Abstract
Systemic deletion of the gene encoding for adipose triglyceride lipase (ATGL) in mice leads to severe cardiac dysfunction due to massive accumulation of neutral lipids in cardiomyocytes. Recently, impaired peroxisome proliferator-activated receptor α (PPARα) signaling has been described to substantially contribute to the observed cardiac phenotype. Disturbances of the ubiquitin–proteasome system (UPS) have been implicated in numerous cardiac diseases including cardiomyopathy, ischemic heart disease, and heart failure. The objective of the present study was to investigate the potential role of UPS in cardiac ATGL deficiency. Our results demonstrate prominent accumulation of ubiquitinated proteins in hearts of ATGL-deficient mice, an effect that was abolished upon cardiomyocyte-directed overexpression of ATGL. In parallel, cardiac protein expression of the ubiquitin-activating enzyme E1a, which catalyzes the first step of the ubiquitination cascade, was significantly upregulated in ATGL-deficient hearts. Dysfunction of the UPS was accompanied by activation of NF-κB signaling. Moreover, the endoplasmic reticulum (ER)-resident chaperon protein disulfide isomerase was significantly upregulated in ATGL-deficient hearts. Chronic treatment of ATGL-deficient mice with the PPARα agonist Wy14,643 improved proteasomal function, prevented NF-κB activation and decreased oxidative stress. In summary, our data point to a hitherto unrecognized link between proteasomal function, PPARα signaling and cardiovascular disease.
Abbreviations: ATF6, activating transcription factor 6; ATGL, adipose triglyceride lipase; AKO, adipose triglyceride lipase knockout; AMC, amino-4-methylcoumarin; CHOP, C/EBP homologous protein; EDTA, ethylenediaminetetraacetic acid; ER, endoplasmic reticulum; FA, fatty acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GRP78, glucose-regulated protein 78 kDa; GTPCH-1, GTP cyclohydrolase 1; HO-1, heme oxygenase-1; IκB, inhibitor of κB; IKK, IκB kinase; IL-6, interleukin 6; I/R, ischemia/reperfusion; IRE-1, inositol-requiring enzyme 1; MCP-1, monocyte chemotactic protein-1; MG132, carbobenzoxy-Leu-Leu-leucinal; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOX, NADPH oxidase; PDI, protein disulfide isomerase; PGC, PPARγ coactivator; PPARα, peroxisome proliferator receptor α; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SOD-1, Cu,Zn-superoxide dismutase; SucLLVY-AMC, succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin; TAG, triacylglyceride; Tfam, mitochondrial transcription factor A; Tg, transgene; TNFα, tumor necrosis factor α; UBE1a, ubiquitin-activating enzyme 1a; UPR, unfolded protein response; UPS, ubiquitin-proteasome system; WT, wild-type
Keywords: Adipose triglyceride lipase, Cardiac dysfunction, Inflammation, NF-κB, Peroxisome proliferator-activated receptor α
Highlights
-
•
Accumulation of ubiquitinated proteins was increased in ATGL-deficient hearts.
-
•
Cardiac NF-κB signaling was upregulated in ATGL deficiency.
-
•
Chronic PPARα treatment improved proteasomal function.
-
•
Cardiac NOX activity was normalized upon PPARα treatment.
1. Introduction
The ubiquitin–proteasome system (UPS) represents the major degradation pathway of intracellular proteins and is therefore substantially involved in quality control and turnover of cellular proteins (for review, see [1]). Prior to proteolytic degradation, proteins are tagged by covalent linkage to multiple ubiquitin molecules. This process involves sequential actions of ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin-ligases (E3). Degradation of ubiquitinated proteins is mediated by the 26S proteasome, an ATP-dependent proteolytic complex that consists of a 20S core and two 19S regulatory subunits. Effective quality control and protein turnover in the heart are crucial for physiological cardiac performance and dysfunction of the UPS has been implicated in numerous cardiac diseases including cardiomyopathy, ischemic heart disease, and heart failure [2], [3], [4]. In studies of ischemia/reperfusion (I/R) injury, decreased proteasomal activity was associated with oxidative modification of the 20S proteasome [5]. In a transgenic mouse model of desmin-related cardiomyopathy, entry of ubiquitinated proteins into the 20S proteasome was blocked due to the downregulation of key components of the 19S subunit [6]. Furthermore, proteasomal inhibition was described to disturb mitochondrial homeostasis, resulting in impaired mitochondrial electron transport and therefore decreased energy production [7]. In a mouse model of pressure overload-induced cardiac hypertrophy, dysfunction of the UPS was linked to an imbalance of apoptosis regulating proteins, e.g. elevation of apoptotic signals (p53 and Bax) and/or reduction of anti-apoptotic stimuli (Bcl-2, Bcl-XL). As consequence, transition from decompensated cardiac hypertrophy to decompensated heart failure [3] was observed in those animals, indicating a possible role of UPS in development of cardiac dysfunction.
Peroxisome proliferator-activated receptor α (PPARα) acts as key transcriptional regulator of lipid and glucose metabolism and is implicated in the pathogenesis of inflammatory processes. PPARα is predominantly expressed in tissues with high intracellular fatty acid (FA) catabolism, such as the liver, skeletal muscle, and heart. Activation of PPARα by FA or FA derivates results in heterodimerization with nuclear retinoid X receptor and recruitment of PPARγ coactivator-1α (PCG-1α) or PPARγ coactivator 1-ß (for review, see [8]). Haemmerle and colleagues have recently shown that generation of PPARα ligands by the lipolytic enzyme adipose triglyceride lipase (ATGL) is pivotal for efficient mitochondrial substrate oxidation and respiration [9]. The enzyme is predominantly expressed in adipose tissues and catalyzes the initial and rate-limiting step of triglyceride (TAG) hydrolysis [10]. Global deletion of the ATGL gene in mice resulted in a phenotype with massive cardiac lipid accumulation, leading to severe cardiomyopathy and premature death of the animals [11]. Pharmacological treatment of ATGL-deficient (AKO) mice with PPARα agonists ameliorated mitochondrial defects and prevented lethal cardiomyopathy [9]. Langendorff perfusion experiments of isolated hearts showed that chronic treatment of AKO mice with a PPARα agonist effectively attenuated cardiac hypertrophy and improved systolic and diastolic performance [12]. A recent study by Schrammel and colleagues pointed to an essential role of oxidative and inflammatory stress in cardiac dysfunction observed in ATGL deficiency [13]. These observations agree well with a report showing the involvement of oxidative stress in left ventricular dysfunction observed in PPARα-deficient mice [14]. In addition to their lipid lowering actions, PPARα agonists were shown to prevent cardiac inflammation in angiotensin II-infused rats via inhibition of the NF-κB signal transduction pathway [14]. In accordance with this, Staels and coworkers reported in vivo anti-inflammatory actions of PPARα agonists, resulting in decreased interleukin-6 (IL-6) production in hyperlipidemic patients [15].
The aim of the present study was to probe a potential contribution of disturbed UPS in the development of lipid-driven cardiomyopathy. Furthermore, we wanted to clarify if cardiac oxidative inflammatory stress and defective PPARα signaling are linked in ATGL deficiency.
2. Materials and methods
2.1. Mice and experimental groups
Homozygous male and female AKO mice on a C57BL/6 background (n = 30–50) and their corresponding wild-type (WT; n = 30–50) littermates were used in this study. Additionally, WT (n = 15–25) and AKO (n = 15–25) mice with cardiomyocyte-directed overexpression of ATGL driven by the α-myosin heavy chain promoter were used in a subset of experiments and were herein designated as WT/cTg and AKO/cTg, respectively. AKO mice [11] as well as cardiac transgene mice [9] were generated in the laboratory of Rudolf Zechner (University of Graz, Austria). After weaning, mice were kept on standard laboratory chow and were allowed ad libitum access to food and water. Animals were housed in approved cages and kept on a regular 12 h dark/light cycle. At the age of 9–10 weeks, mice were sacrificed and hearts were dissected, cleaned and stored in liquid nitrogen until further analysis.
Animal care was in compliance with the Austrian law on experimentation with laboratory animals (last amendment, 2011) based on the US National Institutes of Health guidelines.
2.2. Wy14,643 treatment
WT and AKO animals (aged 6–7 weeks; 5–8 animals per group) were fed chow mixed with 0.1% Wy14,643 (Cayman Chemical, Ann Arbor, MI, USA) for three weeks [9], [12]. Food intake was similar between the experimental groups. The protocol was approved by the Austrian Federal Ministry of Science and Research (BMWF-66.007/0001-11/3b/2014).
2.3. Real-time PCR
Total RNA was extracted from homogenized cardiac tissues using the GenElute™ Mammalian Total RNA Miniprep Kit including DNAse treatment of samples (Sigma, Austria). For first-strand cDNA synthesis, ~ 800 ng of total RNA was reversely transcribed at 37 °C using the High Capacity Reverse Transcription Kit (Life Technologies, Vienna, Austria). Real-time PCR analysis was performed with ~ 30 ng cDNA using TaqMan® Universal PCR Master Mix and pre-designed TaqMan® Gene Expression Assays as listed in Supplementary Table 1. Amplification was carried out using 7300 Real-Time PCR System (Life Technologies, Vienna, Austria). Cycling conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, 40–50 cycles of 15 s at 95 °C, and finally 1 min at 60 °C. Relative mRNA levels were quantified using the 2− ΔΔCt method, normalized to the reference gene cyclophilin.
2.4. Western blot analysis
Hearts were homogenized in 10 volumes of ice-cold RIPA lysis buffer (Sigma, Vienna, Austria) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA), protease and phosphatase inhibitor cocktails (Complete™; PhosSTOP™; Roche, Vienna, Austria) using a glass potter Elvehjem homogenizer. Protein concentration was determined with the Thermo Scientific Pierce BCA™ Protein Assay Kit (Fisher Scientific Austria GmbH, Vienna, Austria) using bovine serum albumin as standard. Denatured proteins (20–40 μg) were resolved by SDS-PAGE (Mini-PROTEAN T, Bio-Rad) and transferred onto nitrocellulose membranes (0.45 μm). After blocking with 5% non-fat dry milk in Tris-buffered saline containing 0.1% (v/v) Tween-20 for 1 h at ambient temperature, membranes were incubated overnight at 4 °C with primary antibodies (listed in Supplementary Table 2). Thereafter, membranes were washed 3 times for 5 min and incubated with respective horseradish peroxidase-conjugates anti-rabbit or anti-mouse IgG (1:5,000). Immunoreactive bands were visualized with ECL detection reagent (Biozym, Germany) and quantified densitometrically using the E.A.S.Y. 1.3 Win 32 software (Herolab, Vienna, Austria).
2.5. Measurement of chymotrypsin-like proteasomal activity
Hearts were homogenized in 50 mM Tris buffer, pH 7.5, containing 20 mM KCl, 5 mM MgCl2, 250 mM sucrose, and 1 mM dithiothreitol using a glass potter Elvehjem homogenizer. Homogenates were centrifuged at 20,000 g for 15 min at 4 °C. Lipid-free infranatants were collected and protein concentration was determined with the Thermo Scientific Pierce BCA™ Protein Assay Kit (Fisher Scientific Austria GmbH, Vienna, Austria) using bovine serum albumin as standard. Samples containing 30–50 μg protein were incubated in 100 μl of 50 mM Tris buffer (pH 7.5), containing 20 mM KCl, and 5 mM MgCl2, in the presence and absence of 1 μM MG132 (Enzo; Switzerland). The peptide substrate SucLLVY-AMC (Sigma, Austria) was used at a concentration of 100 μM. Assays were carried out in 96 well microtiter plates and fluorescence was measured after 10 min incubation at 37 °C using a fluorescence plate reader (excitation wavelength of 355 nm, emission wavelength of 460 nm; SpectraMax, Molecular Devices, Germany). Chymotrypsin-like activity was quantified and expressed as cleaved AMC per mg protein using AMC (Sigma, Austria) as a standard.
2.6. Measurement of NADPH oxidase activity
Frozen hearts were homogenized in 10 volumes of PBS containing Complete™ using a glass potter Elvehjem homogenizer. Total homogenates (~ 100 μg protein) were incubated in PBS containing diethylenetriamine pentaacetic acid (100 μM) at 37 °C for 10 min in the absence or presence of Cu,Zn-superoxide dismutase (SOD-1; 500 U/ml). Thereafter, NADPH (300 μM) was added to activate NADPH oxidases, followed by addition of lucigenin at a non-redox cycling concentration of 5 μM [16]. Lucigenin-derived chemiluminescence was measured every 30 s for 5 min using a TriCarb® 2100TR Liquid Scintillation Counter (PerkinElmer, Vienna, Austria). Results were corrected for protein-deficient blanks and expressed as cpm per μg protein.
2.7. Statistics
Results were expressed as mean values ± S.E.M. Comparison between 2 groups was performed using Student's t-test and comparison between multiple groups was performed using one-way ANOVA and Student–Newman–Keuls as post-hoc test. p-Values of < 0.05 were considered as statistically significant.
3. Results
3.1. Accumulation of ubiquitinated proteins in cardiac ATGL deficiency
Fig. 1A shows a representative Western blot of ATGL expression in cardiac homogenates of WT, AKO, WT/cTg, and AKO/cTg mice. Two different exposure times were chosen to better illustrate the massive ATGL overexpression in transgene animals (WT/cTg and AKO/cTg). To examine the involvement of UPS in the pathogenesis of cardiac dysfunction observed in AKO mice, total levels of ubiquitinated proteins as well as protein expression of the ubiquitin-activating enzyme E1 (UBE1a) were measured in cardiac homogenates of WT, AKO, WT/cTg, and AKO/cTg mice (Fig. 1B). As shown in Fig. 1C, homogenates of AKO hearts showed markedly increased levels of ubiquitinated proteins, whereas only minor accumulation was observed in WT/cTg and AKO/cTg mice compared to WT controls (Fig. 1C). Protein expression of UBE1a, which catalyzes the initial step of the UPS cascade (i.e. activation of ubiquitin moieties through thioester formation), was more than 2-fold increased in AKO hearts (Fig. 1D). Expression of UBE1a was normalized to WT levels upon cardiomyocyte-directed overexpression of ATGL in AKO mice. To investigate whether increased protein ubiquitination was a consequence of 26S proteasomal dysfunction, chymotrypsin-like activity was measured in cardiac cytosols of WT and AKO animals. Peptidase activity was monitored over a range of ATP concentrations (0–4 mM) to ensure maximal activation of the 26S proteasome. ATP bidirectionally modulated proteasomal activity with maximal activation observed at 10–30 μM ATP (Fig. 1E). Normalizing proteasomal activity to basal values (measured in the absence of exogenous ATP) resulted in a similar activation profile of the UPS in cardiac homogenates of WT and AKO mice (Fig. 1F). The selective proteasome inhibitor MG132 (1 μM) decreased chymotrypsin-like activity to a similar extent (~ 90%) in WT and AKO animals (Fig. 1E). Protein expression of the 19S regulatory cap was not affected by ATGL deficiency (Fig. 1G). Western blot analysis of the mitochondrial marker citrate synthetase revealed that protein expression was significantly decreased in AKO animals by 41 ± 8%. Similar results were obtained for prohibitin (53 ± 18%), however, results failed to reach statistical significance (Fig. 1H).
Fig. 1.
UPS and cardiac ATGL deficiency. (A) Representative Western blots of ATGL protein expression in cardiac homogenates of WT, AKO, WT/cTg, and AKO/cTg mice. Two different exposure times were chosen to better illustrate supraphysiological ATGL levels in transgene animals. (B) Representative Western blots showing total levels of ubiquitinated proteins as well as UBE1a protein expression in WT, AKO, WT/cTg, and AKO/cTg hearts. (C) Accumulation of ubiquitinated proteins was quantified in cardiac homogenates of WT (open bars), AKO (solid bars), WT/cTg (striped bars), and AKO/cTg (gray bars) mice. Total protein ubiquitination was significantly increased in ATGL deficiency. Data represent mean values ± S.E.M. of 9 individual experiments. (D) Protein expression of UBE1a was upregulated in AKO hearts. Data were expressed as folds of WT control (WT = 1) and represent mean values ± S.E.M. of 6 individual experiments. (E) Chymotrypsin-like proteasomal activity was measured in cardiac cytosols of WT (open circles) and AKO (solid circles) mice over a range of ATP concentrations (0–4 mM). Chymotrypsin-like activity was inhibited in the presence of the peptide aldehyde MG132 (1 μM). (F) Normalization of proteasomal activity to basal values (basal activity = 1) resulted in a similar activation profile. Data represent mean values ± S.E.M. of 5–6 individual experiments; *p < 0.05 vs WT. (G) Protein expression of the 19S regulatory subunit was not affected by ATGL deficiency. (H) Cardiac homogenates of AKO mice showed decreased expression of mitochondrial marker proteins citrate synthetase and prohibitin. Data were expressed as folds of WT control (WT = 1) and represent mean values ± S.E.M. of 6 individual experiments; *p < 0.05 vs WT.
3.2. Endoplasmic reticulum (ER) stress and apoptosis
Dysfunction of the proteasome and subsequent accumulation of damaged/misfolded proteins have been described to cause an ER-resident complex cellular stress response termed as unfolded protein response (UPR; for review, see [17]). To judge if activated UPR is operative in cardiac ATGL deficiency we measured protein/mRNA expression of selected parameters of the system. Western blot analysis of cardiac homogenates showed that protein expression of glucose-regulated protein 78 kDa (GRP78; Fig. S1A), activating transcription factor 6 (ATF6; Fig. S1B), and inositol-requiring kinase 1 (IRE1; Fig. S1C) was not affected by ATGL deficiency, arguing against an involvement of ER stress in cardiac dysfunction of AKO mice. Interestingly, protein expression of the ER-resident chaperon protein disulfide isomerase (PDI) was elevated more than 6-fold in AKO hearts and normalized upon overexpression of ATGL in AKO cardiomyocytes (Fig. S1D). Cardiac protein expression of the proapoptotic marker C/EBP homologous protein (CHOP) was similar in all experimental groups (Fig. S1E). Representative Western blots are shown in Fig. S1F. To test if UPS dysfunction is linked to an imbalance of apoptosis regulating stimuli, protein expression of pro-apoptotic p53 and anti-apoptotic Bcl-2 was measured in cardiac homogenates of WT, AKO, WT/cTg, and AKO/cTg animals. As shown in Figs. S1G and 1H, neither protein expression of p53 nor of Bcl-2 was significantly affected by ATGL knockout.
3.3. Activation of the NF-κB pathway in ATGL deficiency
To investigate the mechanism underlying cardiac inflammation in ATGL deficiency [13] we analyzed crucial parameters of canonical NF-κB signaling (for review, see [18]). As illustrated in Fig. 2A, cardiac ATGL deficiency led to increased protein expression of the IκB kinase (IKK) complex as IKKα protein levels were significantly elevated in homogenates of AKO mice. By contrast, IKKβ protein expression was similar in all experimental groups (Fig. 2B). Furthermore, AKO hearts showed a more than 3-fold increased NF-κB RelA/p65 protein expression that was normalized to WT levels in hearts of AKO/cTg mice (Fig. 2C). Phosphorylation of NF-κB RelA/p65 at serine 536, which is thought to activate the NF-κB complex independently of IκB release [19], was also markedly elevated in AKO hearts (Fig. 2D). However, the ratio of phosphorylated to total NF-κB protein was unaffected by ATGL knockout (data not shown). Cardiac protein levels of IκB (which reacts with NF-κB to form an inactive cytosolic complex) were increased nearly 2-fold in AKO mice (Fig. 2E). All markers of NF-κB signaling that were increased in AKO mice were restored in AKO/cTg mice. Representative Western blots are shown in Fig. 2F. Activation of cardiac NF-κB signaling was supported by qPCR experiments showing that mRNA levels of selected NF-κB target genes including TNFα, monocyte chemoattractant protein-1 (MCP-1), IL-6, heme oxygenase-1 (HO-1), and GTP cyclohydrolase 1 (GTPCH-1) were significantly increased in AKO hearts (Fig. 2G).
Fig. 2.
Activation of NF-κB signaling in AKO hearts. Protein expression was measured in cardiac homogenates of WT (open bars), AKO (solid bars), WT/cTg (striped bars), and AKO/cTg (gray bars) mice. Protein levels of (A) IKKα but not of (B) IKKβ were significantly increased in ATGL deficiency. Protein expression of (C) NF-κB, (D) p-NF-κB and (E) IκB were raised in AKO hearts. Data were expressed as folds of WT control (WT = 1) and represent mean values ± S.E.M. of 6 individual experiments; *p < 0.05 vs WT; #p < 0.05 vs AKO. (F) Representative Western blots. (G) Cardiac mRNA levels of NF-κB target genes TNFα, MCP-1, IL-6, HO-1, and GTPCH-1 determined by qPCR were upregulated in ATGL deficiency. Data represent mean values ± S.E.M. of 5–10 individual experiments. *p < 0.05 vs WT.
3.4. Treatment of WT and AKO mice with the PPARα agonist Wy14,643
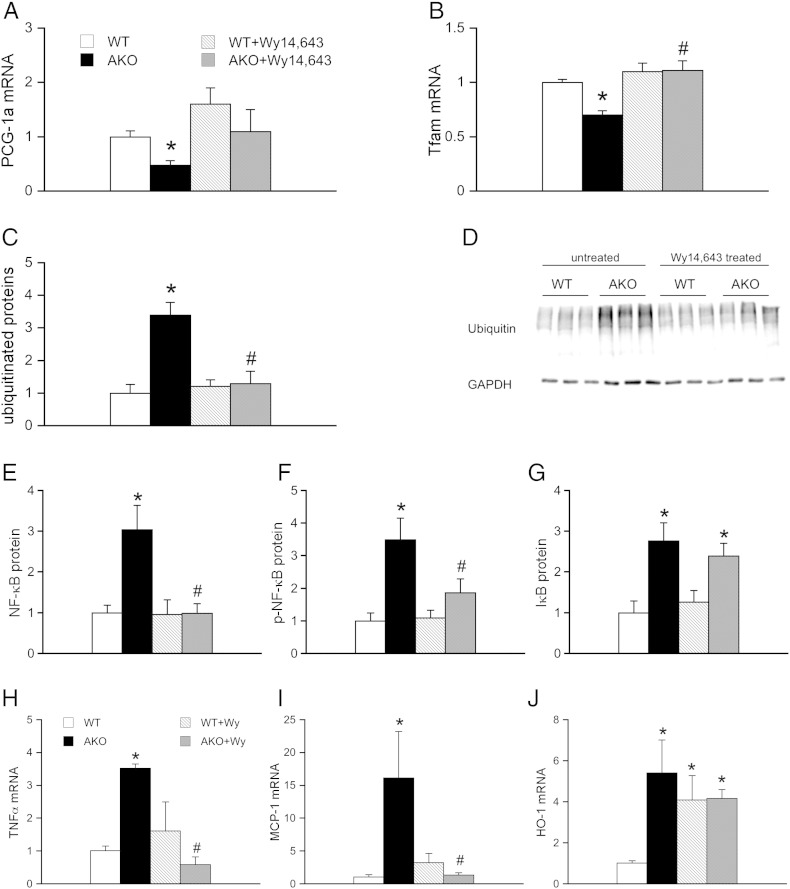
Since chronic treatment of AKO mice with PPARα agonists has been described to substantially improve cardiac performance in prior studies [9], [12], we investigated whether the drug has a similar effect on cardiac proteasomal function. For this purpose, 6-week-old WT and AKO mice were fed a chow containing 0.1% Wy14,643 according to a well-established protocol [9]. To evaluate the outcome of the treatment, cardiac mRNA expression of PPARα coactivator PGC-1α (Fig. 3A) and mitochondrial transcription factor A (Tfam; Fig. 3B) was analyzed. Both, PGC-1α and Tfam mRNA were significantly decreased in ATGL-deficient hearts (Figs. 3A and B) by 52 ± 16% and 30 ± 4%, respectively. Chronic treatment with the PPARα agonist Wy14,643 restored PGC-1α and Tfam mRNA levels in AKO animals to WT niveau. These results are in good accordance with that reported by Haemmerle and colleagues [9]. As shown in Figs. 3C and D, feeding of AKO mice with the PPARα agonist reduced cardiac protein expression of ubiquitinated proteins to WT levels suggesting restored function of the UPS in those hearts. To investigate whether the observed effect was linked to altered NF-κB activation we analyzed protein expression and phosphorylation status of NF-κB RelA/p65. As illustrated in Figs. 3E and F, both parameters were reduced to WT levels upon Wy14,643 treatment. In contrast, cardiac IκB protein expression was not normalized upon feeding mice with the PPARα agonist (Fig. 3G). Furthermore, we observed that mRNA expression of inflammatory markers TNFα (Fig. 3H) and MCP-1 (Fig. 3I) were reduced to WT levels while increased expression of protective HO-1 persisted after Wy14,643 treatment (Fig. 3J).
Fig. 3.
Feeding of WT and AKO mice with the PPARα agonist Wy14,643. Protein and mRNA expression was measured in cardiac homogenates prepared from Wy14,643-treated WT (striped bars) and AKO (gray bars) mice and compared to that of non-treated WT (open bars) and AKO (solid bars) controls. Improvement of PPARα signaling by Wy14,643 treatment was confirmed by reversal of (A) PGC-1α and (B) Tfam mRNA expression. Feeding of AKO mice with Wy14,643 reduced cardiac expression of (C, D) ubiquitinated proteins to WT levels. Protein expression of (E) NF-κB and (F) p-NF-κB but not (G) IκB was reversed in Wy14,643-treated AKO mice. Cardiac mRNA levels of the NF-κB target genes (H) TNFα and (I) MCP-1 were reduced to WT levels while increased expression of (J) HO-1 persisted after Wy14,643 treatment. Data represent mean values ± S.E.M. of 5–6 individual experiments. *p < 0.05 vs WT; #p < 0.05 vs AKO.
3.5. Cardiac oxidative stress and Wy14,643 treatment
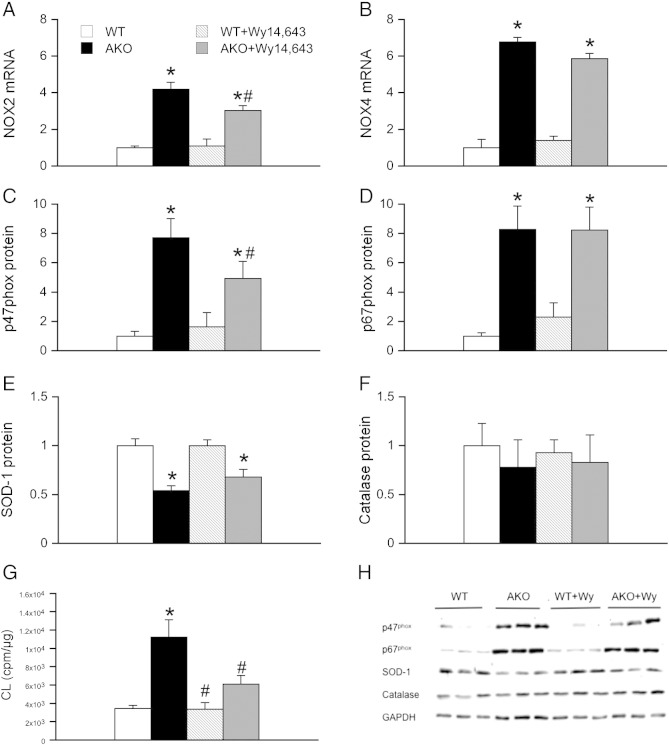
Cardiac oxidative stress was assayed as mRNA expression of the two major cardiac NADPH oxidase isoforms (NOX), NOX2 and NOX4, as well as protein expression of the NOX2 cytosolic activator subunits p47phox and p67phox. As shown in Fig. 4A, NOX2 mRNA was significantly upregulated in AKO animals. This effect was significantly decreased in AKO animals treated with the PPARα agonist Wy14,643. In contrast, upregulation of NOX4 mRNA in AKO animals persisted upon Wy14,643-treatment (Fig. 4B). In accordance with NOX2 mRNA levels, p47phox protein showed an ~ 8-fold upregulation in cardiac homogenates of AKO mice. Upon Wy14,643 treatment, protein expression of p47phox was significantly reduced compared to untreated AKOs but was still ~ 6-fold increased compared to WT controls (Fig. 4C). By contrast, ~ 8-fold increased protein expression of p67phox observed in AKO hearts persisted upon Wy14,643 treatment (Fig. 4D). To test for the anti-oxidative capacity, catalase and SOD-1 protein expression was measured in cardiac homogenates of WT, AKO, WT/cTg, and AKO/cTg mice. SOD-1 protein was significantly decreased in AKO hearts, an effect that was not affected by Wy14,643 treatment (Fig. 4E). Protein expression of catalase, which triggers decomposition of hydrogen peroxide, was similar in all experimental groups (Fig. 4F). To investigate if partially reduced mRNA and protein levels of distinct NOX subunits have an impact on NADPH oxidase activity, lucigenin-derived chemiluminescence was measured in cardiac homogenates of untreated and Wy14,643-supplemented animals. As shown in Fig. 4G, ATGL deficiency resulted in more than 3-fold higher chemiluminescence production compared to WT. Treatment with the PPARα agonist Wy14,643 significantly reversed the effect of ATGL deficiency. Preincubation with the superoxide scavenging enzyme SOD-1 diminished chemiluminescence in all experimental groups to an equal extent (56 ± 2.6%). Representative Western blots are illustrated in Fig. 4H.
Fig. 4.
Oxidative stress in Wy14,643-treated AKO mice. Cardiac mRNA expression was measured in homogenates of WT (open bars), AKO (solid bars), Wy14,643-treated WT (striped bars), and Wy14,643-treated AKO/cTg (gray bars) mice. (A) NOX2 mRNA was significantly upregulated in AKO animals. This effect was decreased by treatment with the PPARα agonist Wy14,643. (B) Upregulation of NOX4 mRNA in AKO animals persisted upon Wy14,643 supplementation. (C) Protein expression of p47phox was significantly increased in cardiac homogenates of AKO mice and reduced by 25% upon Wy14,643-treatment. (D) Upregulation of cardiac p67phox protein expression was unaffected by Wy14,643. (E, F) Protein expression of SOD-1 was significantly decreased in untreated and Wy14,643-treated AKO hearts, while cardiac levels of catalase were similar in all experimental groups. Data were expressed as folds of WT control (WT = 1) and represent mean values ± S.E.M. of 6 individual experiments; *p < 0.05 vs WT; #p < 0.05 vs AKO. (G) NADPH oxidase activity was measured as lucigenin-derived chemiluminescence. (H) Representative Western blots.
4. Discussion
UPS-mediated protein degradation is crucial to maintain the dynamic equilibrium between intact and misfolded/damaged proteins. In cardiomyocytes, which are exposed to heavy mechanical workload, imbalances between protein biosynthesis and degradation were reported to contribute to cardiac diseases including desmin-related cardiomyopathy, cardiac hypertrophy, and heart failure [2], [4], [6].
In the present study we demonstrate that AKO mice suffer from significant disturbances in cardiac UPS that are associated with marked activation of cellular NF-κB signaling. Chronic treatment of mice with the PPARα agonist Wy14,643 improved proteasomal function and prevented NF-κB activation and associated oxidative inflammatory stress.
The observation that accumulation of ubiquitinated proteins was completely reversed by cardiomyocyte-directed overexpression of ATGL suggests a causal relationship between ATGL deficiency and dysfunction of the UPS. Increased protein ubiquitination may arise from defective proteasomal activity on the one hand and accumulation of oxidatively damaged, misfolded and/or aggregated proteins on the other hand. Despite decreased basal proteasomal activity in AKO hearts, ATP-dependent activation was similar between WT and AKO hearts, suggesting that maximal stimulation of the 26S proteasome is still preserved. According to Powell and coworkers, the 19S regulatory cap, which facilitates substrate access to the 20S catalytic core in an ATP-dependent manner, may represent a potential target of oxidative modification [20]. However, our results show that protein expression of the 19S particle was not affected by ATGL deficiency. Nevertheless we cannot exclude oxidative damage of distinct ATPase subunits within the 19S complex.
Haemmerle and coworkers demonstrated that AKO hearts suffer from prominent mitochondrial dysfunction evident as impaired oxygen consumption under basal and succinate-stimulated conditions [9]. Mitochondrial defects are often associated with decreased ATP levels [21]. Since ATP is required for proteasomal assembly and protein processing of the 26S proteasome (for review, see [22]), it is conceivable that low intracellular ATP levels in AKO hearts might be an explanation for insufficient basal proteasomal activity. This hypothesis is corroborated by the observation that PPARα treatment normalized both cardiac protein ubiquitination (this study) and mitochondrial function [9]. Additionally, decreased expression of the mitochondrial marker proteins citrate synthetase (and prohibitin) point to reduced mitochondrial content/function in AKO hearts and therefore imply potential differences in endogenous ATP production between WT and AKO hearts.
Considering the fact that AKO mice suffer from oxidative inflammatory stress [13], oxidative modification of a crucial site within the proteasome is feasible. This hypothesis is supported by a recent study of coronary I/R injury, in which decreased proteasomal trypsin-like activity was linked to oxidative modification of the proteasome [23]. Chronic treatment of AKO mice with the PPARα agonist Wy14,643 resulted in reduced NADPH oxidase activity which together with rescued cardiac protein ubiquitination argues for a link between oxidative stress and UPS dysfunction. In accordance with our study, Barlaka and colleagues demonstrated that Wy14,643 treatment ameliorated parameters of cardiac I/R injury by reduction of oxidative stress and inhibition of matrix metallopeptidases [24]. In a separate study, Powell and colleagues showed that cardiac I/R injury was accompanied by decreased proteasomal activity and subsequent increase in myocardial ubiquitinated proteins [5], [20]. It is therefore feasible that in the AKO model Wy14,643 exerts its beneficial effect by decreasing oxidative stress and therefore normalizing proteasomal activities.
Proteasomal dysfunction in AKO hearts seems accompanied by upregulation of the E1/E2/E3 machinery since protein expression of UBE1a (which initiates activation of ubiquitin) was significantly increased in AKO hearts. This observation agrees well with reports of increased mRNA expression of the ubiquitin-conjugating enzyme E2G2 observed in human failing hearts [25]. Therefore, it is conceivable that potential compensating mechanisms exist within cells that counteract the increased burden of damaged/misfolded proteins.
Analysis of selected targets of the cellular UPR revealed that protein expression of the ER chaperon PDI (which catalyzes formation and rearrangement of disulfide bonds) was markedly increased in AKO hearts. A recent study suggests a protective role of PDI in the myocardium of infarct patients since its expression was inversely correlated with apoptotic rates and presence of heart failure [26]. Furthermore, increased activity of superoxide dismutase in PDI-overexpressing cardiomyocytes was observed. Therefore, upregulated PDI protein in AKO mice appears to be more related to oxidative stress than to UPR activation [26]. In line with this, we observed similar expression levels of the pro-apoptotic transcription factors CHOP and p53 in WT and AKO hearts, arguing against an apoptotic scenario in cardiac ATGL deficiency. We therefore conclude that PDI is upregulated to preserve proper protein folding and thereby reduce working load of the UPS. This is in agreement with observations from Fuchs and colleagues, showing a protective role of ATGL in tunicamycin-induced hepatic ER stress [27].
The observation of enhanced NF-κB p65 signaling in cardiac ATGL deficiency is in line with the idea that chronic activation of NF-κB signaling promotes inflammatory processes and hypertrophic growth of cardiomyocytes [28]. Our data point to another less described implication of NF-κB in the development of cardiac heart failure, that is NF-κB-mediated oxidative inflammatory stress as pivotal step leading to accumulation of ubiquitinated proteins. A similar correlation was suggested by Marfella and colleagues [34], who provided evidence for an important role of the UPS in NF-κB-dependent inflammation in atherosclerotic plaques of hypertensive patients. In this context, it is also conceivable that the beneficial effects of Wy14,643 treatment on oxidative inflammatory stress in AKO animals were mediated via inhibition of the inflammatory actions of NF-κB.
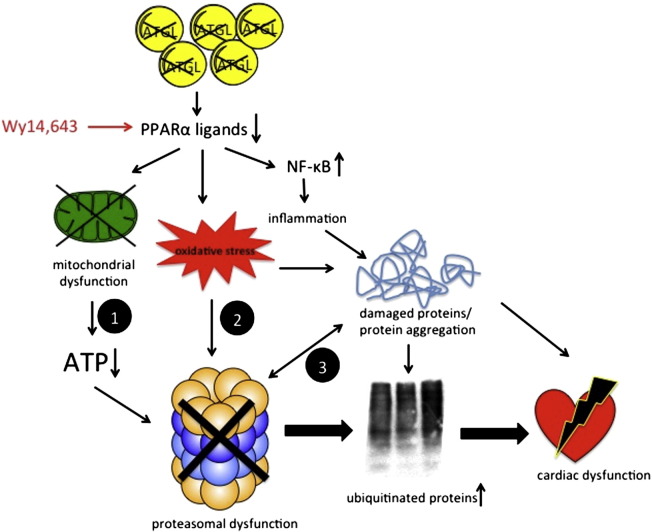
Our current working hypothesis is depicted in Scheme 1. ATGL deficiency in cardiomyocytes results in massively reduced triacylglyceride hydrolysis and consequently decreased production of lipid ligands for PPARα activation (e.g. fatty acids, acyl-CoA and various fatty acid-derived compounds) [9]. As PPARα signaling is an important key player in energy metabolism and inflammation, its downregulation leads to deleterious consequences such as disrupted mitochondrial substrate oxidation and consequent decreased ATP production. Since ATP is required for 19S-mediated channel opening and substrate unfolding, limited ATP availability causes decreased activation of the 26S proteasome (path 1). Proteasomal dysfunction entails insufficient degradation of target proteins, thus resulting in accumulation and aggregation of ubiquitinated products. In a circulus vitiosus, these aggregates further inhibit proteasomal activities, which causes additional cellular stress [29]. Considering the presence of oxidative inflammatory stress in AKO animals, disturbances of the cardiac UPS might also originate from oxidative modifications of critical residues within the catalytic core of the proteasome (path 2). Recent studies have shown that distinct subunits are susceptible to oxidative modifications, including carbonylation, hydroxynonenal-mediated alkylation, and S-glutathionylation [23], [30], [31], [32]. In addition, enhanced superoxide production may also cause protein crosslinking through formation of Schiff bases and/or disulfide bridges [33] which might lead to saturation of the proteasome by non-degradable aggregates (path 3). Defective proteasomal activity together with mitochondrial dysfunction is implicated in the development of cardiac dysfunction and premature death of AKO animals. Pharmacological intervention with the PPARα agonist Wy14,643 improves mitochondrial and proteasomal functions as well as oxidative and inflammatory stress.
Scheme 1.
Interplay of signaling pathways leading to cardiac dysfunction. ATGL deficiency in cardiomyocytes results in decreased production of lipid ligands for PPARα activation. Consequent mitochondrial dysfunction results in decreased intracellular ATP levels, leading to reduced activation of the 26S proteasome (path 1). Proteasomal dysfunction causes accumulation and aggregation of ubiquitinated proteins. Increased oxidative inflammatory stress may damage critical residues/domains within the catalytic core of the proteasome (path 2). Damaged and/or misfolded proteins impair proteasomal activity by saturation of the 20S core with non-degradable aggregates (path 3). Restored PPARα signaling with Wy14,643 reduces oxidative inflammatory stress, improves mitochondrial function, and consequently increases UPS activity and cardiac performance.
In summary, our data demonstrate pronounced accumulation of ubiquitinated proteins in hearts of AKO mice, associated with decreased basal chymotrypsin-like proteasomal activity. These effects were accompanied by mitochondrial dysfunction and inflammatory oxidative stress. Chronic treatment with the PPARα agonist Wy14,643 reversed proteasomal dysfunction and reduced oxidative and inflammatory stress in AKO mice. Our data point to a hitherto unrecognized link between proteasomal function, PPARα signaling and inflammatory oxidative stress in cardiovascular disease.
Disclosures
None declared.
Acknowledgments
This work was supported by the Austrian Science Fund (Grants F3003, P24005, and W901 DK Molecular Enzymology to B.M.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2014.09.028.
Contributor Information
Marion Mussbacher, Email: marion.mussbacher@uni-graz.at.
Heike Stessel, Email: heike.stessel@uni-graz.at.
Gerald Wölkart, Email: gerald.woelkart@uni-graz.at.
Guenter Haemmerle, Email: guenter.haemmerle@uni-graz.at.
Rudolf Zechner, Email: rudolf.zechner@uni-graz.at.
Bernd Mayer, Email: mayer@uni-graz.at.
Astrid Schrammel, Email: astrid.schrammel-gorren@uni-graz.at.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Finley D. Recognition and processing of ubiquitin–protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Predmore J.M., Wang P., Davis F., Bartolone S., Westfall M.V., Dyke D.B. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukamoto O., Minamino T., Okada K., Shintani Y., Takashima S., Kato H. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun. 2006;340:1125–1133. doi: 10.016/j.bbrc.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 4.Divald A., Powell S.R. Proteasome mediates removal of proteins oxidized during myocardial ischemia. Free Radic Biol Med. 2006;40:156–164. doi: 10.1016/j.freeradbiomed.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Powell S.R., Wang P., Katzeff H., Shringarpure R., Teoh C., Khaliulin I. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–546. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q., Liu J.B., Horak K.M., Zheng H., Kumarapeli A.R., Li J. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–1026. doi: 10.161/01.RES.0000189262.92896.0b. [DOI] [PubMed] [Google Scholar]
- 7.Ling Y.H., Liebes L., Zou Y., Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 8.Robinson E., Grieve D.J. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol Ther. 2009;122:246–263. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.38/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 11.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 12.Wölkart G., Schrammel A., Dörffel K., Haemmerle G., Zechner R., Mayer B. Cardiac dysfunction in adipose triglyceride lipase deficiency: treatment with a PPARα agonist. Br J Pharmacol. 2012;165:380–389. doi: 10.1111/j.476-5381.2011.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrammel A., Mussbacher M., Winkler S., Haemmerle G., Stessel H., Wölkart G. Cardiac oxidative stress in a mouse model of neutral lipid storage disease. Biochim Biophys Acta. 2013;1831:1600–1608. doi: 10.016/j.bbalip.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guellich A., Damy T., Conti M., Claes V., Samuel J.L., Pineau T. Tempol prevents cardiac oxidative damage and left ventricular dysfunction in the PPAR-α KO mouse. Am J Physiol Heart Circ Physiol. 2013;304:H1505–H1512. doi: 10.152/ajpheart.00669.2012. [DOI] [PubMed] [Google Scholar]
- 15.Staels B., Koenig W., Habib A., Merval R., Lebret M., Torra I.P. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Zhu H., Kuppusamy P., Roubaud V., Zweier J.L., Trush M.A. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 17.Groenendyk J., Sreenivasaiah P.K., Kim do H., Agellon L.B., Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.61/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 18.Gordon J.W., Shaw J.A., Kirshenbaum L.A. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res. 2011;108:1122–1132. doi: 10.61/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki C.Y., Barberi T.J., Ghosh P., Longo D.L. Phosphorylation of RelA/p65 on serine 536 defines an IκBα-independent NF-κB pathway. J Biol Chem. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 20.Powell S.R., Davies K.J., Divald A. Optimal determination of heart tissue 26S-proteasome activity requires maximal stimulating ATP concentrations. J Mol Cell Cardiol. 2007;42:265–269. doi: 10.1016/j.yjmcc.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kratky D., Obrowsky S., Kolb D., Radovic B. Pleiotropic regulation of mitochondrial function by adipose triglyceride lipase-mediated lipolysis. Biochimie. 2014;96:106–112. doi: 10.1016/j.biochi.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majetschak M. Regulation of the proteasome by ATP: implications for ischemic myocardial injury and donor heart preservation. Am J Physiol Heart Circ Physiol. 2013;305:H267–H278. doi: 10.1152/ajpheart.00206.2012. [DOI] [PubMed] [Google Scholar]
- 23.Bulteau A.L., Lundberg K.C., Humphries K.M., Sadek H.A., Szweda P.A., Friguet B. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 24.Barlaka E., Lédvenyiová V., Galatou E., Ferko M., Čarnická S., Ravingerová T. Delayed cardioprotective effects of WY-14643 are associated with inhibition of MMP-2 and modulation of Bcl-2 family proteins through PPAR-α activation in rat hearts subjected to global ischaemia–reperfusion. Can J Physiol Pharmacol. 2013;91:608–616. doi: 10.1139/cjpp-2012-0412. [DOI] [PubMed] [Google Scholar]
- 25.Hwang J.J., Allen P.D., Tseng G.C., Lam C.W., Fananapazir L., Dzau V.J. Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol Genomics. 2002;10:31–44. doi: 10.1152/physiolgenomics.00122.2001. [DOI] [PubMed] [Google Scholar]
- 26.Severino A., Campioni M., Straino S., Salloum F.N., Schmidt N., Herbrand U. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–1037. doi: 10.16/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs C.D., Claudel T., Kumari P., Haemmerle G., Pollheimer M.J., Stojakovic T. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270–280. doi: 10.1002/hep.25601. [DOI] [PubMed] [Google Scholar]
- 28.Purcell N.H., Tang G., Yu C., Mercurio F., DiDonato J.A., Lin A. Activation of NF-κB is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci U S A. 2001;98:6668–6673. doi: 10.1073/pnas.111155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiken C.T., Kaake R.M., Wang X., Huang L. Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110. [R110.006924] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T., Akagawa M., Naito Y., Handa O., Takagi T., Mori T. Pro-oxidant action of pyrroloquinoline quinone: characterization of protein oxidative modifications. Biosci Biotechnol Biochem. 2010;74:663–666. doi: 10.1271/bbb.90764. [DOI] [PubMed] [Google Scholar]
- 31.Demasi M., Silva G.M., Netto L.E. 20 S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J Biol Chem. 2003;278:679–685. doi: 10.1074/jbc.M209282200. [DOI] [PubMed] [Google Scholar]
- 32.Demasi M., Shringarpure R., Davies K.J. Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch Biochem Biophys. 2001;389:254–263. doi: 10.1006/abbi.2001.332. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman E.R. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 34.Marfella R., Siniscalchi M., Portoghese M., Di Filippo C., Ferraraccio F., Schiattarella C. Morning blood pressure surge as a destabilizing factor of atherosclerotic plaque: role of ubiquitin-proteasome activity. Hypertension. 2007;49:784–791. doi: 10.1161/01.HYP.0000259739.64834.d4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.