficiencies (E) of 1.94–2.03. Gene expression values were calculated using the E−Ct method, where Ct corresponds to the threshold cycle. Expression levels of each gene were normalized by the corresponding wild-type expression level. For each gene, comparison between strains was performed using an unpaired t-test with P-values adjusted using false discovery rate correction (Prism, v. 6.03, La Jolla, CA).
Motility assay
Overnight LBS cultures supplemented with 2.5 μg mL−1 chloramphenicol were diluted 1:100 into LBS supplemented with chloramphenicol and 100 μmol/L IPTG. Cultures were standardized to OD = 0.2 and inoculated into Tryptone-based Seawater (TBSW) motility plates containing chloramphenicol and Isopropyl Beta-D-1-thiogalactopyranoside (IPTG). Assays were performed as described previously (DeLoney-Marino et al. 2003).
Western blotting
Western blot analysis was used to analyze the levels of epitope (FLAG)-tagged luxQ from KV6529 containing pVAR48 (wild-type luxQ-FLAG), pVAR50 (luxQ-A216P-FLAG), or pVAR51 (luxQ-H378A-FLAG) and either the vector control (pKV282) or the sypK overexpression plasmid pVAR70. Briefly, cultures were grown overnight with shaking in LBS containing tetracycline and chloramphenicol. Samples were collected and standardized to an OD600 = 3.5, resuspended in 500-μL 2x SDS-loading buffer (4% SDS, 10% 2-mercaptoethanol, 0.005% bromophenol blue, 20% glycerol, 0.1 mol/L Tris pH 7), boiled for 5 min, and then loaded onto a 10% SDS polyacrylamide gel. After electrophoresis, proteins were transferred to a polyvinylidene fluoride membrane (PVDF) and probed with an anti-FLAG antibody (Sigma-Aldrich, St. Louis, MO). Protein bands were visualized using a horseradish peroxidase-conjugated secondary antibody and ECL reagents (Pierce Biotechnology, Rockford, IL).
Results
Identification of syp genes that affect qrr1 expression
We have previously shown that the LuxU-LuxO phosphorelay activates qrr1 to control the level of the transcription factor LitR (Miyashiro et al. 2010). To further characterize this branch within the QS network of V. fischeri, we initiated a genetic screen by introducing the qrr1 transcriptional reporter plasmid pTM268 into a Tn5 transposon-mutant library of the wild-type V. fischeri strain ES114. The plasmid pTM268 contains the qrr1 promoter cloned upstream of gfp, and the constitutively expressed tetA promoter cloned upstream of mCherry. The GFP/mCherry fluorescence ratio of cells harboring pTM268 provides a quantitative measure of qrr1 expression. In colonies of wild-type V. fischeri harboring pTM268, the level of GFP fluorescence is low (data not shown), presumably due to the high cell density conditions within colonies repressing qrr1 expression.
We screened over 100,000 colonies and isolated ˜100 clones with elevated levels of GFP. In this study, we report our characterization of two mutants isolated from this genetic screen. Both mutants displayed qrr1 expression levels that were approximately threefold higher than wild-type cells (Fig. 2A). Sequencing of the transposon insertion site in each mutant revealed an insertion in either VF_A1028 (sypI) or VF_A1029 (sypJ) (Fig. 2B). To determine whether the phenotype of elevated qrr1 expression was linked to the transposon, we reintroduced the sypI transposon insertion into ES114 by transformation. The resulting strain, DRO222 (designated sypI::Tn5 [NT]), showed qrr1 expression levels comparable to the original transposon insertion mutant (sypI::Tn5), indicating that the transposon insertion in sypI is linked to elevated qrr1 expression (Fig. 2A). Hereafter, the sypI::Tn5 [NT] mutant is termed the sypI mutant.
Figure 2.
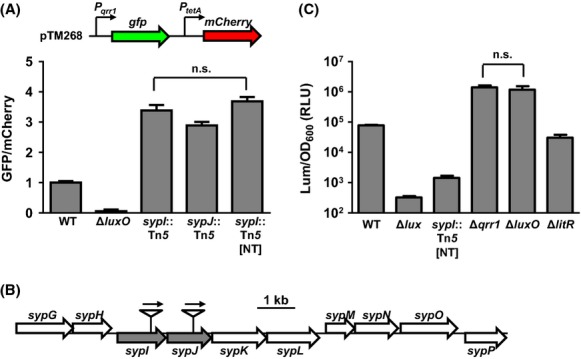
Mutants with a transposon insertion in the sypIJKL operon have enhanced qrr1 expression. (A) Levels of qrr1 expression in WT (ES114), ΔluxO (TIM306), sypI::Tn5 (DRO5F11), sypJ::Tn5 (DRO1B3), and sypI::Tn5 [NT] (DRO222) harboring the reporter plasmid pTM268. The nonfluorescent strain ES114 harboring pVSV105 was used to calculate cellular levels of GFP and mCherry. Graphical and error bars represent the averages and standard deviations of triplicate biological replicates, respectively. One-way ANOVA with Tukey's multiple comparisons test show significance (P-value <0.01) between columns, except for the comparison labeled not significant (n.s.). Experiment was performed three times, with similar results. (B) Transposon insertion sites of mutants examined in (A). Genes disrupted by a transposon are highlighted in gray. Arrows above transposon insertions indicate direction of erm gene transcription. (C) Luminescence levels of WT (ES114), Δlux (EVS102), sypI::Tn5 [NT] (DRO222), Δqrr1 (TIM305), ΔluxO (TIM306), and ΔlitR (TIM358) in response to 120 nmol/L 3-oxo-C6. Graphical and error bars represent the averages and standard deviations of triplicate biological replicates, respectively. One-way ANOVA with Tukey's multiple comparisons test on log-transformed data show significance (P-value <0.01) between columns, except for the comparison labeled not significant (n.s.). Experiment was performed three times, with similar results.
LitR indirectly enhances luminescence in V. fischeri by binding the intergenic region between luxR and luxI to positively regulate luxR expression (Fidopiastis et al. 2002; Miyashiro et al. 2010). Because Qrr1 posttranscriptionally represses litR, cells expressing qrr1 are predicted to exhibit low luminescence levels. Relative to wild-type cells, Δqrr1 and ΔluxO mutants become 18- and 15-fold brighter, respectively, and a ΔlitR mutant is 2.5-fold dimmer (Fig. 2C). Consistent with high levels of qrr1 expression, the sypI mutant is 55-fold dimmer than wild-type cells. Together, these results suggest that the syp locus can affect qrr1 expression and QS phenotypes in V. fischeri.
Polar effect of transposon insertion on syp expression
Both sypI and sypJ are predicted to encode glycosyltransferases, and the effects of their disruption on syp-mediated biofilm formation have recently been determined (Shibata et al. 2012). Whereas sypJ is required for biofilm formation, a deletion of sypI only delays biofilm formation. We were unable to formulate a simple model that could account for the ability of the two different glycosyltransferases to affect qrr1 expression. However, in each transposon mutant, the promoter associated with the erythromycin resistance marker (erm) was oriented in the same direction as the syp locus (Fig. 2B). In addition, the insertions, which were within different genes in the same operon, resulted in similarly high levels of qrr1 expression (Fig. 2A). Therefore, we hypothesized that the elevated level of qrr1 expression detected in each mutant was due to the activation of genes downstream of the transposon insertion rather than to the disruption of either sypI or sypJ.
To test this hypothesis, we examined the transcript levels of various genes in the sypI mutant. We found that litR levels were reduced 3.3-fold in the sypI mutant relative to wild-type cells (Fig. 3), consistent with the elevated expression of qrr1 in the mutant. We also found that the sypI mutant exhibited higher transcription levels of sypJ (4.7-fold), sypK (18.5-fold), and sypL (2.8-fold). Because our original screen also identified a sypJ mutant, we conclude that the elevated level of qrr1 expression in the sypI mutant is independent of sypJ. To determine whether the transposon insertion resulted in a general activation of the syp locus, we also examined several other syp genes. While the level of sypM, which is contained within the adjacent operon (Fig. 2B), was elevated 3.2-fold (Fig. 3), three other genes (sypA, sypF, and sypP) displayed wild-type levels of transcription in the sypI mutant. These results demonstrate that the transposon insertion leads to the increased expression of several downstream genes, but not of the entire locus.
Figure 3.
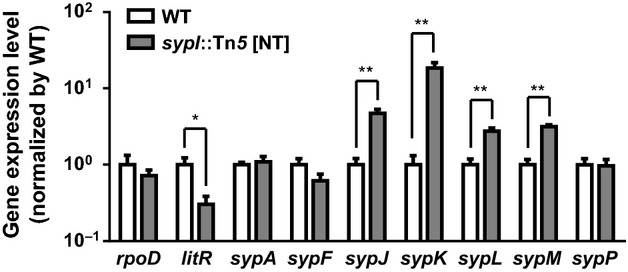
Polar effects of transposon insertion on syp gene expression. Quantitative reverse-transcriptase PCR analysis of various genes in the sypI mutant (DRO222) relative to WT (ES114). Genes tested are VF_2254 (rpoD), VF_2177 (litR), VF_A1020 (sypA), VF_A1025 (sypF), VF_A1029 (sypJ), VF_A1030 (sypK), VF_A1031 (sypL), VF_A1032 (sypM), and VF_A1035 (sypP). Values are of quadruplicate biological replicates and normalized by wild-type levels. Error bars indicate ± 1 SD. Comparisons with significance based on unpaired t-tests are shown with *(P-value <0.01) and **(P-value <0.001), where P-values are adjusted using false discovery rate correction.
Induction of sypK results in qrr1 expression
To determine whether increased expression of sypK or sypL leads to qrr1 expression, we separately cloned sypK and sypL on a plasmid downstream of the IPTG-inducible trc promoter. For experiments involving overexpression vectors, we monitored qrr1 expression using a GFP transcriptional reporter integrated into the chromosome at the Tn7 insertion site. We found that basal expression of sypK from the trc promoter was sufficient to increase qrr1 levels at least threefold higher than wild-type levels (Fig. 4). Addition of IPTG to the growth medium resulted in a slight but significant increase in qrr1 expression. In contrast, we did not detect any change in qrr1 expression when using the IPTG-inducible sypL construct. These results indicate that overexpression of sypK is sufficient to activate qrr1 expression.
Figure 4.
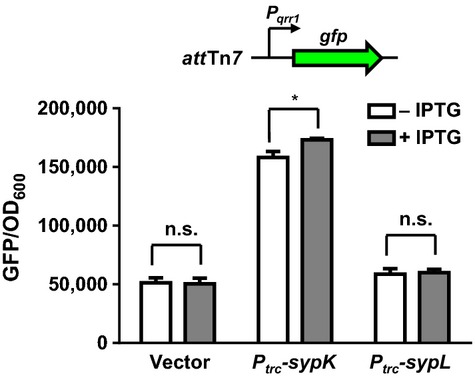
Overexpression of sypK activates qrr1 expression. Levels of qrr1 expression in the reporter strain TIM303 harboring vector (pTM214), Ptrc-sypK (pTM367), or Ptrc-sypL (pTM368) grown ±100 μmol/L IPTG. The wild-type strain ES114 harboring pTM214 was used as the GFP-negative control for quantifying GFP levels. Graphical and error bars represent the averages and standard deviations of triplicate biological replicates, respectively. Comparisons with significance based on two-way ANOVA with Tukey's multiple comparisons are shown with *(P-value <0.01). Comparisons between pTM367-harboring strains with strains harboring either pTM214 or pTM368 are significant (P-value <0.001). Experiment was performed three times, with similar results.
Previous studies have shown that V. fischeri also uses the LuxU-LuxO QS pathway to regulate motility (Lupp and Ruby 2005; Cao et al. 2012). In particular, V. fischeri cells become hypermotile in the absence of litR. To test whether SypK has an effect on motility, we examined on soft agar the motility of cells expressing sypK. Compared to wild-type motility levels, cells with induced sypK expression were hypermotile (Fig. 5A and B). Furthermore, this hypermotility phenotype depended on Qrr1, as shown by the overall reduced motility associated with mutants containing the Δqrr1 allele. Together, these results demonstrate that expression of sypK leads to higher motility due to increased qrr1 transcription.
Figure 5.
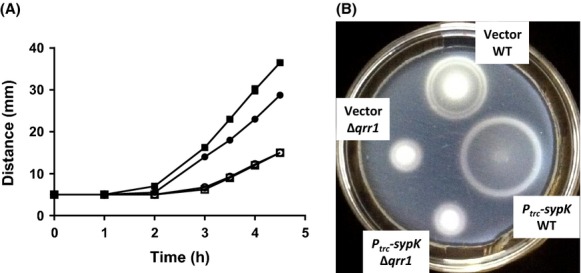
Overexpression of sypK enhances qrr1-dependent motility. (A) Soft-agar motility migration distances by WT (ES114; closed symbols) and Δqrr1 (TIM305; open symbols) harboring vector (pTM214; circles) or Ptrc-sypK (pTM367; squares). TBSW motility plates contained chloramphenicol and 100 μmol/L IPTG. Points and error bars (too small to visualize) represent means and standard deviations of spot diameter (in mm) for quadruplicate biological replicates, respectively. Experiment was performed twice, with similar results. (B) Image of motility plate used in (A) at 4 h.
SypK modulates LuxO phosphorylation via LuxQ
The sypK gene is predicted to encode an oligosaccharide translocase. SypK is required for V. fischeri to form syp-mediated biofilms as well as to colonize E. scolopes juvenile squid (Shibata et al. 2012). In addition, SypK also contributes to the production of outer membrane vesicles under biofilm-inducing conditions (Shibata and Visick 2012). We concluded that, because of its predicted function as an oligosaccharide translocase, SypK is unlikely to directly regulate qrr1 expression. Therefore, we assayed qrr1 expression in response to sypK induction in various QS mutants of V. fischeri to determine whether, and at what step, SypK interacts with the known QS pathway.
We first examined the response of qrr1 expression to sypK induction in the absence of either LuxO or LuxU, which are the phosphorelay proteins that directly control qrr1 expression (Fig. 1). We found that qrr1 expression remained at or lower than the wild-type level in either ΔluxO or ΔluxU mutants, regardless of whether sypK was induced (Fig. 6), indicating that both proteins are required for SypK-dependent activation of qrr1. We next examined qrr1 expression in a strain containing a luxOD47E allele, which expresses a variant of LuxO that mimics the phosphorylated state of LuxO (Lupp and Ruby 2005). We found that the level of qrr1 expression in the luxOD47E mutant was higher than in wild-type cells (Fig. 6). Importantly, qrr1 expression in the luxOD47E mutant was independent of sypK induction, suggesting that an intact LuxU-LuxO phosphorelay is required for SypK to increase qrr1 expression (Fig. 6). Together, these results indicate that SypK acts upstream of the LuxU-LuxO phosphorelay to affect qrr1 expression.
Figure 6.
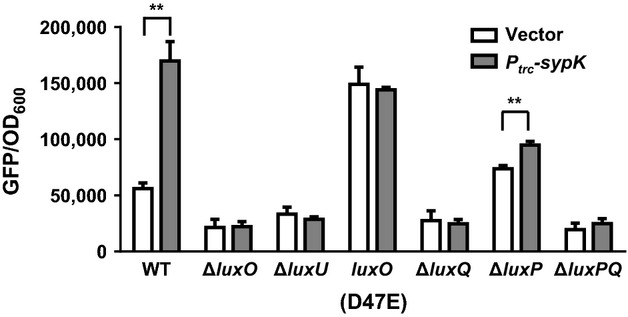
SypK modulates the LuxU-LuxO phosphorelay via LuxQ. Levels of qrr1 expression in WT (TIM303), ΔluxO (TIM311), ΔluxU (KV6530), luxOD47E (DRO216), ΔluxQ (KV6529), ΔluxP (KV6549), ΔluxPQ (TIM374) harboring vector (pTM214) or Ptrc-sypK (pTM367) grown in the presence of 100 μmol/L IPTG. The wild-type strain ES114 harboring pTM214 was used as the GFP-negative control for quantifying GFP levels. Graphical and error bars represent the averages and standard deviations of triplicate biological replicates, respectively. Comparisons with significance based on unpaired t-tests are shown with **(P-value <0.001), where P-values are adjusted using false discovery rate correction. Experiment was performed three times, with similar results.
Because LuxO phosphorylation is controlled by upstream kinases, we next examined the effect of the signaling proteins controlling the LuxU-LuxO phosphorelay. Specifically, we assessed whether SypK activation of the LuxU-LuxO phosphorelay depends on the sensor kinase LuxQ or the AI-2 receptor protein LuxP, which has been shown in V. harveyi and V. cholerae to control the kinase and phosphatase activities of LuxQ (Neiditch et al. 2005, 2006; Shikuma et al. 2009). Induction of sypK in either ΔluxQ or ΔluxPQ mutants did not result in qrr1 expression (Fig. 6), indicating that SypK-mediated activation of qrr1 requires LuxQ. Relative to wild-type cells, deletion of luxP resulted in increased qrr1 expression (Fig. 6), which is consistent with the inhibitory function LuxP exhibits toward the kinase activity of LuxQ in V. harveyi (Neiditch et al. 2005). We found a slight but significant increase in qrr1 expression when sypK was induced in the ΔluxP mutant, suggesting that the ability of SypK to activate LuxQ may also involve LuxP. Taken together, our results suggest that SypK activates qrr1 by stimulating the LuxP/Q complex, which modulates the LuxU-LuxO phosphorelay.
Like many histidine kinases, LuxQ is bifunctional, with both kinase and phosphatase activities (Freeman and Bassler 1999a, 1999b). To determine which enzymatic activity of LuxQ is modulated by SypK, we used two LuxQ variants, A216P or H378A, that exhibit only kinase (K+/P−) or phosphatase (K−/P+) activities, respectively (Neiditch et al. 2006; Ray and Visick 2012). Consistent with the results shown in Figure 6, we found that qrr1 expression remained low in a ΔluxQ mutant, regardless of whether sypK was overexpressed (Fig. 7A). Introduction of luxQ into the ΔluxQ mutant in trans resulted in an increased expression of qrr1. The level of qrr1 expression in this strain was even higher than in wild-type cells (Fig. 6), presumably due to the overexpression of luxQ from the multicopy plasmid pVAR48. In V. cholerae, a similar activation of the analogous LuxU-LuxO phosphorelay by overexpression of the luxQ homologue has been observed (Shikuma et al. 2009). Similar to the results shown in Figure 6, the overexpression of sypK in V. fischeri harboring luxQ in trans also led to the increased qrr1 expression (Fig. 7A). In the presence of the K+/P− mutation luxQA216P, qrr1 was expressed, albeit at lower levels than in the presence of luxQ. This lower level of qrr1 expression may be attributed to the overall lower levels of the LuxQA216P variant relative to wild-type LuxQ (Fig. 7B). Overexpression of sypK in this background resulted in a high level of qrr1 expression that was comparable to the level induced by sypK in cells expressing wild-type LuxQ (Fig. 7A). Finally, the expression of qrr1 was low in cells harboring the K−/P+ variant of LuxQ regardless of sypK induction. As shown in Figure 7B, the LuxQH378A variant was produced at levels comparable to wild-type LuxQ. Taken together, these data suggest that the kinase activity of LuxQ is required for SypK-dependent regulation of qrr1 expression.
Figure 7.
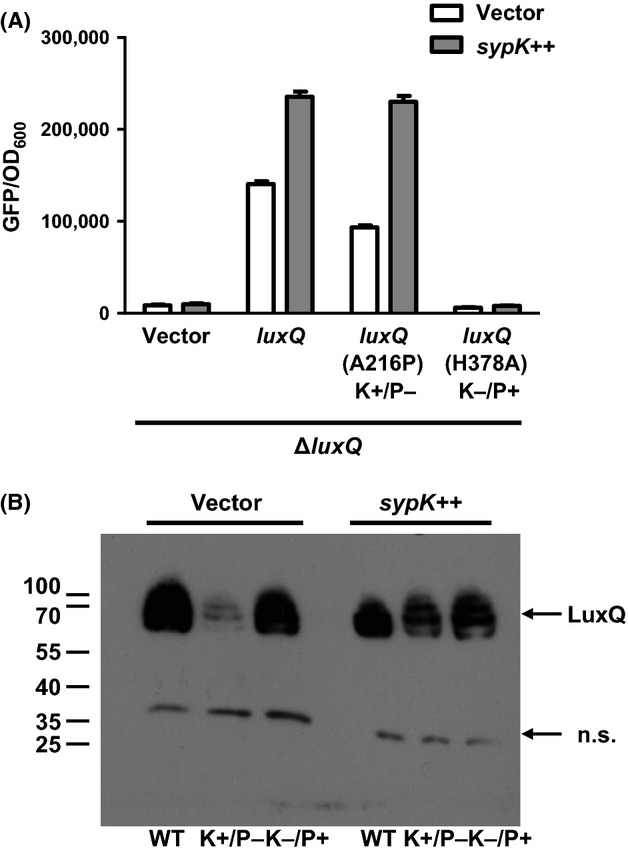
Kinase activity of LuxQ is required for SypK activation of qrr1 expression. (A) Levels of qrr1 expression in ΔluxQ strain KV6529 harboring a luxQ-FLAG variant plasmid (vector [pVSV105], luxQ [pVAR48], luxQ [A216P] K+/P− [pVAR50], or luxQ [H378A] K−/P+ [pVAR51]) and a sypK plasmid (vector [pKV282] or sypK++ [pVAR70]). The wild-type strain ES114 harboring pVSV105 and pKV282 was used as the nonfluorescent control for quantifying fluorescence levels. The copy of sypK in plasmid pVAR70 contains the FLAG tag. Graphical and error bars represent the averages and standard deviations of triplicate biological replicates, respectively. Experiment was performed three times, with similar results. (B) Western blot of ΔluxQ strain KV6529 harboring a luxQ-FLAG variant plasmid (WT [pVAR48], K+/P− [pVAR50], or K−/P+ [pVAR51]) and a sypK plasmid (vector [pKV282] or sypK++ [pVAR70]). Anti-FLAG antibodies were used to detect the variants of LuxQ-FLAG. Numbers to the left indicate molecular-weight marker positions (in kDa). A nonspecific band detected throughout the samples is designated as “n.s.”. A band corresponding to SypK-FLAG was not detected in this experiment. Experiment was performed twice, with similar results.
Induction of the syp locus results in qrr1 expression
Our results indicate that overexpression of sypK is sufficient to activate qrr1 expression in wild-type cells. Therefore, we hypothesized that conditions that activate transcription of the syp locus would also lead to increased qrr1 expression in a sypK-dependent manner. Because the formation of syp-mediated biofilms is linked to SypK, and cellular physiology significantly varies between planktonic and biofilm states, we sought conditions that would activate transcription of the syp locus but prevent biofilm formation. Previous work has demonstrated that in trans expression of sypG, which encodes a transcription factor, results in transcription of the syp locus without concomitant biofilm formation (Yip et al. 2005; Hussa et al. 2008). Further analysis of this condition has determined that the lack of biofilm formation is due to an inhibitory activity by the regulator SypE (Morris and Visick 2013).
We thus assayed qrr1 expression in cells harboring either a multicopy plasmid containing sypG (pCLD56) or a vector control (pKV282). Wild-type cells that overexpressed sypG exhibited ˜2.4-fold higher qrr1 expression levels than did vector-containing cells (Fig. 8). This level of qrr1 activity was comparable to that in cells overexpressing sypK (Fig. 4). The level of qrr1 activation in response to sypG decreased to 1.5-fold in the absence of sypK (Fig. 8). In addition, the 2.7-fold qrr1 activation by sypG in a ΔsypL mutant was comparable to the response in wild-type cells, confirming our results that showed qrr1 expression is independent of sypL induction (Fig. 4). Thus, our results show that conditions that lead to transcription of the syp locus, that is, biofilm formation, also activate qrr1 expression.
Figure 8.
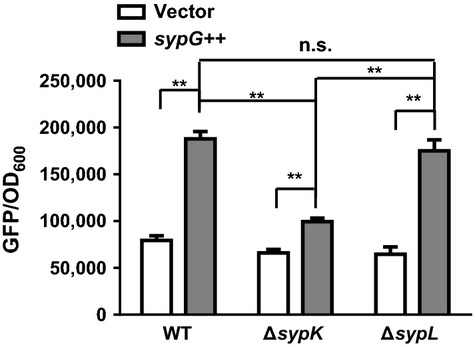
Expression of qrr1 increases with SypG-mediated activation of syp locus. Levels of qrr1 expression in WT (TIM303), ΔsypK (TIM395), and ΔsypL (KV6629) harboring pKV282 (vector) or pCLD56 (sypG++). The wild-type strain ES114 harboring pCLD56 was used as the nonfluorescent control for quantifying fluorescence levels. Graphical and error bars represent average and standard deviation of three biological replicates, respectively, from a representative experiment performed twice. Comparisons with significance based on two-way ANOVA with Tukey's multiple comparisons test shows significance **(P-value <0.001) between columns, except for the comparison labeled nonsignificant (n.s.) and for comparisons between columns for vector controls, which are also nonsignificant.
Discussion
We initiated this study to identify novel regulators of qrr1, which encodes a sRNA conserved among members of the Vibrionaceae family (Lenz et al. 2004; Miyashiro et al. 2010; Weber et al. 2011; Shao and Bassler 2012). Our results show that the putative oligosaccharide translocase SypK can interact with the QS network to control qrr1 levels in V. fischeri. In particular, our data are consistent with the conclusion that SypK exerts its impact at or above the LuxP/Q complex in a manner that depends on the kinase activity of LuxQ (Figs. 6, 7). Because SypL, which is another predicted inner membrane protein, does not activate qrr1 expression (Fig. 4), we posit that the increase in LuxQ signaling by SypK is not due to a general perturbation of the protein composition within the inner membrane. The activation of the LuxU-LuxO phosphorelay resulting from SypK overexpression, either as an individual protein or in the context of activation of the syp locus (Fig. 8), leads to the expression of qrr1. Finally, our results show that the interaction between SypK and the QS pathway is sufficient to affect cellular behaviors associated with quorum sensing in V. fischeri, for example, bioluminescence and motility (Figs. 2C, 5).
From these results, we have generated the model presented in Figure 9. Under conditions that result in transcription of the syp locus, the sypK gene will be expressed, leading to the formation of this putative oligosaccharide translocase within the inner membrane. While contributing to biofilm formation, SypK can also interact with the QS pathway via LuxQ. Our results in Figure 7B suggest that the levels of the K+/P− variant of LuxQ may be influenced by SypK. One interpretation of these data consistent with our other results is that SypK stabilizes the kinase form of LuxQ; however, a clear understanding of this pathway awaits a more complete study. Regardless, the net result of sypK induction is activation of the LuxU-LuxO phosphorelay and, consequently, qrr1 expression. Whether the resulting increase in LuxU phosphorylation can further enhance syp transcription via SypG (Ray and Visick 2012) remains unknown; further characterization of the signaling pathway directly controlling the syp locus is required to determine whether a potential positive feedback loop is present. Interestingly, this regulatory link between SypK and LuxQ suggests a mechanism that enables V. fischeri cells densely packed within a biofilm to activate the LuxU-LuxO phosphorelay even in the presence of AI-2, which is an autoinducer broadly used for bacterial QS.
Figure 9.
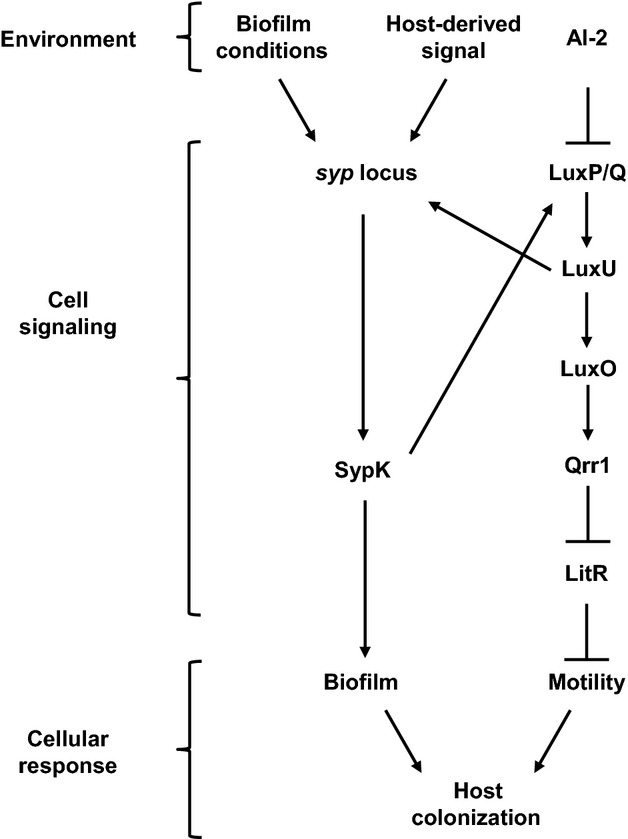
Model of integrated biofilm and QS regulatory networks in Vibrio fischeri. Environmental cues, including conditions associated with the host during initial colonization of the squid light organ, activate the syp locus. In addition to participating in biofilm formation, SypK activates the LuxU-LuxO phosphorelay via the LuxP/Q complex. The resulting expression of qrr1 leads to enhanced flagellar-based motility, which may contribute to host colonization (see Discussion).
The threefold increase in qrr1 expression observed in the transposon insertion mutants resulted in a 55-fold decrease in luminescence (Fig. 2A and C). Such dramatic effects on cellular luminescence from small changes in the expression of genes involved in QS have been previously reported. For instance, deletion of arcA, which encodes a response regulator that responds to redox conditions, results in a 500-fold effect on luminescence despite only changing luxI transcription by 10-fold (Bose et al. 2007; Septer and Stabb 2012). Similarly, deletion of litR results in less than a twofold effect on luxR expression, but decreases luminescence by 10-fold (Miyashiro et al. 2010). The results presented here provide another example of the exquisite sensitivity of bioluminescence to QS.
We also found that the sypI mutant is 21-fold dimmer than a ΔlitR mutant (Fig. 2C). Because Qrr1 represses litR mRNA levels, we anticipated that the luminescence levels of strains overexpressing qrr1 would be higher than the ΔlitR mutant. Our surprising result with the sypI mutant may be due, in part, to an indirect effect from overexpressed Qrr1 titrating free Hfq, a condition that occurs in E. coli when an sRNA is overexpressed (Moon and Gottesman 2011). In V. fischeri, lower levels of free Hfq may result in inefficient posttranscriptional regulation by other sRNAs involved in regulating luminescence.
We found that overexpression of SypG also enhances qrr1 expression in a SypK-independent manner (Fig. 8). A recent study has revealed that the SypG regulon extends beyond the syp locus (Ray et al. 2013), and several of these genes appear to impact bioluminescence (V. A. Ray and K. L. Visick, unpubl. results). Future studies will determine how these SypG-regulated genes affect bioluminescence and whether the mechanism involves qrr1.
All sequenced Vibrionaceae members possess a luxQ homologue within their genomes; however, the presence of sypK and the remaining syp genes appears to be species dependent. In addition to V. fischeri, the pathogens Vibrio parahaemolyticus and Vibrio vulnificus have been reported to each contain the syp locus, including a sypK homologue (Yip et al. 2005). Homologues of sypK are also present within the genomes of Aliivibrio salmonicida (VSAL_II0302), V. harveyi (VIBHAR_02224), Photobacterium profundum (PBPRA1735), and Vibrio splendidus (VS_2150). Notably, the syp locus is absent from the genomes of V. cholerae, Vibrio anguillarum, and Vibrio furnissii. As future studies uncover the mechanism underlying the interaction between SypK and LuxQ, they will also provide opportunities to determine how sypK and, more generally, the syp locus have coevolved with the core QS network of the Vibrionaceae, and whether the regulatory link is conserved.
Our finding that a protein involved in biofilm formation can also function in signaling through the QS pathway inverts the traditional view of the role of bacterial QS during biofilm development. Generally, many genes that are involved in forming biofilms are regulated by QS systems. For example, in V. cholerae, expression of the vps exopolysaccharide gene cluster is downregulated by HapR, the LitR homologue in V. cholerae, (Hammer and Bassler 2003; Zhu and Mekalanos 2003). In V. vulnificus, the LitR homologue SmcR was recently shown to control transcription of the capsular polysaccharide (CPS) gene cluster (Lee et al. 2013). From this latter work, it has been proposed that QS within mature biofilms results in the production of cell-associated CPS, which decreases the hydrophobicity of the cell surface. As a result, V. vulnificus cells are released from biofilms with high cell density, thereby providing a mechanism to control the overall size of a biofilm. QS control of biofilm formation has also been observed in non-Vibrionaceae bacteria, including the pathogen Pseudomonas aeruginosa, which uses hierarchically arranged LasR/LasI and RhlR/RhlI QS systems to control biofilm development, in addition to virulence, motility, and antibiotic resistance (Williams and Camara 2009). The study we report here demonstrates that induction of a gene involved in biofilm development is able to influence QS. Whether this regulatory link represents a general phenomenon in bacteria or is instead specific to V. fischeri remains unknown.
Is the regulatory link between SypK and the QS network relevant to the known biology of V. fischeri? Because biofilm formation is often correlated with a sessile, community lifestyle, it seems somewhat counterintuitive for microbes to activate a signaling cascade associated with the planktonic, that is, low cell density, state while actively developing a biofilm. However, the general developmental cycle of a biofilm includes dispersal, which describes the stage when a subset of cells leaves the matrix to initiate biofilm formation on another surface (McDougald et al. 2012). In V. cholerae, mutation of luxO represses biofilm formation, reduces motility, and promotes cellular detachment from biofilms (Zhu et al. 2002; Zhu and Mekalanos 2003). Consequently, inactivation of the LuxU-LuxO phosphorelay via QS can prime cells within mature, densely packed biofilms for dispersal. In V. fischeri, the effect on motility by the LuxU-LuxO phosphorelay is similar to that observed in V. cholerae: mutation of either luxO or qrr1 results in attenuated motility (Lupp and Ruby 2005) (Fig. 5). Therefore, the increased motility from SypK-dependent qrr1 activation may enhance V. fischeri dispersal from syp-mediated biofilms.
Activation of qrr1 expression by SypK may also play a role during the initiation of the squid-Vibrio symbiosis. The current model of initial host colonization is a two-step process, in which V. fischeri cells first attach individually to host cilia and then aggregate in a syp-dependent manner outside the light-organ pores (Altura et al. 2013). The syp genes, including sypK, are required for V. fischeri to efficiently colonize juvenile squid (Yip et al. 2005; Shibata et al. 2012). Our model predicts that activation of the syp locus will result in high qrr1 expression (Fig. 9), which has been shown to repress litR mRNA levels (Miyashiro et al. 2010). V. fischeri cells containing a litR deletion allele are able to outcompete wild-type cells in host colonization (Fidopiastis et al. 2002; Miyashiro et al. 2010). We hypothesize that by linking SypK and the QS network, V. fischeri symbionts can escape via flagellar-based motility from the exopolysaccharide matrix secreted during the aggregation stage. The residual biofilm may hinder other cells from entering the light organ, thereby contributing to the winnowing process during the initial establishment of symbiosis (Nyholm and McFall-Ngai 2004). Future molecular-based studies will help resolve the interconnectivity of SypK in biofilm formation, host colonization, and quorum sensing in V. fischeri.
Acknowledgments
This work was supported by the National Institutes of Health Grant R00 GM 097032 to T. M., the National Institutes of Health Grant GM 059690 to K. L. V., the National Institutes of Health Grant OD 011024 to E. G. R. and M.J. McFall-Ngai, and the National Institutes of Health Grant GM 099507 to E. G. R. We also thank three anonymous reviewers for their suggestions.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Primers used in this study.
References
- Altura MA, Heath-Heckman EA, Gillette A, Kremer N, Krachler AM, Brennan C, et al. The first engagement of partners in the Euprymna scolopesVibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 2013;15:2937–2950. doi: 10.1111/1462-2920.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ. Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JL, Kim U, Bartkowski W, Gunsalus RP, Overley AM, Lyell NL, et al. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol. Microbiol. 2007;65:538–553. doi: 10.1111/j.1365-2958.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Bose JL, Rosenberg CS. Stabb EV. Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch. Microbiol. 2008;190:169–183. doi: 10.1007/s00203-008-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Studer SV, Wassarman K, Zhang Y, Ruby EG. Miyashiro T. The novel sigma factor-like regulator RpoQ controls luminescence, chitinase activity, and motility in Vibrio fischeri. MBio. 2012;3:e00285-11. doi: 10.1128/mBio.00285-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoney-Marino CR, Wolfe AJ. Visick KL. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl. Environ. Microbiol. 2003;69:7527–7530. doi: 10.1128/AEM.69.12.7527-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL. Stabb EV. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 2006;72:802–810. doi: 10.1128/AEM.72.1.802-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA. Ruby EG. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 2002;45:131–143. doi: 10.1046/j.1365-2958.2002.02996.x. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Neu TR. Wozniak DJ. The EPS matrix: the “house of biofilm cells”. J. Bacteriol. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA. Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999a;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA. Bassler BL. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 1999b;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR. Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- Gilson L, Kuo A. Dunlap PV. AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 1995;177:6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Dunlap PV. Ruby EG. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BK. Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Herrero M, de Lorenzo V. Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussa EA, Darnell CL. Visick KL. RscS functions upstream of SypG to control the syp locus and biofilm formation in Vibrio fischeri. J. Bacteriol. 2008;190:4576–4583. doi: 10.1128/JB.00130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough JH. Stabb EV. Substrate specificity and function of the pheromone receptor AinR in Vibrio fischeri ES114. J. Bacteriol. 2013;195:5223–5232. doi: 10.1128/JB.00913-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux F, Binesse J, Saulnier D. Mazel D. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl. Environ. Microbiol. 2007;73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Kim JA, Hwang W, Park SJ. Lee KH. Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol. Microbiol. 2013;90:841–857. doi: 10.1111/mmi.12401. [DOI] [PubMed] [Google Scholar]
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS. Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Lupp C. Ruby EG. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 2005;187:3620–3629. doi: 10.1128/JB.187.11.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MJ, Stabb EV. Ruby EG. Comparative genomics-based investigation of resequencing targets in Vibrio fischeri: focus on point miscalls and artefactual expansions. BMC Genomics. 2008;9:138. doi: 10.1186/1471-2164-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J, Stabb EV, Millikan DS. Ruby EG. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 2003;69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougald D, Rice SA, Barraud N, Steinberg PD. Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- Milton DL. Quorum sensing in vibrios: complexity for diversification. Int. J. Med. Microbiol. 2006;296:61–71. doi: 10.1016/j.ijmm.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Miyashiro T. Ruby EG. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 2012;84:795–806. doi: 10.1111/j.1365-2958.2012.08065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Wollenberg MS, Cao X, Oehlert D. Ruby EG. A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol. Microbiol. 2010;77:1556–1567. doi: 10.1111/j.1365-2958.2010.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Klein W, Oehlert D, Cao X, Schwartzman J. Ruby EG. The N-acetyl-D-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol. Microbiol. 2011;82:894–903. doi: 10.1111/j.1365-2958.2011.07858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K. Gottesman S. Competition among Hfq-binding small RNAs in Escherichia coli. Mol. Microbiol. 2011;82:1545–1562. doi: 10.1111/j.1365-2958.2011.07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR. Visick KL. Inhibition of SypG-induced biofilms and host colonization by the negative regulator SypE in Vibrio fischeri. PLoS One. 2013;8:e60076. doi: 10.1371/journal.pone.0060076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR, Darnell CL. Visick KL. Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri. Mol. Microbiol. 2011;82:114–130. doi: 10.1111/j.1365-2958.2011.07800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiditch MB, Federle MJ, Miller ST, Bassler BL. Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol. Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL. Bassler BL. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV. McFall-Ngai MJ. The winnowing: establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- Pollack-Berti A, Wollenberg MS. Ruby EG. Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ. Microbiol. 2010;12:2302–2311. doi: 10.1111/j.1462-2920.2010.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray VA. Visick KL. LuxU connects quorum sensing to biofilm formation in Vibrio fischeri. Mol. Microbiol. 2012;86:954–970. doi: 10.1111/mmi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray VA, Eddy JL, Hussa EA, Misale M. Visick KL. The syp enhancer sequence plays a key role in transcriptional activation by the sigma54-dependent response regulator SypG and in biofilm formation and host colonization by Vibrio fischeri. J. Bacteriol. 2013;195:5402–5412. doi: 10.1128/JB.00689-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Urbanowski M, Campbell J, Dunn A, Faini M, Gunsalus R, et al. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA. 2005;102:3004–3009. doi: 10.1073/pnas.0409900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septer AN. Stabb EV. Coordination of the arc regulatory system and pheromone-mediated positive feedback in controlling the Vibrio fischeri lux operon. PLoS One. 2012;7:e49590. doi: 10.1371/journal.pone.0049590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y. Bassler BL. Quorum-sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets. Mol. Microbiol. 2012;83:599–611. doi: 10.1111/j.1365-2958.2011.07959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S. Visick KL. Sensor kinase RscS induces the production of antigenically distinct outer membrane vesicles that depend on the symbiosis polysaccharide locus in Vibrio fischeri. J. Bacteriol. 2012;194:185–194. doi: 10.1128/JB.05926-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Yip ES, Quirke KP, Ondrey JM. Visick KL. Roles of the structural symbiosis polysaccharide (syp) genes in host colonization, biofilm formation, and polysaccharide biosynthesis in Vibrio fischeri. J. Bacteriol. 2012;194:6736–6747. doi: 10.1128/JB.00707-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma NJ, Fong JC, Odell LS, Perchuk BS, Laub MT. Yildiz FH. Overexpression of VpsS, a hybrid sensor kinase, enhances biofilm formation in Vibrio cholerae. J. Bacteriol. 2009;191:5147–5158. doi: 10.1128/JB.00401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV. Ruby EG. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 2002;358:413–426. doi: 10.1016/s0076-6879(02)58106-4. [DOI] [PubMed] [Google Scholar]
- Stabb EV. Visick KL. Vibrio fischeri: a bioluminescent light-organ symbiont of the bobtail squid Euprymna scolopes. In: Rosenberg E, DeLong EF, Stackebrand E, Lory S, Thompson F, editors; The Prokaryotes – Prokaryotic Biology and Symbiotic Associations. Berlin Heidelberg: Springer; 2013. pp. 497–532. [Google Scholar]
- Verma SC. Miyashiro T. Quorum sensing in the squid-Vibrio symbiosis. Int. J. Mol. Sci. 2013;14:16386–16401. doi: 10.3390/ijms140816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL. An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol. Microbiol. 2009;74:782–789. doi: 10.1111/j.1365-2958.2009.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Lindell K, El Qaidi S, Hjerde E, Willassen NP. Milton DL. The phosphotransferase VanU represses expression of four qrr genes antagonizing VanO-mediated quorum-sensing regulation in Vibrio anguillarum. Microbiology. 2011;157:3324–3339. doi: 10.1099/mic.0.051011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Camara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009;12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Yildiz FH. Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009;17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip ES, Grublesky BT, Hussa EA. Visick KL. A novel, conserved cluster of genes promotes symbiotic colonization and sigma-dependent biofilm formation by Vibrio fischeri. Mol. Microbiol. 2005;57:1485–1498. doi: 10.1111/j.1365-2958.2005.04784.x. [DOI] [PubMed] [Google Scholar]
- Yip ES, Geszvain K, DeLoney-Marino CR. Visick KL. The symbiosis regulator rscS controls the syp gene locus, biofilm formation and symbiotic aggregation by Vibrio fischeri. Mol. Microbiol. 2006;62:1586–1600. doi: 10.1111/j.1365-2958.2006.05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL. Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used in this study.


