Abstract
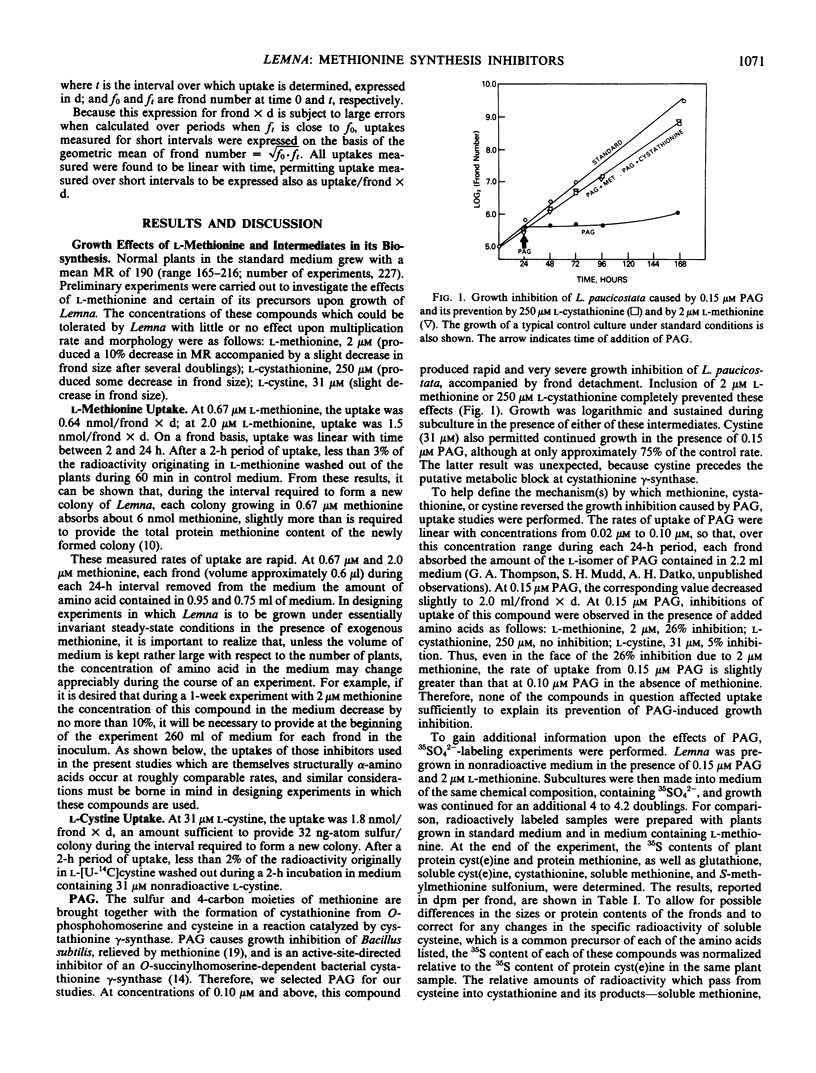
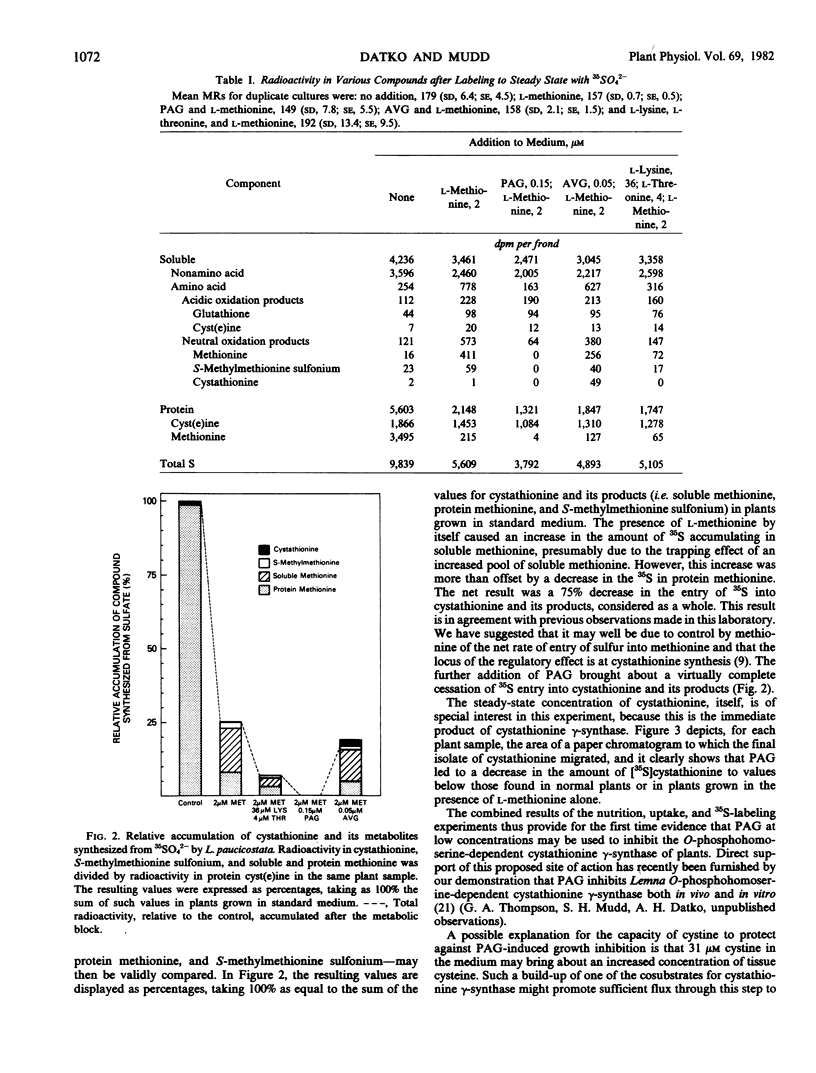
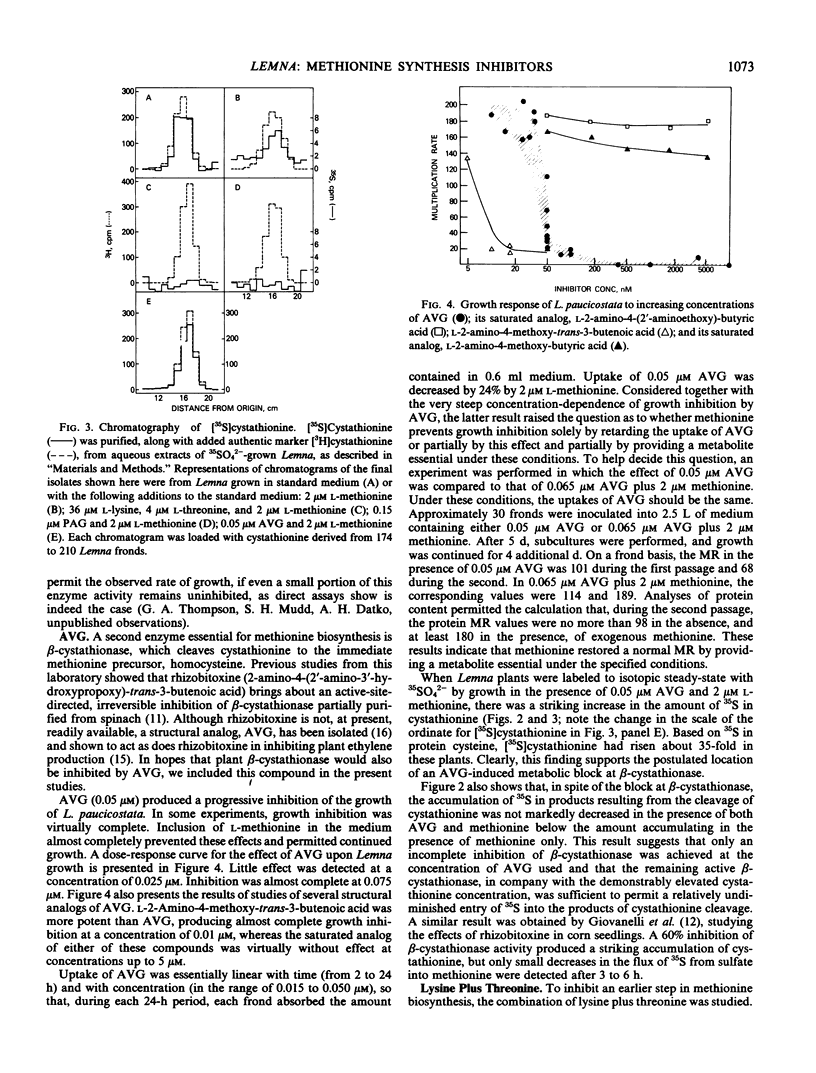
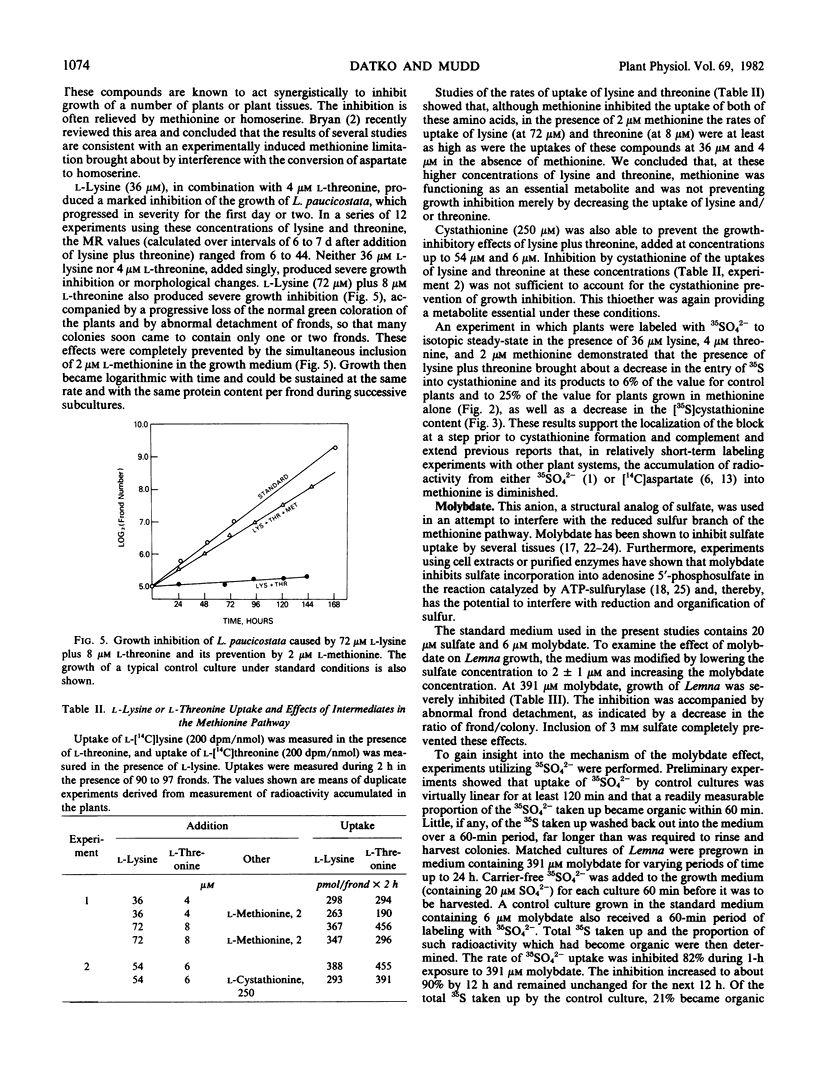
A search was made for compounds that would inhibit methionine biosynthesis in Lemna paucicostata Hegelm. 6746. dl-Propargylglycine (0.15 micromolar) produced growth inhibition and morphological changes which were prevented by exogenous methionine. Also, dl-propargylglycine inhibits cystathionine γ-synthase activity. l-Aminoethoxyvinylglycine (0.05 micromolar) produced growth inhibition and morphological changes partially preventable by exogenous methionine. l-Aminoethoxyvinylglycine impairs the cleavage of cystathionine to homocysteine. Lysine and threonine, at concentrations which individually had little effect on growth or morphology of Lemna, together produced growth inhibition and morphological changes preventable by exogenous methionine. The resulting metabolic block prevented conversion of cysteine to cystathionine, presumably secondary to depletion of the supply of O-phosphohomoserine.
Inhibition of Lemna growth resulted when the molybdate:sulfate ratio in the medium was increased to 20:1 or more. Such inhibition was prevented by lowering this ratio to 0.3 or less. A non-steady-state experiment (molybdate:sulfate, 20:1) showed that molybdate inhibited sulfate uptake, but it provided no evidence of a further impairment in the organification of sulfate. Molybdate-induced growth inhibition of Lemna was prevented by cystine but not by cystathionine or methionine. Cystathionine is not converted by Lemna to cysteine rapidly enough to sustain growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham V. L., Bryan J. K. Synergistic effects of metabolically related amino acids on the growth of a multicellular plant. II. Studies of 14C-amino acid incorporation. Plant Physiol. 1971 Jan;47(1):91–97. doi: 10.1104/pp.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Transsulfuration in higher plants. Partial purification and properties of beta-cystathionase of spinach. Biochim Biophys Acta. 1971 Mar 10;227(3):654–670. doi: 10.1016/0005-2744(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. Mechanism of inhibition of spinach beta-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971 Mar 10;227(3):671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. beta-Cystathionase In Vivo Inactivation by Rhizobitoxine and Role of the Enzyme in Methionine Biosynthesis in Corn Seedlings. Plant Physiol. 1973 Mar;51(3):492–503. doi: 10.1104/pp.51.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Jankowski D., Marcotte P., Tanaka H., Esaki N., Soda K., Walsh C. Suicide inactivation of bacterial cystathionine gamma-synthase and methionine gamma-lyase during processing of L-propargylglycine. Biochemistry. 1979 Oct 16;18(21):4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- Pruess D. L., Scannell J. P., Kellett M., Ax H. A., Janecek J., Williams T. H., Stempel A., Berger J. Antimetabolites produced by microorganisms. X. L-2-amino-4-(2-aminoethoxy)-trans-3-butenoic acid. J Antibiot (Tokyo) 1974 Apr;27(4):229–233. doi: 10.7164/antibiotics.27.229. [DOI] [PubMed] [Google Scholar]
- Scannell J. P., Pruess D. L., Demny T. C., Weiss F., Williams T. Antimetabolites produced by microorganisms. II. L-2-amino-4-pentynoic acid. J Antibiot (Tokyo) 1971 Apr;24(4):239–244. doi: 10.7164/antibiotics.24.239. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Specificity of transport processes for sulfur, selenium, and molybdenum anions by filamentous fungi. Biochim Biophys Acta. 1970 Jan 6;196(1):95–106. doi: 10.1016/0005-2736(70)90170-7. [DOI] [PubMed] [Google Scholar]
- Vallée M., Jeanjean R. Le système de transport de SO4= chez Chlorella pyrenoidosa et sa régulation. I. Etude cinétique de la perméation. Biochim Biophys Acta. 1968 Jun 11;150(4):599–606. doi: 10.1016/0005-2736(68)90049-7. [DOI] [PubMed] [Google Scholar]
- Varma A. K., Nicholas D. J. Purification and properties of ATP-sulphurylase from Nitrobacter agilis. Biochim Biophys Acta. 1971 Feb 10;227(2):373–389. doi: 10.1016/0005-2744(71)90069-6. [DOI] [PubMed] [Google Scholar]


